Introduction
Over the past two decades, owing to advances in
various imaging techniques, small hepatocellular carcinomas (HCCs)
have been detected (1).
Percutaneous ethanol injection therapy (PEIT) (2–6),
percutaneous microwave coagulation therapy (PMCT) (7–11)
and percutaneous radiofrequency ablation (PRFA) (12–14)
are minimally invasive ablation procedures that are used to treat
such small tumors at numerous institutions. PEIT, which was the
first to become more widely used owing to its low cost and
convenience, has certain disadvantages, including insufficient
local control owing to the efflux of ethanol into the blood
vessels, and the inadequate diffusion of ethanol due to the
presence of a fibrous capsule or septum in tumors (5,6).
To overcome these disadvantages of PEIT, thermal
ablation treatments, such as PRFA and PMCT, were developed and
offered local control that is better than that of PEIT (10,13).
At present, PRFA is employed more commonly for local therapy since
the range of tissue coagulation achieved by a single puncture is
larger with PRFA than with PMCT. Therefore, a new type of microwave
electrode was designed to overcome this disadvantage of
conventional PMCT. This new type of microwave electrode is a
perfusion microwave electrode (PME), which continuously supplies a
solution to target tissues and provides continuous and stable heat.
In contrast to a conventional microwave system, this system may
expand the range of coagulation.
To evaluate the coagulation capability of a PME as a
key component of microwave coagulation therapy, the present
preliminary experimental study was conducted.
Using an ex vivo bovine liver, the range of
tissue coagulation was measured for various volumes of infused
saline and microwave outputs. The efficiency of coagulation using
the PME was then compared with that using an RFA electrode
(cool-tip needle) in an in vivo porcine liver.
Materials and methods
Coagulation experiments on an ex vivo bovine
liver and an in vivo porcine liver were conducted using the
PME. The system employs a microwave generator (Microtaze AZM-520;
Alfresa-Pharma, Osaka, Japan), which produces microwaves at 2450±50
MHz, to be transmitted to the electrode via a coaxial cable. During
microwave irradiation, thermal coagulation is induced in the tissue
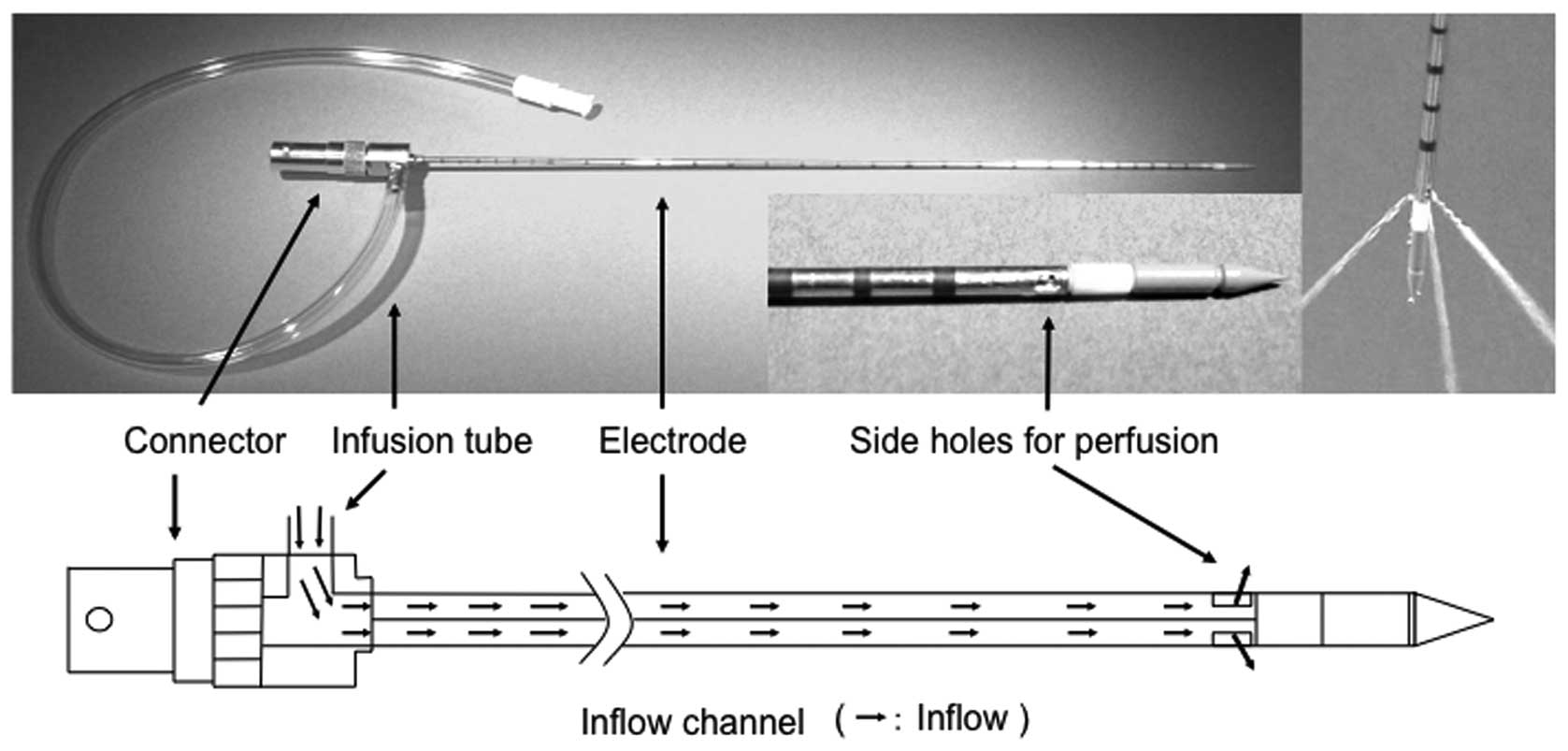
by dielectric heating at the tip of the electrode. PME is 14 G in
diameter and 25 cm in length. The electrode has three rectangular
side holes (each measuring 1×2 mm) at a position 15 mm from the tip
of the electrode. A perfusion solution flows out in three
directions through these holes (Fig.
