Introduction
Non-small cell lung cancer (NSCLC) is the most
common malignancy in northern China, despite its declining
incidence. The estimated overall 5-year survival rate is only 16%
(1). A more complete understanding
of the molecular mechanism of lung cancer will aid in the
development of new treatment modalities, diagnostic technologies
and preventive approaches. Thus, increased numbers of molecular
markers need to be investigated in order to clarify the features of
NSCLC and to ensure effective treatment.
Recent research indicates that the expression of
chemokine receptors plays an important role in determining the
metastatic potential of tumor cells (2). C-X-C chemokine receptor type 4
(CXCR4) is functionally expressed on the cell surface of various
types of cancer cells and plays a role in the cell proliferation
and migration of these cells (3).
CXCR4 belongs to the superfamily of seven transmembrane domain
heterotrimeric G protein-coupled receptors that are physiologically
involved in the migration of various hematopoietic cells to
home-specific anatomical sites through local interaction with their
specific ligands. CXCR4 is overexpressed in colorectal cancer
(4), hepatocellular carcinoma
(5), ovarian cancer (6), and renal cell carcinoma (7), and is associated with chemotaxis,
invasion, angiogenesis and cell proliferation.
Signal transducer and activator of transcription 3
(STAT3) is a key pathway that regulates metastasis in human cancer
cells (8), and persistent
activation of STAT3 may lead to oncogenesis by promoting tumor
angiogenesis, cell proliferation, and resistance to apoptosis
(9). In invasive breast cancer
tissue, elevated levels of STAT3 phosphorylation were significantly
associated with increased expression of downstream targets of
STAT3, including inducers of tumor angiogenesis [vascular
endothelial growth factor (VEGF), metalloproteinase (MMP)-2, MMP-10
and cyclooxygenase (COX)-2] (10).
Tumor growth and development is a complex, multistep process; one
essential step for tumor growth is angiogenesis, which plays a
critical role in tumor invasion and metastasis (11). VEGF is believed to be the principal
growth stimulatory factor for tumor-related angiogenesis.
Constitutive activation of STAT3 is able to up-regulate VEGF-A
expression in human pancreatic and colorectal cancer cells
(12,13).
Therefore, the clinical significance and possible
prognostic value of CXCR4, P-STAT3 and VEGF-A expression in
patients with completely resected NSCLC was evaluated in the
present study to determine the effects of CXCR4 in tumor
angiogenesis and metastasis.
Materials and methods
Patients and samples
A total of 208 cases with pathologically confirmed
NSCLC were involved in this study. The patients underwent
potentially curative tumor resection at the Tumor Hospital of
Harbin Medical University from 2002 to 2004. The patients received
neither chemotherapy nor radiation therapy prior to surgery.
Routinely processed formalin-fixed, paraffin-embedded blocks
containing principal tumors were selected for the study. Serial
sections (4 μm) were prepared from the cut surface of the blocks at
the maximum cross-sectional location of the tumor. Informed consent
was obtained from all patients. The study was approved by the
Ethics Committee of the Harbin Medical University.
Immunohistochemistry
Immunohistochemical staining for the CXCR4, P-STAT3
and VEGF-A antigen was performed using the standard
streptavidin-peroxidase biotin technique (SP technique) with a SP
kit (Zhongshan Co., Beijing, China). Paraffin sections (4 μm) were
deparaffinized in xylene and then rehydated through graded alcohol.
Hydrated autoclave pre-treatment was carried out by boiling for 5
min in citrate buffer (10 mM, pH 6.0). After endogenous peroxidase
was quenched in 3% hydrogen peroxide and blocked for 10 min, the
sections were incubated overnight at 4˚C with antibodies against
CXCR4 (R&D Systems) at a 1:200 dilution, P-STAT3 (Cell
Signalling Technology) at a 1:150 dilution and VEGF-A (Neomarkers)
at a 1:200 dilution. Biotinylated immunoglobulin and streptavidin
conjugated to peroxidase were then added. Finally,
3,3′-diaminobenzidine was added for color development, and
hematoxylin was used for counterstaining. Negative control slides
processed without the primary antibody were included for each
staining. The mean percentage of positive tumor cells was
determined in at least five areas at x200 magnification. All slides
were evaluated by experienced pathologists who reviewed the slides
together and reached a consensus. Positive expression for P-STAT3
was defined as >25% nuclear staining with greater than moderate
staining intensity of tumor cells. The staining of both CXCR4 and
VEGF-A was mainly localized in the cytoplasm. CXCR4 and VEGF were
scaled as follows: 0, <5%; 1, 5–25%; 2, 25–50%; 3, 50–75%; and
4, >75% positively stained cells. The intensity of
immunostaining was scored as follows: 0, none; 1+, weak; 2+,
moderate; 3+, intense. The scores for the percentage of positive
tumor cells and staining intensity were multiplied together to
achieve a weighted score for each case. Cases with weighted scores
0 or 1 were defined as negative; cases with scores ≥2 were defined
as positive.
