Introduction
Radiofrequency (RF) ablation (RFA) is recognized as
a minimally invasive treatment for hepatocellular carcinoma (HCC)
(1–5). The ideal goal of RFA for HCC is to
obtain a reproducible ablation volume that encompasses the tumor,
surrounded by a margin of hepatic parenchyma (6,7). The
critical steps in attaining this goal are correct electrode
positioning according to the planned ablation algorithm and the
acquisition of a constant ablation volume. In RFA, the physician
positions the electrode needle in the center of the tumor under
ultrasound guidance. However, insufficient ablation may occur when
the ablation volume is more irregular or smaller than expected.
One of several commercially available RF devices is
the RF 3000TM RFA system (Boston Scientific Corporation, Natick,
MA, USA) using an umbrella-shaped expandable electrode (8). The conventional electrode (LeVeen
eletrode) has a 15 G cannula and 10 tines and was the first to
become commercially available, but it is thicker and less sharp
than another RFA device (internally cooled electrode, Radionics,
Burlington, MA, USA). Two new umbrella-shaped expandable electrodes
have become available as improved versions; one of these, the
SuperSlim electrode, has a thinner cannula and thinner tines
compared to the conventional electrode and became available in
Japan in October 2003. It enables easy puncture into the tumor and
may reduce severe complications, such as hemorrhaging (9). The other, the CoAccess electrode, is
used in combination with a coaxial insulated needle, though it has
the same thick cannula and tines as the conventional LeVeen
electrode. It became available in Japan in August 2005. The
14-gauge coaxial insulated needle enables easy puncture, and is
highly cuspidate due to its diamond-cut tip, even though its
coaxial needle is 3 gauges thicker compared to that of the
SuperSlim electrode. Although both electrodes offer improved
puncture compared with the conventional LeVeen electrode, no
previous studies have investigated the ablation effect of the two
electrodes. A number of ablation algorithms have been proposed to
achieve more efficient ablation using these electrodes; e.g., the
multi-step expansion method or additional low-power ablation
(10,11). The purpose of the present study was
to investigate the size and configuration of the ablation zones
created by the SuperSlim and CoAccess electrodes, using various
ablation algorithms in ex vivo bovine liver and in clinical
cases.
Material and methods
Experimental study
We compared two types of improved LeVeen®
electrode (Boston Scientific Corporation, Natick, MA, USA)
incorporating a 3-cm array: the SuperSlim and the CoAccess
electrode (Fig. 1). The SuperSlim
electrode has a 17 G cannula and 10 electrode tines (tine diameter:
proximal site, 0.305 mm; distal site, 0.104 mm), while the CoAccess
electrode has a 15 G cannula and 10 electrode tines (tine diameter:
proximal site, 0.34 mm; distal site, 0.162 mm) similar to the
conventional LeVeen electrode. In the CoAccess electrode procedure,
the liver was punctured initially with a 14 G coaxial insulated
needle, the stylet was removed, and the CoAccess electrode was
inserted through the coaxial needle. The cannula and the electrode
tines of the SuperSlim electrode are thinner compared to those of
the CoAccess electrode.
Explanted fresh bovine livers were prepared for
ablation studies: 2 kg of liver were placed on a copper plate with
2 grounding pads at room temperature (Fig. 2). Under sonographic guidance, the
electrode was inserted into the bovine liver from the upper side.
We compared the ablative states between the SuperSlim and the
CoAccess electrodes, both with a 3-cm array diameter, and the
following 4 algorithms. These 4 algorithms were selected based upon
manufacturer-recommended and existing clinical algorithms, namely
the combination of incremental power supply, stepwise expansion and
additional low-power ablation: algorithm 1, the tines were fully
expanded and RF energy was then applied to the tissue using an
initial power setting of 20 W, which was subsequently increased in
increments of 10 W/min until the impedance rose markedly; algorithm
2, RF energy was applied to the tissue using a fixed power setting
of 20 W. The electrode tines were expanded incrementally in 3
steps: array diameter was 15 mm at the first step, 25 mm at the
second, and 30 mm (fully expanded) at the third. At each step, RF
energy was applied at 20 W until the impedance rose markedly;
algorithm 3, the tines were expanded incrementally as in algorithm
2. RF energy was then applied to the liver using an initial power
setting of 20 W, which was subsequently increased in increments of
10 W/min until the impedance rose markedly, at each step; algorithm
4, ablation additional to that performed in algorithm 3 was applied
at 70% of maximum power at the full extension of the array until
the impedance rose markedly, or for 15 min.