1). The solution is injected into the electrode through an
injection port located near the electrode connector. Infusion is
performed using a pump that permits the rate of infusion to be
changed appropriately.
Coagulation of an ex vivo bovine
liver
When an ex vivo bovine liver was coagulated
using the PME, physiological saline was injected (isotonic sodium
chloride, Physisalz-PL, 0.9% w/v sodium chloride; Fuso
Pharmaceutical Industries, Osaka, Japan). The flow rate was set at
0, 1, 2, 3, 4 or 5 ml/min (n=5 for each). Saline was injected using
a pump (TE-161S, Terumo, Tokyo, Japan). A flow rate of saline >6
ml/min results in an overflow that exits to the surface of the
liver through the insertion line. Therefore, the upper limit of the
saline flow rate was set at 5 ml/min. A physiological saline
solution at room temperature (approximately 25°C) was used for
infusion.
In the present study, the microwave output was set
at 60 or 80 W and the microwave irradiation time at 5 min.
According to the manufacturer, 5 min is optimal for obtaining the
maximal coagulated area by a conventional microwave electrode. In
reality, clinical microwave coagulation therapy is performed under
these power levels and irradiation time in Japan.
To confirm whether the temperature sufficient for
tissue coagulation was continuously maintained, the temperature of
the tissue at the tip of the electrode was measured during
microwave irradiation at 1-min intervals. Moreover, the temperature
of the electrode at a position 5 cm from the tip was measured to
confirm whether the temperature rose excessively at the shaft of
the electrode.
A portable thermocouple thermometer (PTC-201; Unique
Medical, Tokyo, Japan) and cannula-type temperature sensors
(PTI-200; Unique Medical) were employed. The sensors were attached
to the tip of the electrode and to the shaft (5 cm from the
tip).
The range of tissue coagulation was measured as the
maximum vertical diameter (a) and maximum transverse diameter (b)
of the largest coagulated area observed in the liver sections that
were cut out following microwave irradiation.
The portion showing a clear discoloration (white
zone) that had been induced by microwave irradiation was judged to
be a coagulated area and a vaguely discolored portion, a
non-coagulated section. The range was measured for the clearly
discolored section only.
Coagulation of in vivo porcine liver
Using healthy pigs, in compliance with the
Guidelines for Animal Experiments of Kansai Medical University
(Japan), the following protocol was prepared for this study. The
protocol was approved by the Animal Experimentation Committee,
Kansai Medical University. The animals were handled according to
the guidelines of the National Institutes of Health (Guide for the
Care and Use of Laboratory Animals, NIH publication no. 90-23,
revised 1990).
For the experiment in in vivo porcine liver,
live pigs weighing approximately 40 kg were used (liver weight,
approximately 950 g). Under light anesthesia induced by an
intramuscular injection of ketamine hydrochloride (Ketalar for
intravenous injection, 200 mg; Daiichi Sankyo, Tokyo, Japan) at a
dose of 500 mg, endotracheal intubation was performed (Traquilon;
inner diameter 7.0 mm, outer diameter 9.3 mm; 28 Fr Terumo, Tokyo,
Japan). The tube was connected to a respirator (Model 55-0715;
Harvard Apparatus, MA, USA). The frequency of respiration and tidal
volume were set at 20 min and 15 ml/kg, respectively. An animal
anesthesia apparatus was used (SN-487; Shinano Manufacturing,
Tokyo, Japan) and anesthesia was maintained with a mixture of gases
(oxygen and air) and 0.5–5% isoflurane (Isoful; Dainippon Sumitomo
Pharma, Osaka, Japan). Under general anesthesia, a midline incision
was made in the abdomen and the liver was identified. Coagulation
was performed with the aid of an SSD-3500 ultrasonic apparatus
(Aloka, Tokyo, Japan). The electrode was inserted under ultrasonic
guidance to avoid major blood vessels and was placed at a depth of
5 cm.