Statistical analysis
All data were analyzed by statistical software (SPSS
13.0 for Windows; SPSS, Inc.). The association between CXCR4,
P-STAT3 and VEGF-A expression and clinicopathological parameters
was analyzed using the χ2 test. Correlations among the
levels of CXCR4, P-STAT3 and VEGF-A protein in NSCLC tissues were
determined using Pearson’s correlation coefficient (r) analysis.
The survival curves were plotted according to the Kaplan-Meier
method and validated by the log-rank test. Univariate and
multivariate regression analyses were performed using the Cox
proportional hazards regression model to analyze the independent
factors related to prognosis. For all of the tests, P<0.05 was
considered to be statistically significant.
Results
Patient characteristics
Data from a total of 208 patients, 128 male and 80
female, were analyzed. The clinicopathologic features are
summarized in Table I. The mean
age of the patients was 59.8 years (range, 35–76). There were 106
patients who had never been smokers (50.1%). Pathological tumor
stage determined according to the American Joint Committee on
Cancer classification included 88 (42.3%) stage I, 70 (33.7%) stage
II and 50 (24%) stage III cases. Of the total cases, 106 tumors
were squamous, 90 were adenocarcinoma and 12 were large-cell
carcinomas. Among the patients with stage III disease, 41 (82%) had
lymph node-positive (N2) disease, and the remaining patients had T4
or T3 tumors. The median follow-up was 67 months (range, 1–78.2),
the median disease-free survival was 32.6 months (95% confidence
interval, 12–53.2 months), and the median overall survival was 46.2
months. There were 76 patients who had died by the time of the
current analysis, with the most common cause of death being disease
progression (57 patients).
 | Table I.Correlation between expression of
CXCR4, P-STAT3 and VEGF-A and clinicopathological features. |
Table I.
Correlation between expression of
CXCR4, P-STAT3 and VEGF-A and clinicopathological features.
| | CXCR4
| P-STAT3
| VEGF-A
|
|---|
| Features | All patients