During ablation, RF energy and ablation time were
recorded. After ablation, the liver was cut along the puncture line
of the RF electrode. To determine the area ablated by RFA, the
ablation area was measured from an image of the cut surface
analyzed using the freely available Image J software (National
Institute of Health, Bethesda, Maryland, USA) (http://rsbweb.nih.gov/ij/index.html). This
experimental study was performed by a single operator (M.K.).
Clinical study
From January 2004 to November 2006, 243 nodules in
186 patients were treated by RFA. Patients included in this study
fulfilled the following criteria: i) the size of the HCC nodule was
<3 cm in diameter, ii) the number of HCC nodules was ≤3, iii) no
portal thrombosis or extrahepatic metastasis were present, and iv)
RFA was performed with 3-cm array SuperSlim or CoAccess electrodes
at one position, that is, overlapping ablation at another position
was not done to enlarge ablation volume. The SuperSlim group
consisted of patients treated from January 2004 to August 2005 and
the CoAccess group of patients treated from September 2005 to
November 2006. A total amount of 24 patients (12 patients in the
SuperSlim group and 12 in the CoAccess group, respectively) were
consecutively enrolled to this study; 1 patient in the SuperSlim
group who was not evaluated by enhanced CT after treatment was
excluded due to contrast agent allergy. The study was approved by
the Ethics Committee of our institute (no. 1607) and this analysis
was conducted retrospectively. The nature of the study was fully
explained to the patients, and informed consent was obtained. We
analyzed 23 patients with HCC who were treated with percutaneous
RFA at our hospital. The preoperative clinical features of these 23
patients are listed in Table I.
All patients had underlying chronic liver disease: chronic
hepatitis in 4 patients and cirrhosis in 19, Child-Pugh grade A in
14 patients and grade B in 5. Hepatitis B surface antigen was
positive in 4 patients, hepatitis C virus antibody was positive in
17, and the remaining 2 patients were cryptogenic and alcoholics,
respectively. There were no significant differences in tumor size
between the 2 groups.
 | Table ICharacteristics of patients in the
SuperSlim and CoAccess groups. |
Table I
Characteristics of patients in the
SuperSlim and CoAccess groups.
| Characteristics | SuperSlim (n=11) | CoAccess (n=12) | p-value |
|---|
| Male/female | 9/2 | 10/2 | NS |
| Age (years) | 67.7±8.70 | 73.2±7.80 | NS |
| Etiology | | | |
| Hepatitis B | 3 | 1 | |
| Hepatitis C | 6 | 11 | NS |
| Alcoholic | 1 | 0 | |
| Cryptogenic | 1 | 0 | |
| Underlying liver
disease | | | |
| Chronic
hepatitis | 0 | 4 | |
| Cirrhosis | 11 | 8 | NS |
| Child-Pugh A | 9 | 5 | |
| Child-Pugh B | 2 | 3 | |
| Tumor size (mm) | 18.5±5.90 | 21.3±3.60 | NS |
| Mean follow-up period
(months) | 38.5±10.7 | 32.1±8.95 | NS |
Prior to RFA, all patients underwent US, enhanced CT
and MRI, and/or CT scan under arteriography and portography through
the superior mesenteric artery via a catheter. On enhanced CT or
MRI, hyper-enhancement in the arterial phase with washout in the
portal phase was designated as HCC.
RFA algorithm
After local anesthesia, RFA therapy was performed
under sonographic guidance using a real-time convex scanner with
3.75-MHz probes (SSA-360A; Toshiba, Tokyo, Japan) and a biopsy
guide device. We used the RF3000 generator system with 2 types of
electrodes (CoAccess and SuperSlim) according to algorithm 4, which
was the most efficient algorithm from the results of the
experimental studies.