Microwave coagulation with the PME was performed
with the infusion of physiological saline at an approximate
temperature of 25°C at 3 ml/min, a microwave output of 80 W, and an
irradiation time of 5 min. On the basis of the experiment with the
ex vivo bovine liver, it was decided that the aforenoted
condition was efficient for an in vivo liver study. RFA was
performed using a high-current 480 kHz monopolar RF generator
(CC-1-100; Radionics, Burlington, MA, USA) that was capable of a
2,000 mA (200 W) output. The electrode was equipped with a cool-tip
RF system (Tyco Healthcare Japan, Tokyo), 25 cm in length, with a
3-cm tip and an outer diameter of 17 G. Under general anesthesia,
paired polar plates were attached to both hindlimbs of each pig.
The application of RF energy was initiated with the generator in
the impedance-control mode at the maximal output that is clinically
required (approximately 120 W).
RFA time was set at 5 and 12 min. RFA was conducted
for 5 min to compare it with the coagulation range of microwave
irradiation, also conducted for 5 min. The time recommended by the
manufacturer for clinical use was 12 min.
The range of tissue coagulation was measured in
specimens that had been resected immediately following coagulation
by both PME and RFA (n=5 for each electrode type). The maximum
vertical diameter (a) and the maximum transverse diameter (b) were
measured for the largest coagulated area on the cut surface of the
sections obtained from the liver where the electrode was inserted.
The two methods were then compared. The portion showing a clear
discoloration (white zone including the hemorrhagic rim) induced by
microwave irradiation and RFA was judged to be the coagulated area
in accordance with a previous report (15).
Statistical analysis
Statistical differences were identified using the
parametric Student’s t-test. P<0.01 was considered to be
statistically significant.
Results
Temperature measurement in the ex vivo
bovine liver
As shown in Fig. 2,
which displays the results of temperature measurement during
microwave irradiation, the tip of the electrode maintained a
temperature that was sufficient for tissue coagulation (>80°C)
even when the physiological saline was injected. Meanwhile, the
temperature increase in the electrode shaft was suppressed to
<45°C by perfusion of physiological saline through the electrode
at flow rates of 3 ml/min or greater. Measurements were not taken
under the conditions of a microwave output equal to 80 W and a flow
rate of 1 or 2 ml/min since coagulated and carbonized tissue that
adhered to the side holes of the electrode interfered with reliable
injection of physiological saline.
Range of PME coagulation in the ex vivo
bovine liver
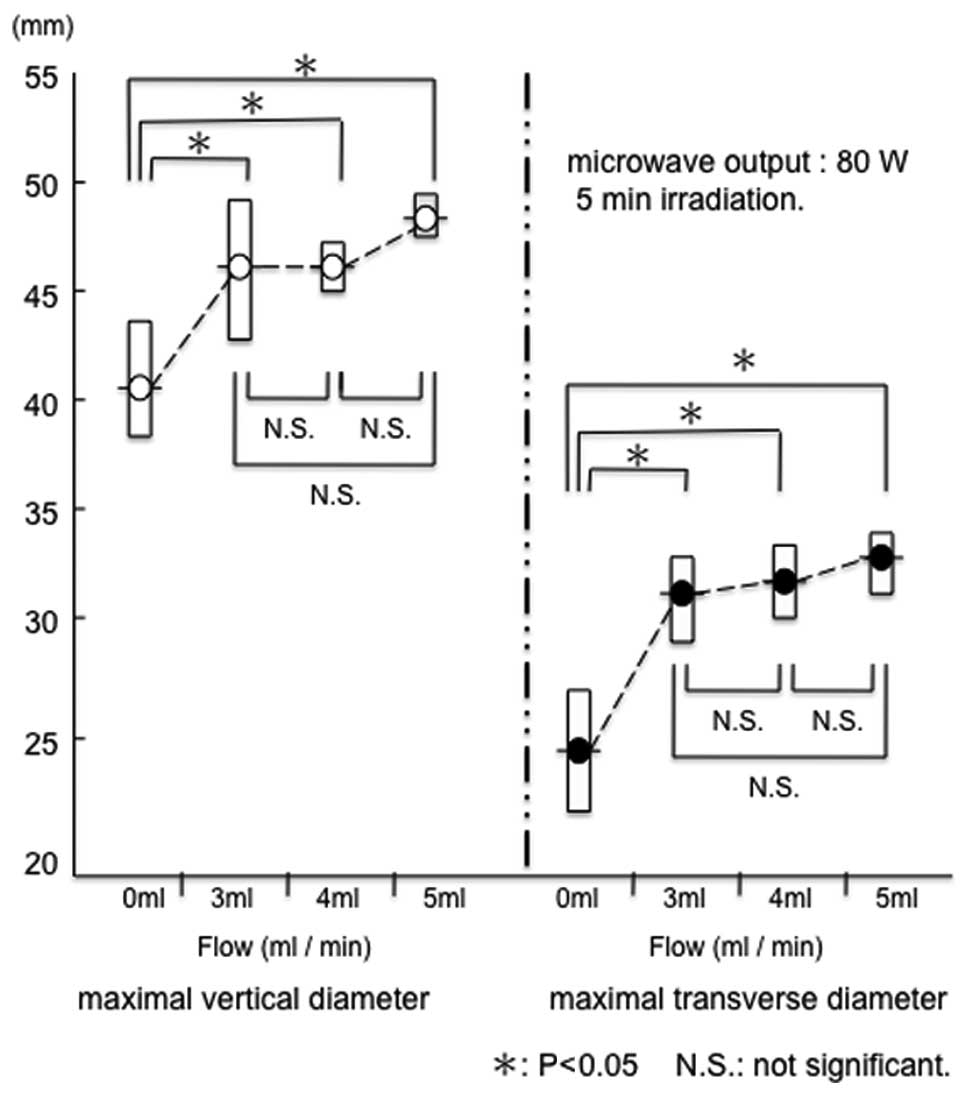
Both the maximal vertical diameter (a) and maximal
transverse diameter (b) were larger at 80 W than at 60 W
(P<0.05) (Fig. 3).