(n=208) | Negative (n=91) | Positive (n=117) | P-value | Negative (n=112) | Positive (n=96) | P-value | Negative (n=100) | Positive (n=108) | P-value |
|---|
| Age (years) | | | | | | | | | | |
| <60 | 89 | 37 | 52 | 0.584 | 51 | 38 | 0.387 | 48 | 41 | 0.144 |
| ≥60 | 119 | 54 | 65 | | 61 | 58 | | 52 | 67 | |
| Gender | | | | | | | | | | |
| Male | 128 | 54 | 74 | 0.566 | 67 | 61 | 0.582 | 59 | 69 | 0.469 |
| Female | 80 | 37 | 43 | | 45 | 35 | | 41 | 39 | |
| Smoking | | | | | | | | | | |
| Never | 106 | 47 | 59 | 0.859 | 62 | 44 | 0.320 | 53 | 53 | 0.360 |
| Former | 81 | 36 | 45 | | 41 | 40 | | 40 | 41 | |
| Current | 21 | 8 | 13 | | 9 | 12 | | 7 | 14 | |
| Stage | | | | | | | | | | |
| I | 88 | 54 | 34 | 0.000 | 52 | 36 | 0.231 | 52 | 36 | 0.004 |
| II | 70 | 32 | 38 | | 38 | 32 | | 33 | 37 | |
| III | 50 | 5 | 45 | | 22 | 28 | | 15 | 35 | |
| Tumor
classification | | | | | | | | | | |
| T1 | 83 | 49 | 34 | 0.000 | 53 | 30 | 0.090 | 48 | 35 | 0.002 |
| T2 | 73 | 31 | 42 | | 37 | 36 | | 39 | 34 | |
| T3 | 41 | 8 | 33 | | 17 | 24 | | 10 | 31 | |
| T4 | 11 | 3 | 8 | | 5 | 6 | | 3 | 8 | |
| Lymph node
status | | | | | | | | | | |
| N0 | 66 | 38 | 28 | 0.011 | 44 | 22 | 0.016 | 43 | 23 | 0.003 |
| N1 | 101 | 41 | 60 | | 52 | 49 | | 39 | 62 | |
| N2 | 41 | 12 | 29 | | 16 | 25 | | 18 | 23 | |
| Tumor size | | | | | | | | | | |
| <2 cm | 71 | 43 | 28 | 0.001 | 37 | 34 | 0.935 | 37 | 34 | 0.462 |
| 2–5 cm | 113 | 42 | 71 | | 62 | 51 | | 54 | 59 | |
| >5 cm | 24 | 6 | 18 | | 13 | 11 | | 9 | 15 | |
| Histology | | | | | | | | | | |
| Squamous-cell
carcinoma | 106 | 50 | 56 | 0.241 | 54 | 52 | 0.689 | 49 | 57 | 0.714 |
|
Adenocarcinoma | 90 | 34 | 56 | | 51 | 39 | | 44 | 46 | |
| Large-cell
carcinoma | 12 | 7 | 5 | | 7 | 5 | | 7 | 5 | |
| Pathological
stage | | | | | | | | | | |
| G1 | 41 | 20 | 21 | 0.449 | 27 | 14 | 0.198 | 23 | 18 | 0.518 |
| G2 | 91 | 42 | 49 | | 48 | 43 | | 42 | 49 | |
| G3 | 76 | 29 | 47 | | 37 | 39 | | 35 | 41 | |
Expression of CXCR4, P-STAT3 and VEGF in
NSCLC
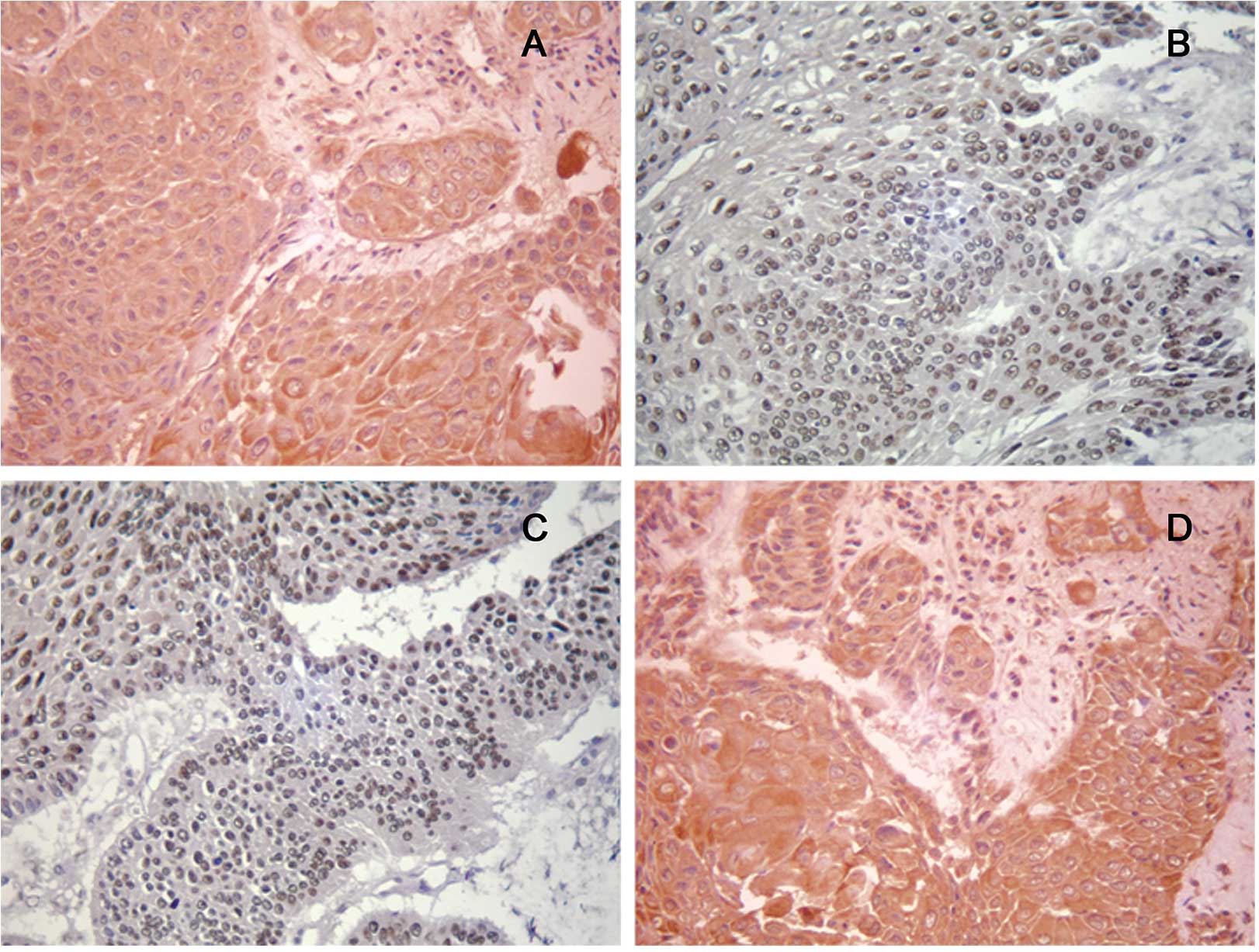
The staining of CXCR4 and VEGF-A was observed
predominantly in the cytoplasm of the tumor cells, whereas staining