In brief, the electrode was positioned in the tumor
and the array was then expanded in 3 steps of array diameters (15,
25 and 30 mm). In the first step, hooks were deployed at an array
diameter of 15 mm and RF power was initially applied at 30 W, which
was increased by 10 W/min until the impedance rose markedly. The
second step began at the RF power level reached in the first step,
and RF power was increased by 10 W/min until the impedance rose
markedly. This cycle was repeated at each step to full extension of
the array. Additional ablation was applied at 70% of maximum power
until the impedance rose markedly or for 15 min.
Imaging analysis
For post-treatment evaluation, helical multiphasic
CT examinations were performed 1 month after RFA using a
multi-detector scanner (Somatom Plus; Siemens, Forchheim, Germany)
with the following imaging protocol: tube voltage, 120 kV; tube
current, automatic mA setting; reconstruction section and interval
thickness, 3 mm; detector configuration, 32x1 mm; pitch, 27; and
gantry speed, 0.5 sec per rotation. Unenhanced CT images were
acquired, followed by triple-phase contrast-enhanced images during
power injection of 100 ml of iopamidol (Iopamiron; Nihon-Schering,
Osaka, Japan) at a rate of 2.7 ml/sec. The entire liver was scanned
3 times. Early arterial phase imaging was initiated at 10 sec, late
arterial phase imaging at 20 sec, and portal venous phase imaging
at 120 sec after initiation of the injection. All scans were
obtained with a 5 mm slice pitch. After treatment, the volume of
the RF-induced ablation zone was evaluated using Image J by
measuring the unenhanced area for each slice of dynamic CT and
summing these values. The shape of the RF-induced ablation zone was
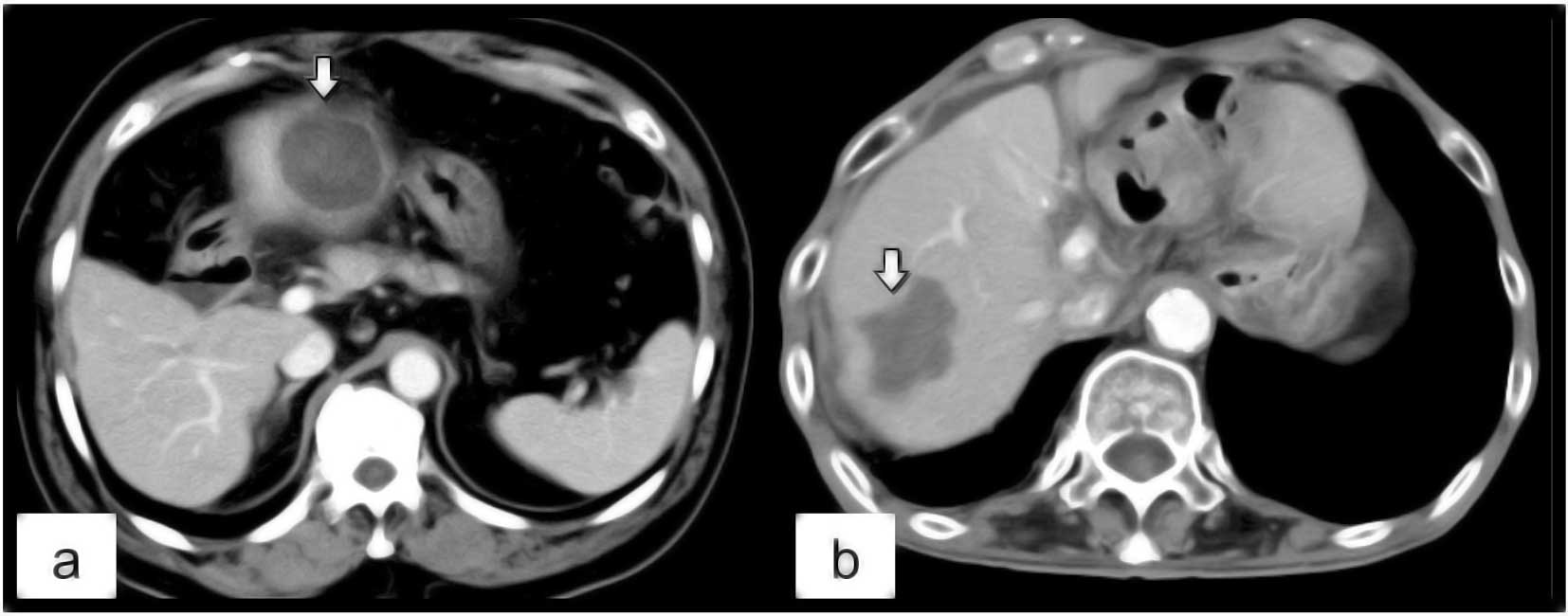
classified into 2 types: irregular and round. We defined ‘round’ as
a smooth spherical contour in the margin of the ablation zone
(Fig. 3a) and ‘irregular’ as a
lobulated, non-spherical contour (Fig.