The range of coagulation increased when
physiological saline was injected. Under conditions of a microwave
output equal to 80 W with no flow, the range of tissue coagulation
was 41.8±2.5 mm (a) x 25.6±2.6 mm (b). When the microwave output
was 80 W and the flow rates were 3, 4 and 5 ml/min, the range of
tissue coagulation was 46.4±3.0 mm (a) x 32.2±1.8 mm (b), 46.4±1.1
mm (a) x 33.0±1.4 mm (b) and 48.8±0.8 mm (a) x 33.8±1.3 mm (b),
respectively.
A significant difference was observed between no
flow and saline infusion (Fig. 4).
However, multiple comparisons of the range of coagulation revealed
no significant differences between flow rates of 3, 4 and 5 ml/min
at a microwave output of 80 W (Fig.
4). Therefore, a flow rate of at least 3 ml/min was selected as
the irradiation setting for coagulation in the in vivo
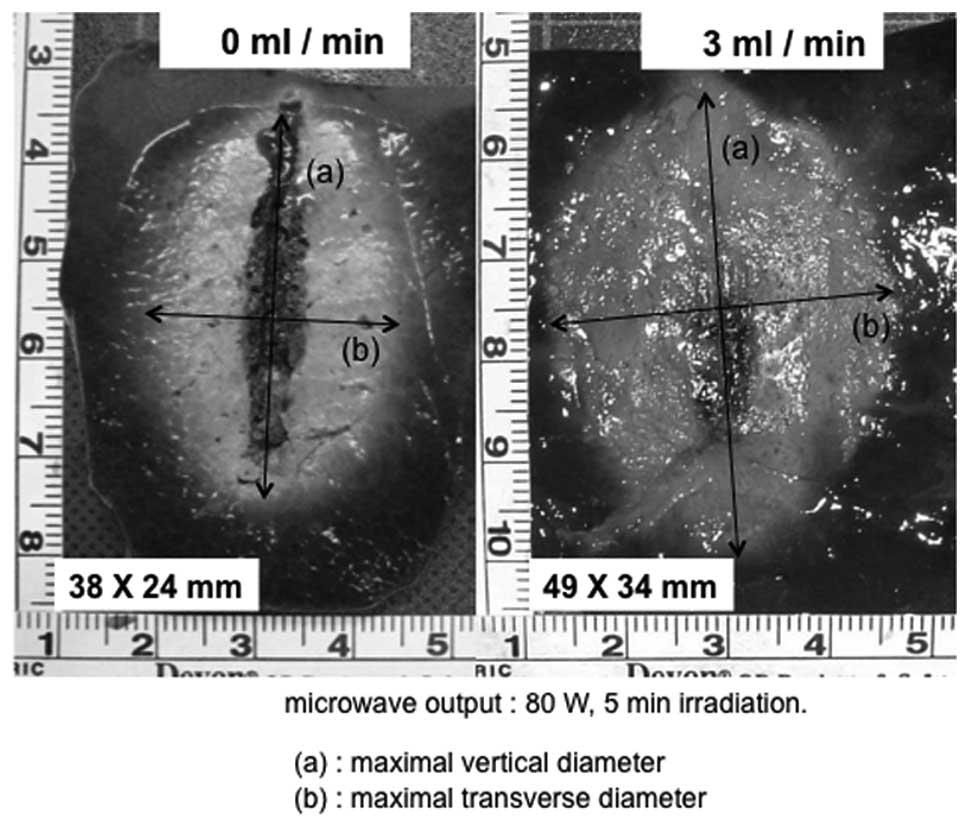
porcine liver. Moreover, when compared with the no-flow condition,
the carbonization of the tissue surrounding the electrode was
suppressed significantly by injecting physiological saline
(Fig. 5).
Range of coagulation in in vivo porcine
liver using PME or RFA
When the in vivo porcine liver was coagulated
using the PME under conditions of a microwave output of 80 W, 5 min
irradiation, and a flow rate of 3 ml/min, the range of coagulation
was 44.8±2.8 mm (a) x 31.2±2.4 mm (b). The range of PME coagulation
was compared between the result of the experiment using the ex
vivo bovine liver (microwave output, 80 W; flow rate, 3 ml/min)
and that of the experiment using the in vivo porcine liver.