for P-STAT3 appeared in the tumor cell nucleus. Representative
immunohistochemical staining of CXCR4, P-STAT3 and VEGF-A are
provided in Fig. 1. Expression of
CXCR4, P-STAT3 and VEGF-A was detected in 117 (56.3%) samples, 96
(46.2%) samples, and 108 (51.9%) samples, respectively. A
correlation was noted between P-STAT3 and CXCR4 (r=0.136, P=0.050),
P-STAT3 and VEGF-A (r=0.231, P=0.001) and CXCR4 and VEGF-A
(r=0.165, P=0.017) (Table II).
 | Table II.Correlations between CXCR4, P-STAT3
and VEGF-A expression in the NSCLC tissues. |
Table II.
Correlations between CXCR4, P-STAT3
and VEGF-A expression in the NSCLC tissues.
| P-STAT3
| VEGF-A
|
|---|
| Negative
expression | Positive
expression | r | P-value | Negative
expression | Positive
expression | r | P-value |
|---|
| CXCR4 | | | | | | | | |
| Negative
expression | 56 | 35 | 0.136 | 0.050 | 54 | 37 | 0.165 | 0.017 |
| Positive
expression | 56 | 61 | | | 50 | 67 | | |
| VEGF | | | | | | | | |
| Negative
expression | 68 | 36 | 0.231 | 0.001 | | | | |
| Positive
expression | 44 | 60 | | | | | | |
Relationship between protein expression
and clinical parameters
The relationship between CXCR4, P-STAT3 and VEGF-A
expression and clinicopathological features of NSCLC is shown in
Table I. Statistical analyses were
performed to examine the relationship between the expression of
CXCR4 and the clinicopathological features of NSCLC. CXCR4 was
found to be significantly correlated with tumor classification,
lymph node metastasis, stage and tumor size (P<0.05). The
expression of P-STAT3 was significantly associated with lymph node
metastasis (P<0.05). Moreover, a weak association was found
between P-STAT3 and tumor classification (P=0.09). The expression
of VEGF-A was significantly correlated with stage, tumor
classification and lymph node metastasis (P<0.05).
Overall survival for patients with
resected NSCLC according to expression levels of CXCR4, P-STAT3 and
VEGF-A
The overall survival time of patients with CXCR4-,
P-STAT3- and VEGF-A-positive cancers was significantly lower
compared with the survival time of patients with CXCR4-,
P-STAT3-and VEGF-A-negative cancers (all P<0.05) (Fig. 2).