3b). Post-ablational CT studies were independently reviewed on
a compute workstation by two abdominal imaging radiologists (M.K.
and S.T., who had 25 and 11 years experience, respectively). The
reviewers knew the diagnosis of HCC but were blinded to other
clinical data. Discrepancies between the two readers were resolved
by discussion to reach consensus.
Patients were followed-up every 3 months with
measurement of serum α-fetoprotein (normal: <12 ng/ml) and
des-γ-carboxy prothrombin (normal: <40 mAU/ml) levels, and
enhanced CT or enhanced MRI. When recurrence was suspected, the
diagnosis of intrahepatic recurrence was made in the case of
positive findings in at least two of the following: CT, MRI,
sonography, angiography and needle biopsy.
Statistical analysis
All measurements in the experimental studies were
performed 5 times except for algorithm 2 in the CoAccess electrode,
where one measurement was excluded due to the failure of ablation.
The results are shown as the means ± standard deviation (SD).
Statistical comparisons for ablation time and the area ablated by
RFA were made using the Mann-Whitney U-test with Statview software
(SAS Institute, Cary, NC, USA).
Results
Experimental study
The intra-observer coefficient of variation for the
ablation area and time were 10.7 and 9.8% in SuperSlim electrodes,
and 4.7 and 8.5% in CoAccess electrodes, respectively. The results
of ablation by SuperSlim and CoAccess electrodes for each algorithm
are shown in Table II. The total
ablation time and area of the ablation zone were significantly
greater for the CoAccess electrode compared to the SuperSlim
electrode for algorithms 1–3 (Table
II; p<0.05 for all comparisons). In algorithm 4, there was
no difference in the total ablation time between the CoAccess and
SuperSlim electrodes (p=0.726). The ablation area by the CoAccess
electrode was larger compared to that by the SuperSlim electrode,
although this difference was not statistically significant
(p=0.108).
 | Table IITotal ablation time (sec) and area
(mm2) by SuperSlim and CoAccess electrodes in ex
vivo bovine liver for the 4 algorithms. |
Table II
Total ablation time (sec) and area
(mm2) by SuperSlim and CoAccess electrodes in ex
vivo bovine liver for the 4 algorithms.
| Algorithms | | SuperSlim | No. | CoAccess | No. | p-value |
|---|
| 1 | Total ablation
time | 301±99 | | 518±120 | | 0.0325 |
| Maximal RF power
(w) | 57.5±15.0 | 5 | 83.7±11.1 | 5 | 0.0306 |
| Total ablation
area | 647±340 | | 1062±149 | | 0.0209 |
| 2 | Total ablation
time | 532±118 | 5 | 1326±189 | 4 | <0.0001 |
| Total ablation
area | 512±111 | | 1037±142 | | 0.0002 |
| 3 | Total ablation
time | 280±51 | 5 | 447±117 | 5 | 0.0407 |
| Total ablation
area | 515±59 | | 723±22 | | 0.0006 |
| 4 | Total ablation
time | 406±51 | 5 | 423±89 | 5 | 0.7260 |
| Total ablation
area | 587±198 | | 756±66 | | 0.1080 |
Clinical study
We performed retrospective evaluation of RFA with
the CoAccess and the SuperSlim electrodes. In all patients in both
groups, the increased impedance was achieved at each step of
ablation. Mean ablation volumes in the CoAccess group were
significantly larger compared to those in the SuperSlim group
(CoAccess, 22.6±9.1 cm3; SuperSlim, 14.8±5.9
cm3; p= 0.0242). Ablation times in the CoAccess group
were significantly longer compared to those in the SuperSlim group
(CoAccess, 21.1±4.1 min; SuperSlim, 15.7±4.8 min; p=0.009).