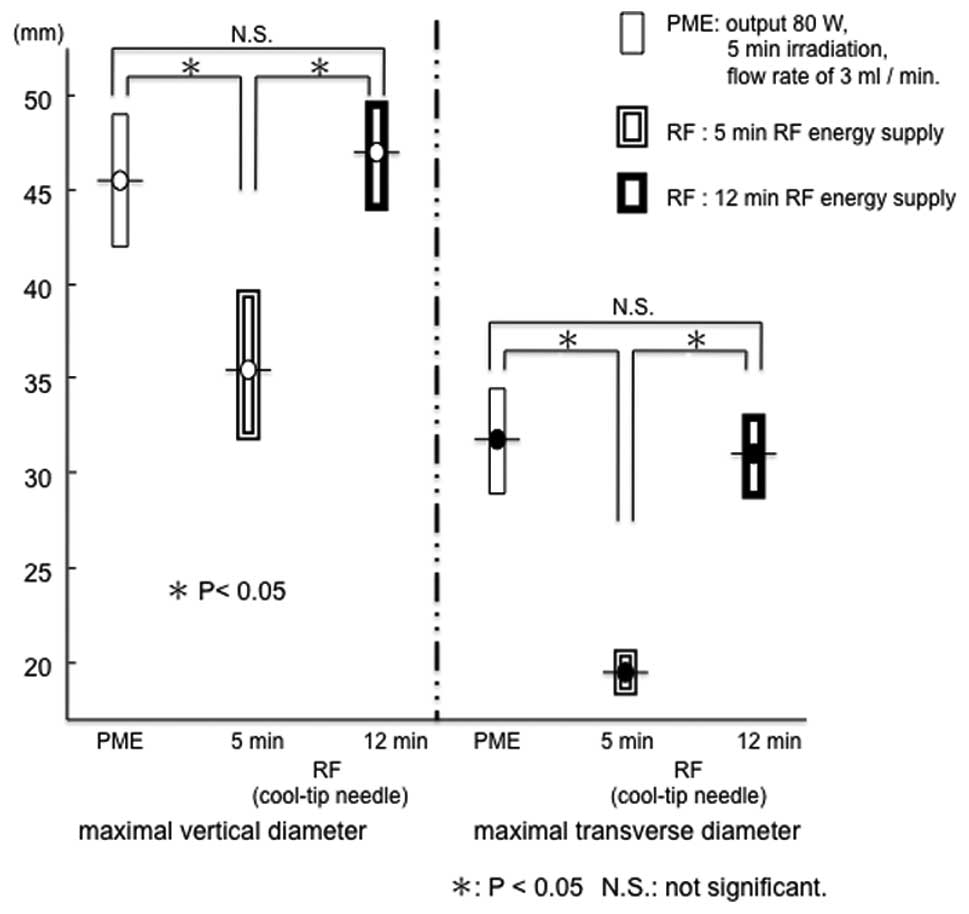
As shown in Fig. 6, no significant
difference in the range of PME coagulation was evident between the
ex vivo bovine liver [46.4±3.0 mm (a) x 32.2±1.8 mm (b)] and
the in vivo porcine liver.
The range of RFA for 5 min in the in vivo
porcine liver was 34.6±2.4 mm (a) x 18.6±0.9 mm (b). Under 5 min
treatment, both the maximal vertical diameter and maximal
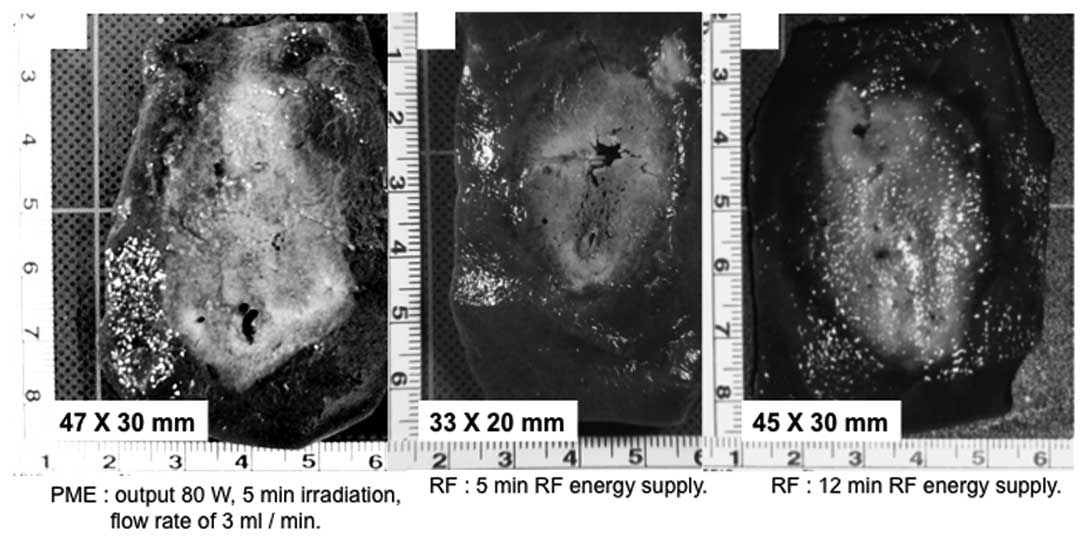
transverse diameter were larger with PME than RFA (Fig. 7). Conversely, the range of RFA for
12 min was 46.0±2.0 mm (a) x 30.2±2.0 mm (b). This range did not
differ from that of PME coagulation under the conditions of a
microwave output of 80 W, 5 min irradiation, and a flow rate of 3
ml/min (Figs. 7 and 8). In the in vivo porcine liver,
the same range of coagulation obtained by RFA for 12 min could be
produced by the PME over 5 min of microwave irradiation.