Univariate and multivariate analyses of
prognostic factors for survival of the NSCLC patients
Univariate analysis showed that smoking, tumor size,
lymph node status, stage, tumor classification, pathological stage,
CXCR4, P-STAT3 and VEGF expression were significantly correlated
with overall patient survival. We carried out multivariate survival
analysis as described in statistical analysis. The findings further
revealed that tumor size, lymph node metastasis, tumor
classification, stage, CXCR4 and VEGF expression were identified as
independent predictive factors (Table
III).
 | Table III.Univariate and multivariate analyses
of prognostic factors for survival of the NSCLC patients. |
Table III.
Univariate and multivariate analyses
of prognostic factors for survival of the NSCLC patients.
| Univariate analysis
| Multivariate
analysis
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<60 vs. ≥60
years) | 1.151 | 0.807–1.642 | 0.438 | 1.162 | 0.802–1.683 | 0.427 |
| Tumor size (<2
vs. ≥2 cm) | 2.076 | 1.386–3.110 | 0.000 | 1.638 | 1.074–2.498 | 0.022 |
| Smoking (never vs.
former, current) | 1.497 | 1.055–2.125 | 0.024 | 1.073 | 0.748–1.537 | 0.703 |
| Lymph node status
(N0 vs. N1, N2) | 3.782 | 2.474–5.781 | 0.000 | 2.341 | 1.684–3.255 | 0.000 |
| Stage (I vs. II,
III) | 3.217 | 2.368–4.372 | 0.000 | 2.275 | 1.636–3.162 | 0.000 |
| Tumor
classification (T1, T2, vs. T4, T3) | 3.572 | 2.463–5.180 | 0.000 | 1.804 | 1.172–2.778 | 0.007 |
| Pathological stage
(G1 vs. G2, G3) | 1.832 | 1.146–2.930 | 0.011 | 1.397 | 0.865–2.259 | 0.172 |
| CXCR4 (negative vs.
positive) | 3.006 | 2.049–4.412 | 0.000 | 2.070 | 1.365–3.140 | 0.001 |
| Phosphorylated
STAT3 (negative vs. positive) | 1.768 | 1.240–2.520 | 0.002 | 1.290 | 0.886–1.877 | 0.184 |
| VEGF (negative vs.
positive) | 2.330 | 1.620–3.353 | 0.000 | 1.751 | 1.194–2.567 | 0.004 |
Discussion
Chemokines, produced by cancer-associated
fibroblasts, a component of stromal cells, influence the metastatic
potential and site-specific dissemination of cancer cells (14). Endogenous CXCR4 expression on
carcinoma cells is known to correlate with a poor prognosis for
several carcinoma types (15–17).
The knockdown of CXCR4 expression by small interfering RNA in
breast carcinoma cells decreases cell invasion and adhesion in
vitro and abrogates tumor growth in vivo (18). In small-cell lung cancer (SCLC)
cells, CXCR4 antagonists such as plerixafor (AMD3100) and T140
analogues (TN14003/ BKT140) disrupt CXCR4-mediated SCLC cell
adhesion to stromal cells, thereby sensitizing SCLC cells to
cytotoxic drugs, such as etoposide, and antagonizing cell
adhesion-mediated drug resistance (19). These findings suggest that CXCR4
may be a potent oncogenic molecule that plays an important role in
NSCLC.
Our study was comprised of a sufficiently large
number of participants to demonstrate that the CXCR4 protein was
expressed with great frequency (56.3%) in 208 NSCLC tissue samples.
Furthermore, CXCR4 exhibited a significant positive correlation
with tumor classification, lymph node metastasis, stage and tumor
size. CXCR4 expression was significantly correlated with P-STAT3
expression, and it was an independent prognostic factor for
survival. The results strongly indicate that CXCR4 has the
potential to be utilized as a novel biomarker to identify the
progression of NSCLC and predict high-risk patients. It should be
noted that CXCR4 staining in our series was primarily located in
the cytoplasm. However, in six cases it was found exclusively in
the nucleus. This phenomenon has also been described by Na et
al (20), and may represent a
functional status of the receptor; however, we cannot reach any
conclusion concerning the different localization as there was not a
sufficient number of examined events.
STAT3 is constitutively activated by numerous
cytokines, growth factors and oncogenic proteins in many types of
human cancers, and it participates in the regulation of malignant
processes (9,21,22);
moreover, it can directly or indirectly regulate genes related to
cell proliferation, metastasis and survival (23,24).
