Introduction
Bone regeneration therapy is becoming common in
regenerative medicine, and is used in the treatment of bone defects
caused by periodontal disease and mandibular tumor resection. Bone
marrow is often used as the typical vital material for bone
regeneration; however, this requires an intricate procedure for
harvesting. Recently, the periosteum has been cited as a bone
supplement material that could be used as an alternative to bone
marrow (1). There have also been
reports that the periosteum has an osteogenic capability that is as
high as bone marrow, which is often used for transplants in the
maxillofacial area (2). A number
of studies have been performed on the osteogenic capability of
periosteal transplants (3,4). At present, collected periosteum is
starting to be cultivated and clinically applied as a bone
supplement (5–8).
However, it is not so easy to obtain the quantity of
periosteum required for bone regeneration. To proliferate a
sufficient quantity of cells for transplant, cell culture is known
to be a good method. Furthermore, it is known that osteogenic
capability is accelerated by changes in the environment, such as
oxygen conditions. Miescher et al (10) as well as others (9,11)
have reported that a hypoxic condition increased red blood cells
and had various other effects on cells, such as the activation of
glycolytic pathways and the induction of angiogenesis. Amemiya K
et al (12) and Amemiya H
et al (13) reported on the
accelerated osteogenic capability of pulp cells and periodontal
ligament cells in hypoxic conditions. However, no comparisons have
been made to date regarding the osteogenic capability of periosteal
cells in hypoxic and normal conditions. The purpose of this
molecular biological study was to investigate the osteogenic
capability of the cultured periosteal cells of rats when incubated
under hypoxic conditions.
Materials and methods
This study was conducted in compliance with the
Guidelines for the Treatment of Experimental Animals at the Tokyo
Dental College (approval number 226102).
Animals and cell culture
Periosteal explants were harvested from the calvaria
of 20 male Sprague-Dawley 7-week-old rats, each weighing
approximately 250 g (Sankyo Labo Service, Tokyo, Japan). The skin
incision was made and underlying muscular fibrous connective tissue
was removed to expose the periosteum. The periosteum was stripped
off mechanically using fine forceps. The obtained periosteum was
mechanically cut into sections approximately 2×2 mm in size. The
cut periosteum was placed with the osteogenic (cambium) side down
onto the surface of 35-mm culture dishes for 30 min, then culture
medium was added and the dishes were cultured for 4 days. For the
culture, Medium 199 containing 10% fetal bovine serum and 50 μg/ml
gentamicin, supplemented with 10−8 M dexametasone, 10 mM
β-glycerophosphate and 50 μg/ml ascorbic acid was used (13).
To set the oxygen conditions, a BL-40 M
CO2 incubator (JuujiField Labo; Bio Labo, Tokyo, Japan)
which regulates the condition of oxygen in the air to nitro-oxygen
was used. For the hypoxic condition group, cells were incubated in
a humidified atmosphere at conditions of 5% O2, 5%
CO2 and 90% N2 at 37°C. For the normal
condition group, cells were incubated in a humidified atmosphere at
normal conditions of 20% O2, 5% CO2 and 75%
N2 at 37°C (15).
Cell proliferation assay
Subcultured cells from each group were harvested
with 0.25% trypsin and 0.02% EDTA, and approximately
3×103 cells were seeded onto the 35-mm culture dishes.
At 1, 2, 3 and 4 days after the culture, the cells were washed with
phosphate-buffered saline (PBS) and harvested using 0.25% trypsin
and 0.02% EDTA. The number of cells was counted using a Vi-CELL™
Coulter counter (Beckman Coulter, Inc., Fullerton, CA, USA).
Quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Approximately 3×104 cells from each group
were seeded in 35-mm dishes and cultured as detailed above. Total
RNA was extracted from each sample using the acid guanidium
thiocyanate/phenol-chloroform method as follows. The culture medium
was removed and cells were rinsed twice using PBS. The cells were
homogenized in 1 ml TRIzol reagent (Invitrogen, Grand Island, NY,
USA) after 1, 2, 3 and 4 days of incubation. Each solution was
transferred to a 1.5 ml tube containing chloroform and mixed. The
tubes were centrifuged at 13,200 rpm at 4°C for 20 min, after which
the supernatants were placed in 1.5 ml tubes containing 250 μl 100%
isopropanol (half the amount of the TRIzol reagent) at −80°C for 1
h. Following centrifugation at 13,200 rpm at 4°C for 20 min, the
supernatants were discarded and the remaining total RNA pellets
were washed with 70% cold ethanol. Total RNAs were dissolved in 50
μl RNase-free (diethylpyrocarbonate-treated) water and then reverse
transcribed and amplified in 20 μl volumes using a reverse
transcription kit (QuantiTect; Qiagen, Germantown, MD, USA)
containing RNA polymerase chain reaction (PCR) buffer (2 U/μl RNase
inhibitor, 0.25 U/μl reverse transcriptase, 0.125 μM oligo(dT)
adaptor primer and 5 mM MgCl2 in RNase-free water)
(13). RT-PCR products were
analyzed by quantitative real-time RT-PCR using a TaqMan gene
expression assay (Applied Biosystems, Inc., Foster City, CA, USA)
for the target genes: Hypoxia-inducible factor (HIF)1α
(Rn00577560_m1, 72 bp), vascular endothelial growth factor (VEGF)
(Rn00582935_m1, 75 bp), alkaline phosphatase (ALP) (Rn01516028_m1,
68 bp), bone sialoprotein (BSP) (Rn01450118_m1, 89 bp), osteocalcin
(OCN) (Rn01455285_g1, 81 bp), Runx2 (Rn01512296_m1, 116 bp). The
TaqMan endogenous control (Applied Biosystems) for the target gene
β-actin (Rn01768120_m1, 63 bp) was used as an endogenous control.
All PCR reactions were performed using a real-time PCR 7500 fast
system (Applied Biosystems). Gene expression quantification using
TaqMan gene expression assays was performed as the second step in a
two-step RT-PCR. Assays were performed in 20 μl single-plex
reactions containing TaqMan Fast Universal PCR Master mix, TaqMan
gene expression assays, distilled water and complementary DNA
according to the manufacturer’s instructions. Reaction conditions
consisted of 50 cycles at 95°C for 3 sec and at 62°C for 30
sec.
ALP activity
ALP activity was measured using a colormetric assay
kit (Alkaline Phosphatase Opt; Roche Diagnostics Japan, Tokyo,
Japan). Cultured cells (3500 cells per well) were washed with
calcium- and magnesium-free PBS at each time-point, and harvested
with demineralized and distilled water for 60 sec using a sonicator
(Sonifier 250D; Branson, Rochester, MI, USA) on ice. Each
homogenate was centrifuged at 800 × g for 5 min and the
supernatants were used for assay. One milliliter of premixed
solution (1 M diethanolamine buffer, pH 9.8, with 0.5 mM
MgCl2 and 10 mM p-nitrophenylphosphate, kept at 37°C)
was added to 10 μl supernatant. Absorption at 405 nm for
p-nitrophenol was measured using a spectrophotometer (Ultrospec
3000; Amersham Pharmacia Biotechnologies, Rochester, NY, USA). To
determine the specific activity of ALP, protein concentrations in
each lysate were determined using the Pierce bicinchoninic acid
protein assay (Pierce, Rockford, IL, USA). A volume of 100 μl of
each cell lysate was added to 100 μl bicinchoninic acid working
reagent (kept at 37°C for 30 min). Absorbance was measured at 595
nm using a microplate reader. ALP activity was calculated according
to the manufacturer’s instructions.