Dynamic CT after 1 month showed no residual tumor in
all HCCs treated with RFA in both groups. In the CoAccess group,
the shape of the ablation zone was round in 11 (91.7%) of 12
nodules and irregular in the remaining nodule (9.3%). In the
SuperSlim group, the shape of the ablation zone was round in 6
(54.5%) of 11 nodules and irregular in 5 (45.5%). There was
significant difference between the 2 groups regarding the shape of
the ablation zone (p=0.0428) (Fig.
3).
On enhanced CT 14 months after RFA, local tumor
progression was detected in only 1 of the 11 nodules treated using
a SuperSlim electrode. There was no local tumor progression in any
nodule treated using a CoAccess electrode. There was no significant
difference. There were no severe complications in either group.
Five patients (45%) in the SuperSlim group and 7 patients (58%) in
the CoAccess group had low or moderate grade fever.
Discussion
In RFA, several needle electrodes have been
developed, i.e., multitined expandable or cooled single electrodes
in an attempt to increase the area of tissue destruction obtained
with one RF delivery (8).
Furthermore, optimization of the algorithm to achieve appropriate
balance among ablation size, duration and precision is a
requirement for developing rational strategies for tumor ablation
(12–14). For the expandable electrode system,
we must take into account several parameters, including the defined
increments of tine extension, the duration of RF application at
each tine extension and the increment of RF supply. The
conventional and manufacturer-recommended algorithm for expandable
electrode is the combination of stepwise expansion, incremental RF
supply and additional low-power ablation.
The findings of the present study show that RF
electrode and parameter selections may influence the ablation area
and time. In the clinical setting, we aimed to obtain a large
ablation area to achieve complete tumor necrosis, while decreasing
the ablation time to reduce the patient's pain during ablation. In
the experimental study, we compared the SuperSlim and CoAccess
electrodes with regard to the ablation area and time for the
application of RF energy to the liver, using several treatment
algorithms. In algorithms 1 and 2, the CoAccess electrode required
significantly longer ablation time compared to the SuperSlim
electrode. By contrast, ablation areas achieved using the CoAccess
electrode were significantly larger compared to those using the
SuperSlim electrode. These results suggest that prolonged ablation
time leads to enlargement of the ablation area, which is reported
to change with the duration of treatment and probe gauge (12). With increased time, there is
additional diffusion of heat and greater tissue ablation until
maximum heat diffusion is achieved. Furthermore, there is a linear
correlation between the needle gauge and the ablation area
(13). The expandable tines of the
SuperSlim electrode are thinner compared to those of the CoAccess
electrode, and the tissue impedance by the SuperSlim electrode rose
earlier compared to that by the CoAccess electrode. Consequently,
ablation areas achieved by SuperSlim electrodes are smaller
compared to those by CoAccess electrodes.
Algorithm 3 employs the stepwise extension technique
proposed by Kobayashi et al (10) to obtain more efficient ablation in
a shorter period. Using this technique, a marked increase in tissue
impedance is always achieved at each step of extension, and the
total time required for ablation is less than the ablation time in
the single-step method, although we found no significant difference
between the two methods regarding ablation area. Berber et
al (15) reported that for the
ablation of tumors larger than 3 cm in diameter, ablation using an
initial smaller deployment of 20 mm to create a nucleus of ablation
can result in a larger ablation area in a shorter total ablation
time, compared with an initial larger deployment of 30 mm with
slower advancement to the final diameter. Kotoh et al
(16) reported that multi-step
ablation requires a shorter time compared to single-step ablation.
Based on the findings of these studies, we used a stepwise
extension technique in the clinical setting. Using algorithm 3, the
ablation time and area by the SuperSlim electrode were shorter and
smaller, respectively, than those by the CoAccess electrode.
In algorithm 4, in which additional ablation was
applied at 70% of maximal power, ablation areas by the SuperSlim
electrode were enlarged (although not significantly) relative to
those by the CoAccess electrode, although ablation times for the
SuperSlim electrode were longer for algorithm 4 than for 3.
According to these experimental results, we applied algorithm 4 to
the clinical RFA treatment.