STAT3 is a key pathway that regulates survival in human NSCLC
(25). In glioblastoma stem cells
(GSCs), knockdown of STAT3 induces apoptosis and significantly
reduces the expression of Bcl-2 and cyclin-D, suggesting that STAT3
is an important target for human GSCs (26). STAT3 is important in the metastatic
process, and blockage of activated STAT3 significantly suppresses
MMP-2 expression, and brain and lung tumor cell metastasis
(27). In the current study 46.2%
of the patients expressed P-STAT3, consistent with previous studies
(28,29). We found a significant association
between P-STAT3 and lymph node status (P=0.016), and a weak
association between P-STAT3 and tumor classification (P=0.090);
this supports the finding that activated STAT3 contributes to the
invasion of NSCLC cells. Therefore, activation of STAT3 may play an
important role in tumor invasion and nodal metastasis in NSCLC.
Moreover, P-STAT3 expression significantly correlated with VEGF
expression, but was not identified as an independent prognostic
factor for overall survival in the present study.
Angiogenesis, the formation of new tumor-feeding
blood vessels from preexisting vasculature, is critical for the
development and subsequent growth of human tumors and is a
prerequisite for metastasis. VEGF is considered to be the principal
vascular growth factor prompting tumor angiogenesis. The expression
of VEGF is associated with poor prognosis and increased resistance
to therapeutic intervention in several types of neoplasms (11,30).
Our study revealed that the overexpression of VEGF-A as determined
in the NSCLC tumor tissue samples was significantly correlated with
lymph node metastasis, stage and tumor classification.
Co-expression was noted between VEGF-A and CXCR4 expression, which
is in accordance with a study by Oda et al where similar
co-expression was reported (17).
In the present study, the overexpression of VEGF-A was a poor
independent prognostic factor for overall survival.
In the present study, co-expression was found
between CXCR4 and P-STAT3, P-STAT3 and VEGF-A, and finally CXCR4
and VEGF-A. CXCR4 expression was observed in 56.3% of the NSCLC
samples and was correlated with tumor classification, lymph node
metastasis, stage and tumor size. Furthermore, 46.2% of the
patients expressed P-STAT3, and a significant association was found
between P-STAT3 and lymph node status and a weak association
between P-STAT3 and tumor classification. Overexpression of VEGF-A
in the NSCLC tumor tissues was significantly correlated with lymph
node metastasis, stage and tumor classification. The present study
suggests that these proteins may contribute to tumor progression
and angiogenesis, and the results also indicate that CXCR4 may
promote metastasis through the STAT3 signaling pathway. Further
studies are needed in order to reveal the detailed mechanism that
underlies the role that these proteins play in NSCLC.
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2.
|
Muller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Burger JA and Kipps TJ: CXCR4: a key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 107:1761–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schimanski CC, Schwald S, Simiantonaki N,
et al: Effect of chemokine receptors CXCR4 and CCR7 on the
metastatic behavior of human colorectal cancer. Clin Cancer Res.
11:1743–1750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Schimanski CC, Bahre R, Gockel I, et al:
Dissemination of hepatocellular carcinoma is mediated via chemokine
receptor CXCR4. Br J Cancer. 95:210–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jiang YP, Wu XH, Shi B, Wu WX and Yin GR:
Expression of chemokine CXCL12 and its receptor CXCR4 in human
epithelial ovarian cancer: an independent prognostic factor for
tumor progression. Gynecol Oncol. 103:226–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wang L, Wang L, Yang B, et al: Strong
expression of chemokine receptor CXCR4 by renal cell carcinoma
cells correlates with metastasis. Clin Exp Metastasis.
26:1049–1054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Devarajan E and Huang S: STAT3 as a
central regulator of tumor metastases. Curr Mol Med. 9:626–633.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yu H and Jove R: The STATs of cancer – new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
|
|
10.
|
Hsieh FC, Cheng G and Lin J: Evaluation of
potential Stat3-regulated genes in human breast cancer. Biochem
Biophys Res Commun. 335:292–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yasumitsu A, Tabata C, Tabata R and
Hirayama N: Clinical significance of serum vascular endothelial
growth factor in malignant pleural mesothelioma. J Thorac Oncol.