Western blot analysis
The expression of HIF1α, bone morphogenetic protein
(BMP)2, Runx2, BSP, OCN, VEGF, Glut1 and periostin in periosteal
cells was assessed by western blot analysis. Blocking was performed
by PVDF blocking reagent to confirm specific and non-specific
bands. Periosteal cells were seeded in 60-mm cell culture dishes
and were cultured for 4 days. After each culture period, the cells
were washed with CMF-PBS, and resuspended in
radio-immunoprecipitation assay buffer for 60 sec using a sonicator
(Branson) on ice. Each homogenate was further stirred using a tube
rotator (As One) for 20 min and then centrifuged at 9,300 × g for
20 min at 4°C. The protein concentration of each supernatant was
measured using the Nowak method (16). Samples (30 μg protein each) were
electrophoresed in 7.5% sodium dodecyl sulfate polyacrylamide gels
and were transferred to nitrocellulose membranes using standard
methods (12). Primary antibodies
were used at a dilution of 1:1000. These antibodies comprised
rabbit polyclonal antibodies against HIF1α, BMP2, Runx2, BSP, OCN,
VEGF, Glut1 and periostin. As a secondary antibody, horseradish
peroxidase (HRP) conjugated anti-rabbit IgG antibody was used.
Specifically bound antibodies were detected on the film with an
enhanced chemiluminescence detection system (ECL Western blot
detecting system; Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Images were analyzed using NIH Image (Scion Corporation, Frederick,
MD, USA) and each density was normalized with actin from the same
sample.
Statistical analysis
The results were analyzed using one-way analysis of
variance (ANOVA) and then compared by Scheffe’s test.
Results
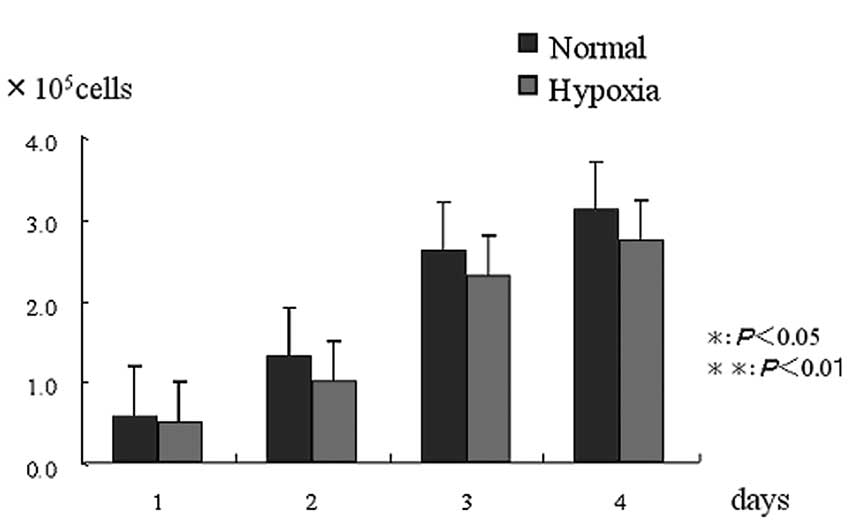
Cell proliferation rate
There was no significant difference in the cell
proliferation rate between the normal and hypoxic condition groups
(Fig. 1).
mRNA expression (RT-PCR)
HIF1α expression was higher in the hypoxic condition
group than in the normal condition group on days 1, 2, 3 and 4
(P<0.05). VEGF expression was significantly higher in the
hypoxic condition group than in the normal condition group on days
3 and 4 (P<0.05). In particular, a significantly high value was
indicated on day 3 (P<0.01). Runx2 expression was significantly
higher in the hypoxic condition group than in the normal condition
group on day 1 (P<0.05). ALP expression was significantly higher
in the hypoxic condition group than in the normal condition group
on days 1 and 2 (P<0.05). BSP expression was significantly
higher in the hypoxic condition group than in the normal condition
group on days 2 and 3 (P<0.05). OCN expression was significantly
higher in the hypoxic condition group than in the normal condition
group on day 4 (P<0.05). In particular, a significantly high
value was indicated on day 3 (P<0.01). Periostin expression was
significantly higher in the hypoxic condition group than in the
normal condition group on day 2 (P<0.05) (Fig. 2).