In the clinical study, ablation volumes in the
CoAccess group were significantly larger compared to those in the
SuperSlim group, although no significant difference in the ablation
area was found using the same ablation algorithm in the
experimental study. One possible explanation for this discrepancy
is that hepatic blood flow may have a cooling effect and could thus
have influenced the ablative state in the clinical cases. By
contrast, ex vivo bovine liver was not perfused with blood
flow (17–19). Previous studies have demonstrated
that a reduction in blood supply, such as in transcatheter arterial
chemoembolization, results in enlarged ablative volume (20–22).
The cooling effect of the blood perfusion in the clinical cases may
have disclosed the advantage of the CoAccess electrode masked in
the ex vivo experiments. The SuperSlim electrode may not be
able to provide sufficient energy deposition to achieve appropriate
tissue heating. Solazzo et al (13) showed that the thickness of the
electrode limits energy deposition even if a high-output generator
is used.
The shape of the ablation zone is important in
achieving complete necrosis of HCC. In RFA with an expandable
electrode, the edge of the ablation zone is initially concave
between the tines as ablation begins at each expanded tine before
encompassing the lesion between the tines, becoming convex after
sufficient ablation. In the present clinical study, most of the
ablation zones by the CoAccess electrode were round in shape. By
contrast, 45% of the ablation zones by the SuperSlim electrode were
irregular, even for algorithm 4. As the expanded tines of a
conventional LeVeen electrode are the same as those of the CoAccess
electrode, our results for the CoAccess electrode are in agreement
with those of Kobayashi et al, who reported that all
ablation zones by a conventional LeVeen electrode are sphere-shaped
(10). As the tissue impedance by
the SuperSlim electrode rose early, sufficient outward enlargement
of the ablation zone could not be achieved. The other possibility
is that the complete hook deployment is interrupted by tumor
capsule or fibrotic tissue in the liver due to thinner tines. It
may be possible to obtain a more uniform ablation zone by
performing overlapping ablation with the SuperSlim electrode; i.e.,
after ablation at full extension, the expanded tines are closed,
rotated and redeployed at full extension, after which RFA is
performed once again.
The present study has certain limitations. Firstly,
the results of the ex vivo liver ablation may lead to
overestimations of the achievable results in clinical practice due
to the lack of blood flow (cooling effect). Secondly, due to the
fact that we could not measure the ablation zone in all three
dimensions in the experimental study, it was difficult to assess
the true volume and ultimate shape of the ablation zones. Thirdly,
the in vivo experimental study, which is more similar to
clinical practice, could not be performed. Fourthly, our clinical
study involved a small number of patients. We could not ethically
continue to compare both electrodes due to statistical differences
in the ablative states between both electrodes.
In conclusion, by comparing the ablative states
achieved by the SuperSlim and CoAccess electrodes in ex vivo
bovine liver and clinical cases, we demonstrated that ablation
zones achieved by the CoAccess electrode were larger and more
uniform in shape compared to those achieved by the SuperSlim
electrode, though they required a longer ablation time. We consider
that the CoAccess electrode is more useful compared to the
SuperSlim electrode for acquiring complete tumor necrosis.
References
|
1
|
Vivarelli M, Guglielmi A, Ruzzenente A, et
al: Surgical resection versus percutaneous radiofrequency ablation
in the treatment of hepatocellular carcinoma on cirrhotic liver.
Ann Surg. 240:102–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Curley SA, Izzo F, Ellis LM, Vauthey JN
and Vallone P: Radiofrequency ablation of hepatocellular cancer in
110 patients with cirrhosis. Ann Surg. 232:381–391. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gervais DA, Goldberg SN, Brown DB, Soulen
MC, Millward SF and Rajan DK: Society of interventional radiology
position statement on percutaneous radiofrequency ablation for the
treatment of liver tumors. J Vasc Interv Radiol. 20:3–8. 2000.
View Article : Google Scholar
|
|
4
|
Livraghi T, Meloni F, Di Stasi M, et al:
Sustained complete response and complications rates after
radiofrequency ablation of very early hepatocellular carcinoma in
cirrhosis: is resection still the treatment of choice? Hepatology.
47:82–89. 2008. View Article : Google Scholar
|
|
5
|
Bhardwaj N, Strickland AD, Ahmad F,
Dennison AR and Lloyd DM: Liver ablation techniques: a review. Surg
Endosc. 24:254–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakazawa T, Kokubu S, Shibuya A, et al:
Radiofrequency ablation of hepatocellular carcinoma: correlation
between local tumor progression after ablation and ablative margin.