5:479–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Huang C, Yang G, Jiang T, Huang K, Cao J
and Qiu Z: Effects of IL-6 and AG490 on regulation of Stat3
signaling pathway and invasion of human pancreatic cancer cells in
vitro. J Exp Clin Cancer Res. 29:512010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cascio S, Ferla R, D’Andrea A, Gerbino A,
Bazan V, Surmacz E and Russo A: Expression of angiogenic
regulators, VEGF and leptin, is regulated by the EGF/PI3K/STAT3
pathway in colorectal cancer cells. J Cell Physiol. 221:189–194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar
|
|
15.
|
Kim J, Takeuchi H, Lam ST, et al:
Chemokine receptor CXCR4 expression in colorectal cancer patients
increases the risk for recurrence and for poor survival. J Clin
Oncol. 23:2744–2753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Maréchal R, Demetter P, Nagy N, Berton A,
Decaestecker C, Polus M, Closset J, Devière J, Salmon I and van
Laethem JL: High expression of CXCR4 may predict poor survival in
resected pancreatic adenocarcinoma. Br J Cancer. 100:1444–1451.
2009.PubMed/NCBI
|
|
17.
|
Oda Y, Tateishi N, Matono H, Matsuura S,
Yamamaoto H, Tamiya S, Yokoyama R, Matsuda S, Iwamoto Y and
Tsuneyoshi M: Chemokine receptor CXCR4 expression is correlated
with VEGF expression and poor survival in soft-tissue sarcoma. Int
J Cancer. 124:1852–1859. 2009.PubMed/NCBI
|
|
18.
|
Lapteva N, Yang AG, Sanders DE, Strube RW
and Chen SY: CXCR4 knockdown by small interfering RNA abrogates
breast tumor growth in vivo. Cancer Gene Ther. 12:84–89. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Burger JA and Stewart DJ: CXCR4 chemokine
receptor antagonists: perspectives in SCLC. Expert Opin Investig
Drugs. 18:481–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Na IK, Scheibenbogen C, Adam C, Stroux A,
Ghadjar P, Thiel E, Keilholz U and Coupland SE: Nuclear expression
of CXCR4 in tumor cells of non-small cell lung cancer is correlated
with lymph node metastasis. Hum Pathol. 39:1751–1755. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bromberg J: Stat proteins and oncogenesis.
J Clin Invest. 109:1139–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zaid Al, Siddiquee K and Turkson J: STAT3
as a target for inducing apoptosis in solid and hematological
tumors. Cell Res. 18:254–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Garcia R, Bowman TL, Niu G, et al:
Constitutive activation of Stat3 by the Src and JAK tyrosine
kinases participates in growth regulation of human breast carcinoma
cells. Oncogene. 20:2499–2513. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Barbieri I, Pensa S, Pannellini T, et al:
Constitutively active Stat3 enhances neu-mediated migration and
metastasis in mammary tumors via upregulation of Cten. Cancer Res.
70:2558–2567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Song L, Turkson J, Karras JG, Jove R and
Haura EB: Activation of STAT-3 by receptor tyrosine kinases and
cytokines regulates survival in human non-small cell carcinoma
cells. Oncogene. 22:4150–4165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Li GH, Wei H, Lv SQ, Ji H and Wang DL:
Knockdown of STAT3 expression by RNAi suppresses growth and induces
apoptosis and differentiation in glioblastoma stem cells. Int J
Oncol. 37:103–110. 2010.PubMed/NCBI
|
|
27.
|
Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F,
Sawaya R and Huang S: Stat3 activation regulates the expression of
matrix metalloproteinase-2 and tumor invasion and metastasis.
Oncogene. 23:3550–3560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Qu P, Roberts J, Li Y, et al: Stat3
downstream genes serve as biomarkers in human lung carcinomas and
chronic obstructive pulmonary disease. Lung Cancer. 63:341–347.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kim HS, Park YH, Lee J, et al: Clinical
impact of phosphorylated signal transducer and activator of
transcription 3, epidermal growth factor receptor, p53, and
vascular endothelial growth factor receptor 1 expression in
resected adenocarcinoma of lung by using tissue microarray. Cancer.
116:676–685. 2010. View Article : Google Scholar
|
|
30.
|
Dassoulas K, Gazouli M, Theodoropoulos G,
et al: Vascular endothelial growth factor and endoglin expression
in colorectal cancer. J Cancer Res Clin Oncol. 136:703–708. 2010.
View Article : Google Scholar : PubMed/NCBI
|