Discussion
The microwave tissue coagulation system developed by
Tabuse has mainly been used during surgery (16,17).
Subsequently, a microwave electrode for percutaneous insertion was
developed by Seki et al (7)
and used for ultrasonically guided percutaneous coagulation therapy
for HCC (7–11) and metastatic liver cancer (9,11).
Thereafter, laparoscopic microwave coagulation therapy under local
anesthesia was reported as a minimally invasive and effective
therapy for HCC that is located near the hepatic surface (18,19).
Microwave coagulation therapy has come to be recognized as a
reliable thermal ablation procedure for liver cancer. Shibata et
al conducted a randomized controlled trial to compare the two
techniques for treating small HCCs and found that PMCT and PRFA
were equivalent in local therapeutic efficacy, complication rates
and local recurrence rates (20).
However, the range of coagulation obtained with a single electrode
was smaller than that obtained by RFA, a method that was
subsequently developed. This is currently the major mode for local
cancer treatments (12–14). In addition, various methods are
being developed to increase the range of microwave coagulation
(21,22). Against this background, the present
study was performed to evaluate the coagulation capability of a new
type of microwave electrode (a PME) and to compare the coagulation
capability between the PME and RFA electrodes (cool-tip
needle).
Microwave tissue coagulation is defined as a mode of
thermal coagulation that uses heat from friction (dielectric
heating), which is generated when polar molecules (those of water
in the body) vibrate markedly in response to microwave irradiation
(7,10). If a conventional electrode is used,
the surrounding tissues will be coagulated due to a rise in
temperature (dielectric heating) caused by microwave irradiation.
However, the tissue near the electrode will be carbonized owing to
a rapid rise in temperature. In this situation, the delivery of
microwave energy may be attenuated by the presence of carbonized
tissue or its adherence to the electrode. As a result, the range of
stable heating is limited.
Therefore, in the present study, a new type of
microwave electrode was designed so that physiological saline could
be injected continuously through it. This electrode is known as a
PME. Physiological saline was injected continuously to inhibit a
rapid increase of temperature at the tip of the electrode and to
prevent tissue carbonization. The attenuation of microwave energy
delivered due to the presence of carbonized tissue or its adherence
to the electrode is averted and the microwave energy is delivered
efficiently. As a result, it is thought that the coagulation range
is expanded. In addition, the temperature rise of the injected
physiological saline itself, by microwave irradiation, may also be
related to an increase in the coagulation range.
In actuality, the carbonization of the electrode by
the surrounding tissue was significantly suppressed by injecting
the physiological saline and the range of coagulation was augmented
in the experiment with the ex vivo bovine liver.
During microwave irradiation, the tip of the PME
maintained a temperature sufficiently high for tissue coagulation,
while an excessive rise in the temperature of the electrode shaft
(5 cm from the tip) was inhibited by increasing the volume of the
injected physiological saline. Therefore, the risk of skin burns
should be reduced by clinical application of this electrode.
In the experiment using in vivo liver, the
coagulation conditions were set on the basis of results obtained in
the experiment in which an ex vivo bovine liver was used.
The microwave output was set at 80 W since the range of coagulation
increased with an increase in the microwave output from 60 to 80
W.
When the flow rate of the physiological saline was
low (1 or 2 ml/min), the injection port at the tip of the electrode
was sometimes occluded by the carbonization of tissue around the
tip and adherence of coagulated tissue to the electrode,
interfering with the injection of physiological saline. When it
exceeded 6 ml/min, it became unsuitable due to saline overflow from
the insertion line. Thus, the ex vivo bovine liver was
coagulated at a flow rate of a few ml of saline (3, 4 or 5
ml/min).
In reality, the range of coagulation may be
augmented by injecting the physiological saline at 80 W for 5 min.
However, multiple comparisons of the range of coagulation displayed
no significant differences between flow rates of 3, 4 and 5 ml/min.
This fact demonstrated that a flow rate of 3 ml/min was sufficient
to prevent tissue carbonization or its adherence to the electrode
at 80 W output for 5 min. A flow rate of at least 3 ml/min was
selected as being most efficient in this study.
In the experiment using in vivo porcine
liver, when the microwave output was 80 W, irradiation time was 5
min and flow rate was 3 ml/min, the range of coagulation was
similar to that observed in the ex vivo bovine liver under
the same conditions. This result indicates that tissue coagulation
using the PME may be achieved efficiently in spite of the presence
of a cooling effect caused by blood flow. This suggests that the
influence of tissue blood flow on coagulation is slight when this
system is used. However, this was a preliminary study. Further
investigation will be required to search for the best coagulation
setting for the PME, such as microwave output and irradiation
time.