AJR. 188:480–488. 2007. View Article : Google Scholar
|
|
7
|
Liu CH, Arellano RS, Uppot RN, Samir AE,
Gervais DA and Mueller PR: Radiofrequency ablation of hepatic
tumours: effect of post-ablation margin on local tumour
progression. Eur Radiol. 20:879–885. 2009.PubMed/NCBI
|
|
8
|
Denys AL, De Baere T, Kuoch V, et al:
Radio-frequency tissue ablation of the liver: in vivo and ex vivo
experiments with four different systems. Eur Radiol. 13:2346–2352.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendiratta-Lala M, Brook OR, Midkiff BD,
et al: Quality initiatives: strategies for anticipating and
reducing complications and treatment failures in hepatic
radiofrequency ablation. Radiographics. 30:1107–1122. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi M, Ikeda K, Someya T, et al:
Stepwise hook extension technique for radiofrequency ablation
therapy of hepatocellular carcinoma. Oncology. 63:139–144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi D, Kim SK, Lim HK, et al: Overlapping
ablation using a coaxial radiofrequency electrode and multiple
cannulae system: experimental study in ex-vivo bovine liver. Korean
J Radiol. 4:117–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldberg SN, Gazelle GS, Dawson SL,
Rittman WJ, Mueller PR and Rosenthal DI: Tissue ablation with
radiofrequency: effect of probe size, gauge, duration, and
temparature on lesion volume. Acad Radiol. 2:399–404.
1995.PubMed/NCBI
|
|
13
|
Solazzo SA, Ahmed M, Liu Z, Hines-Peralta
AU and Goldberg SN: High-power generator for radiofrequency
ablation: larger electrodes and pulsing algorithms in bovine ex
vivo and porcine in vivo settings. Radiology. 242:743–750. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Appelbaum L, Sosna J, Pearson R, et al:
Algorithm optimization for multitined radiofrequency ablation:
comparative study in ex vivo and in vivo bovine liver. Radiology.
254:430–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berber E, Herceg NL, Casto KJ and
Siperstein AE: Laparoscopic radiofrequency ablation of hepatic
tumors. Surg Endosc. 8:390–396. 2004. View Article : Google Scholar
|
|
16
|
Kotoh K, Nakamuta M, Morizono S, et al: A
multi-step, incremental expansion method for radio frequency
ablation: potimization of the procedure to prevent increases in
intra-tumor pressure and to reduce the ablation time. Liver Int.
25:542–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patterson EJ, Scudamore CH, Owen DA, Nagy
AG and Buczkowski AK: Radiofrequency ablation of porcine liver in
vivo: effects of blood flow and treatment time on lesion size. Ann
Surg. 227:559–565. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldberg SN, Hahn PF, Halpern EF, Fogle RM
and Gazelle GS: Radio-frequency tissue ablation: effect of
pharmacologic modulation of blood flow on coagulation diameter.
Radiology. 209:761–767. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu DS, Raman SS, Vodopich DJ, Wang M,
Sayre J and Lassman C: Effect of vessel size on creation of hepatic
radiofrequency lesions in pigs: assessment of the ‘heat sink’
effect. Am J Roentgenol. 178:47–51. 2002.PubMed/NCBI
|
|
20
|
Rossi S, Garbagnati F, Lencioni R, et al:
Percutaneous radio-frequency thermal ablation of nonresectable
hepatocellular carcinoma after occlusion of tumor blood supply.
Radiology. 217:119–126. 2000. View Article : Google Scholar
|
|
21
|
Yamakado K, Nakatsuka A, Ohmori S, et al:
Radiofrequency ablation combined with chemoembolization in
hepatocellular carcinoma: treatment response based on tumor size
and morphology. J Vasc Interv Radiol. 13:1225–1232. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Veltri A, Moretto P, Doriguzzi A, Pagano
E, Carrara G and Gandini G: Radiofrequency thermal ablation (RFA)
after transarterial chemoembolization (TACE) as a combined therapy
for unresectable non-early hepatocellular carcinoma (HCC). Eur
Radiol. 16:661–669. 2006. View Article : Google Scholar
|