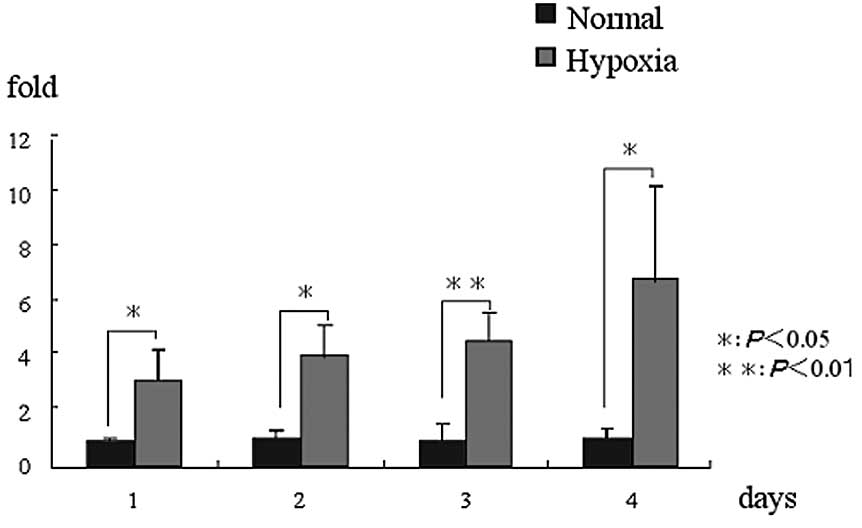 | Figure 2.mRNA expression. (A) HIF1α mRNA
expression in the hypoxic condition group was significantly higher
than that in the normal condition group at all of the time-periods.
(B) VEGF mRNA expression in the hypoxic condition group was
significantly higher than that in the normal condition group on
days 2 and 3, but was not significantly different at other
time-periods. (C) Runx2 mRNA expression in the hypoxic condition
group was significantly higher than that in the normal condition
group on day 1, but was not significantly different at other
time-periods. (D) ALP mRNA expression in the hypoxic condition
group was significantly higher than that in the normal condition
group on days 1 and 2, but was not significantly different at other
time-periods. (E) BSP mRNA expression in the hypoxic condition
group was significantly higher than that in the normal condition
group on days 2 and 3, but was not significantly different at other
time-periods. (F) OCN mRNA expression in the hypoxic condition
group was significantly higher than that in the normal condition
group on day 4, but was not significantly different at other
time-periods. (G) Periostin mRNA expression in the hypoxic
condition group was significantly higher than that in the normal
condition group on day 2, but was not significantly different at
other time-periods. *P<0.05, **P<0.01.
HIF1α, hypoxia-inducible factor; VEGF, vascular endothelial growth
factor; ALP, alkaline phosphatase; BSP, bone sialoprotein; OCN,
osteocalcin. |
ALP activity
In comparison to the normal condition group, the
hypoxic condition group demonstrated a higher (P<0.05)
expression of ALP activity on day 4. However, there were no
significant differences at the other time-periods (Fig. 3).
Protein expression
HIF1α, VEGF, BMP2, Runx2 and BSP were all expressed
more strongly in the hypoxic condition cell group than in the
normal condition group. Glut1, OCN and periostin were all expressed
in both the normal and hypoxic condition groups (Fig. 4).
 | Figure 4.Protein expression. We carried out an
evaluation of the normal and the hypoxic condition group on day 4
after the incubation. HIF1α, VEGF, BMP2, Runx2 and BSP were all
expressed more strongly in the hypoxic condition cell group than in
the normal condition group. Glut1, OCN and periostin were all
expressed in both the normal and hypoxic condition groups. HIF1α,
hypoxia-inducible factor; VEGF, vascular endothelial growth factor;
BMP2, bone morphogenetic protein 2; ALP, alkaline phosphatase; BSP,
bone sialoprotein; OCN, osteocalcin. |
Discussion
It has been reported that osteogenic capability is
just as remarkably high in periosteal cells as it is in bone marrow
cells, which are the material typically used in bone regeneration
therapy (8). Pittenger et
al reported that SH2 and SH3 are mesenchymal stem cell markers
that differentiate into osteoblasts within bone marrow (17). Since then, the same markers have
also been reported in the periosteum (18,19).