Since the range of coagulation obtained with a
single electrode is wider using RFA than microwave coagulation
therapy (MCT) using the existing electrode, RFA is widely performed
at present as a local ablation therapy. In the present study, the
PME and a single-needle RF electrode (cool-tip needle), which is
currently used at many medical institutions, were compared for
their ranges of coagulation. We confirmed that the same range of
coagulation obtained by the cool-tip needle under ordinary
conditions (maximum output, treatment for 12 min) may be obtained
by the PME within a much shorter period (5 min). Rapid-term
treatment is important in clinical applications to minimize the
stress on patients and operators. Moreover, rapid-term treatment
may reduce the frequency of complications. Further experimental
studies under various coagulation settings (e.g., power output and
irradiation time) will be necessary to determine whether the
coagulation ability of PME is superior to that of RFA (cool-tip
needle). Furthermore, a microwave electrode having an internally
cooled shaft antenna was recently developed and used clinically
(22). It was reported that the
range of coagulation was 54±5 (long axis) x 36±4 mm (short axis),
when the in vivo porcine liver was coagulated using the
cooled shaft antenna under conditions of a microwave output of 80
W, for 5 min. Therefore, a comparison study is necessary between
the PME and the cooled shaft antenna.
During clinical application, one may fear that the
PME could cause cancer cell seeding since physiological saline is
injected into the tumor. Because the cancerous tissue around the
electrode tip coagulates immediately upon initiating microwave
irradiation and the temperature of the injected physiological
saline is raised markedly by the microwaves, the possibility of
seeding cancer cells is thought to be low. On this point, further
studies using a tumor model are required (23,24).
The HITT 106 system, an RF electrode designed to
increase the range of coagulation by injecting physiological saline
(at a flow rate of 38–120 ml/h) during the application of RF
energy, has already been developed and used clinically (25,26),
with no data presented on increased cancer cell dispersion or
metastasis (27). Thus, comparison
research on the PME and saline perfusion RFA systems will be
necessary.
In conclusion, the PME increases the range of tissue
coagulation by infusion of saline into the target tissues. In in
vivo porcine liver, the same range of coagulation obtained by
RFA (cool-tip needle) for 12 min may be produced with the PME by 5
min of microwave treatment. We believe that the PME produces
effective treatment results in a short time and is one of the
tissue coagulation systems suitable for local ablation therapy.
References
|
1.
|
T TobeJ UchinoY EndoM OtoE OkamotoM
KojiroT ShikataK TanikawaT TsuzukiR MizumotoM MitoR YamadaS AriiY
HiraishiPercutaneous ethanol injection therapy for hepatocellular
carcinoma: predictive factors for long-term prognosis after partial
hepatectomy for patients with hepatocellular carcinoma in
JapanCancer7427722780199410.1002/1097-0142(19941115)74:10%3C2772::AID-CNCR2820741006%3E3.0.CO;2-V
|
|
2.
|
S ShiinaK TagawaY NiwaT UnumaY KomatsuK
YoshiuraE HamadaM TakahashiY ShiratoriA TeranoPercutaneous ethanol
injection therapy for hepatocellular carcinoma: results in 146
patientsAJR Am J
Roentgenol16010231028199310.2214/ajr.160.5.76823787682378
|
|
3.
|
K KotohH SakaiS SakamotoK KotohH SakaiS
SakamotoS NakayamaM SatohI MorotomiH NawataThe effect of
percutaneous ethanol injection therapy on small solitary
hepatocellular carcinoma is comparable to that of hepatectomyAm J
Gastroenterol8919419819948304302
|
|
4.
|
T LivraghiA GiorgioG MarinA A SalmiI de
SioL BolondiM PompiliF BrunelloS LazzaroniG TorzilliHepatocellular
carcinoma and cirrhosis in 746 patients: long-term results of
percutaneous ethanol
injectionRadiology197101108199510.1148/radiology.197.1.75688067568806
|
|
5.
|
T SekiT NonakaY KubotaT MizunoY
SameshimaUltrasonically guided percutaneous ethanol injection
therapy for hepatocellular carcinomaAm J
Gastroenterol841400140719892479262
|
|
6.
|
S ShiinaK TagawaT UnumaR TakanashiK
YoshiuraY KomatsuY HataY NiwaY ShiratoriA TeranoT
SugimotoPercutaneous ethanol injection therapy for hepatocellular
carcinoma: a histopathologic
studyCancer6815241530199110.1002/1097-0142(19911001)68:7%3C1524::AID-CNCR2820680711%3E3.0.CO;2-O1654196
|
|
7.
|
T SekiM WakabayashiT NakagawaT IthoT
ShiroK KuniedaM SatoS UchiyamaK InoueUltrasonically guided
percutaneous microwave coagulation therapy for small hepatocellular
carcinomaCancer74817825199410.1002/1097-0142(19940801)74:3%3C817::AID-CNCR2820740306%3E3.0.CO;2-88039109
|
|
8.
|
R MurakamiS YoshimatsuY YamashitaT
MatsukawaM TakahashiK SagaraTreatment of hepatocellular carcinoma:
value of percutaneous microwave coagulationAJR Am J
Roentgenol16411591164199510.2214/ajr.164.5.77172247717224
|
|
9.
|
M SatoY WatanabeY KashuT NakataY HamadaK
KawachiSequential percutaneous microwave coagulation therapy for
liver tumorAm J
Surg175322324199810.1016/S0002-9610(98)00007-59568662
|
|
10.