Furthermore, Sakaguchi et al reported that there was no
significant difference between periosteal and bone marrow cells in
terms of the expression of CD44, CD90 and CD105, which are also
mesenchymal stem cell markers (20). We observed that rat cultured bone
marrow cells expressed the same markers as cultured periosteal
cells using exactly the same antibodies as were used in this study
(data not shown). Taken together, these results suggest that both
periosteal and bone marrow cells have similar characteristics, in
terms of their quality and quantity of stem cells and strong
osteogenic capability.
In the maxillofacial area, the mandible has been
cited as a collection site for actual periosteal transplants. The
calvarial periosteum used in this study also originates from
intramembranous ossification as in the mandibular bone. Jeroen
et al (22) conducted
comparisons of the periosteal osteogenic capability of rabbits and
humans, and Krzysztof et al (21) conducted comparisons in chickens,
dogs, mice and rats. Furthermore, Wei et al compared
periosteum of the same size collected from the diaphyseal and
epiphyseal region of the femurs of rats according to age (23,24).
In these studies, there were differences in the speed of
differentiation and the degree of osteogenic capability according
to species, site and age, but they were mostly in agreement
regarding cell kinetics. Therefore, this molecular biological study
of cell kinetics using calvarial periosteal cells of rats may
assist in the clinical application of human mandible periosteal
cells.
Generally, it is known that the cell proliferation
ratio changes according to the type of hypoxic condition that the
cells are placed in (25–30). Also, the hypoxic condition affects
the cell kinetics even when the same cells are used in the
condition. Yoshida et al studied mouse embryonic fibroblasts
in vitro with the oxygen condition set to 1, 5 and
20%, and found the number of colonies to be highest at the
condition of 5% oxygen (31).
Senzui et al reported that the cell count in rat pulp cells
decreased at an oxygen condition of 5% (16). No significant difference was noted
in the hypoxic condition at 5% oxygen in this study. These results
shows that there is a marked difference in cell proliferation
between rat periosteal cells and mouse embryonic fibroblasts under
hypoxic conditions.
HIF1α is a hypoxia-inducible factor, and expressed
in hypoxic conditions. Hanada et al reported that the
expression of BMP2 raised the osteogenic capability of the
periosteum (32). In this study, a
stronger protein expression of BMP2 was noted in the hypoxic
condition group than in the normal condition group. HIF1α induces
hypoxia via integrin-linked kinase (ILK), Akt and the mammalian
target of rapamycin (mTOR), causing BMP2 to be strongly expressed
(33) and accelerating the cell
differentiation.
In this study, the hypoxic condition group exhibited
significantly and chronologically higher values than the normal
condition group which may suggest that periosteal cells are
resistant to hypoxia. In this study, Glut1 and VEGF have been cited
as being strongly expressed at either the protein or mRNA level in
hypoxic conditions. Glut1, which comprises 5% of red blood cell
membrane protein, activates the glycolytic pathways, and VEGF
activates angiogenesis and HIF1α is closely related to osteogenic
capability (34–38). HIF1α binds to hypoxia response
elements located in the gene promoters to regulate the
transcription of VEGF and Glut1 (39). Runx2 is an initial transcription
factor for the differentiation process of osteoblasts. Komori et
al (40) and Otto et al
(41) proved that Runx2 gene is
vital to the differentiation and osteogenesis of osteoblasts since
in Runx2 knockout mice, osteoblast differentiation is clearly
suppressed and osteoblast formation does not take place. Kobayashi
et al proved that although neither endochondral ossification
nor intramembranous ossification occur in Runx2 knockout mice,
since the differentiation of damaged mesenchymal cells into
adipocytes and chondrocytes is possible, it is an essential factor
in the initial stage of osteoblast differentiation (42). Liu et al reported that
although Runx2 functions as an accelerator in the initial stage of
osteoblast differentiation, it inhibits mature osteoblasts and
osteocytes (43). In this study,
both mRNA and protein levels of Runx2 were strongly expressed in
the hypoxic incubation group. This suggests that periosteal cells,
which might include pre-osteoblast stage cells, react to the
hypoxic condition. ALP is known to be a marker for early
osteogenesis, BSP is known to be a marker for pre-calcification,
and OCN is known to be a marker for post-calcification (44). In this study, both these markers
showed higher expression at both the protein and mRNA level in the
hypoxic condition group. In particular, BSP was more strongly
expressed on days 2 and 3 and OCN was more strongly expressed on
day 4 in the hypoxic condition group. It would appear that a
hypoxic condition is also effective for osteoblasts to mature to
osteocytes.