|
T SekiM WakabayashiT NakagawaM ImamuraT
TamaiA NishimuraN YamashikiA OkamuraK InouePercutaneous microwave
coagulation therapy for patients with small hepatocellular
carcinoma: comparison with percutaneous ethanol injection
therapyCancer8516941702199910.1002/(SICI)1097-0142(19990415)85:8%3C1694::AID-CNCR8%3E3.0.CO;2-3
|
|
11.
|
T MatsukawaY YamashitaA ArakawaT
NishiharuJ UrataR MurakamiM TakahashiS YoshimatsuPercutaneous
microwave coagulation therapy in liver tumors: a 3-year
experienceActa Radiol3841041519979191432
|
|
12.
|
S RossiF FornariL BuscariniPercutaneous
ultrasound-guided radiofrequency electrocautery for the treatment
of small hepatocellular carcinomaJ Intervent Radiol8971031993
|
|
13.
|
T LivraghiSN GoldbergS LazzaroniF MeloniL
SolbiatiGS GazelleSmall hepatocellular carcinoma: treatment with
radio-frequency ablation versus ethanol
injectionRadiology210655661199910.1148/radiology.210.3.r99fe4065510207464
|
|
14.
|
T LivraghiS GoldbergS LazzaroniF MeloniT
TiIeraceL SolbiatiGS GazelleHepatocellular carcinoma:
radio-frequency ablation of medium and large
lesionsRadiology214761768200010.1148/radiology.214.3.r00mr0276110715043
|
|
15.
|
K SugimoriA NozawaM MorimotoK ShiratoA
KokawaT SaitoK NumataK TanakaExtension of radiofrequency ablation
of the liver by transcatheter arterial embolization with iodized
oil and gelatin sponge: results in a pig modelJ Vasc Interv
Radiol16849856200510.1097/01.RVI.0000157780.44868.7815947049
|
|
16.
|
K TabuseA new operative procedure of
hepatic surgery using a microwave tissue coagulatorNihon Geka
Hokan481601721979553495
|
|
17.
|
K TabuseBasic knowledge of a microwave
tissue coagulator and its clinical applicationsJ Hepatobiliary
Pancreat Surg5165172199810.1007/s0053400500289745083
|
|
18.
|
S SekiH SakaguchiH KadoyaH MorikawaD HabuS
NishiguchiS ShiomiT KitadaT KurokiLaparoscopic microwave
coagulation therapy for hepatocellular
carcinomaEndoscopy32591597200010.1055/s-2000-901410935786
|
|
19.
|
S SekiH SakaguchiS IwaiH KadoyaS
KabayashiT KitadaH FujiiT TanakaFive-year survival of patients with
hepatocellular carcinoma treated with laparoscopic microwave
coagulation therapyEndoscopy3712201225200516329021
|
|
20.
|
T ShibataY IimuroY YamamotoY MaetaniF
AmetaniK ItohJ KonishiSmall hepatocellular carcinoma: comparison of
radio-frequency ablation and percutaneous microwave coagulation
therapyRadiology223331337200210.1148/radiol.2232010775
|
|
21.
|
SA ShockK MeredithTF WarnerLA SampsonAS
WrightTC Winter IIIDM MahviJP FineFT Lee JrMicrowave ablation with
loop antenna: in vivo porcine liver
modelRadiology231143149200410.1148/radiol.231102134214990816
|
|
22.
|
M KuangMD LuXY XieHX XuLQ MoGJ LiuZF XuYL
ZhengJY LiangLiver cancer increased microwave delivery to ablation
zone with cooled-shaft antenna - experimental and clinical
studiesRadiology242914924200710.1148/radiol.242305202817229876
|
|
23.
|
Y MiaoY NiS MulierJ YuI De WeverF
PenninckxAL BaertG MarchalTreatment of VX2 liver tumor in rabbits
with ‘Wet’ electrode mediated radio-frequency ablationEur
Radiol101881942000
|
|
24.
|
A Hines-PeraltaZJ LiuC HorkanS SolazzoSN
GoldbergChemical tumor ablation with use of a novel multiple-tine
infusion system in a canine sarcoma modelJ Vasc Interv
Radiol1713511358200616517782
|
|
25.
|
D SchmidtJ TrübenbachJ BriegerC KoenigH
PutzhammerSH DudaCD ClaussenPL PereiraAutomated saline-enhanced
radiofrequency thermal ablation: initial results in ex-vivo bovine
liverAJR Am J
Roentgenol180163165200310.2214/ajr.180.1.180016312490496
|
|
26.
|
PL PereiraJ TrübenbachM SchenkJ SubkeS
KroeberI SchaeferCT RemyD SchmidtJ BriegerCD ClaussenRadiofrequency
ablation: in vivo comparison of four commercially available devices
in pig
liversRadiology232482490200410.1148/radiol.232203018415286318
|
|
27.
|
J HänslerM FrieserV TietzD UhlkeT
WissniowskiT BernatikT HothornEG HahnD StrobelPercutaneous
radiofrequency ablation of liver tumors using multiple
saline-perfused electrodesJ Vasc Interv
Radiol18405441200717377187
|