Periostin, which is known to be a specifically
expressed protein of the osteoblastic cells of the periodontal
ligament and periosteum, was strongly expressed at the mRNA level
in the hypoxic condition group in this study. Hiouchi et al
reported that periostin existed only in the periosteum, and was
expressed in the periodontal ligament induced by some kind of
mechanical stress (45). Kudo
et al (47) as well as
others (46,48) reported that periostin affects the
ligands α-V/β3 and α-V/ β5, and is involved in the angiogenesis of
malignant tumors. Another report suggests that periostin is
involved in the phosphorylation of FAK and Akt in αv-integrin
pathways during the recovery process following a myocardial
infarction (49). The fact that
Akt is a factor in the upregulation of HIF1α (50) suggests that there must be a
significant correlation between periostin and hypoxic
conditions.
In conclusion, hypoxic conditions activate the
osteogenic capability of periosteal cells in rats.
References
|
1.
|
Y ZhengJ RingeZ LiangOsteogenic potential
of human periosteum-derived progenitor cells in PLGA scaffold using
allogeneic serumJ Zhejiang
Univ7817824200610.1631/jzus.2006.B081716972324
|
|
2.
|
O HayashiY KatsubeM HiroseComparison of
osteogenic ability of rat mesenchymal stem cells from bone marrow,
periosteum, and adipose tissueCalcif Tissue
Int82238247200810.1007/s00223-008-9112-y18305886
|
|
3.
|
WE GallieDE RobertsonThe periosteumCan Med
Assoc J433361914
|
|
4.
|
K OkudaH TanabeK SuzukiPlatelet-rich
plasma combined with a porous hydroxyapatite graft for the
treatment of intrabony periodontal defects in humans:a comparative
controlled clinical studyJ Periodontal
Res76890898200510.1902/jop.2005.76.6.890
|
|
5.
|
K YamamiyaK OkudaT KawaseTissue-engineered
cultured periosteum sheets combined with platelet-rich plasma and
porous hydroxyapatite graft in treating human osseous defectsJ
Periodontol79811818200810.1902/jop.2008.070518
|
|
6.
|
T KawaseK OkudaH KogamiHuman
periosteum-derived cells combined with superporous hydroxyapatite
blocks used as an osteogenic bone substitute for periodontal
regenerative therapy: an animal implantation study using nude miceJ
Periodontol81420427201010.1902/jop.2009.090523
|
|
7.
|
J MaseH MizunoK OkadaCryopreservation of
cultured periosteum: Effect of different cryoprotectants and
pre-incubation protocols on cell viability and osteogenic
potentialCryobiology52182192200610.1016/j.cryobiol.2005.10.01316360651
|
|
8.
|
K OkudaK YamamiyaT KawaseTreatment of
human infrabony periodontal defects by grafting human cultured
periosteum sheets combined with platelet-rich plasma and porous
hydroxyapatite granules: Case seriesJ Int Acad
Periodontol112062132009
|
|
9.
|
ED ThomasIn vitro studies of
erythropoiesis. II. The effect of anoxia on heme
synthesisBlood10612615195514378280
|
|
10.
|
F MiescherUber die beziehungen zwischen
meereschohe und beschaffenheit des blutesCor-Bl f Schweiz
Aerzte238091893
|
|
11.
|
C WanJ ShaoRG ShawnRole of HIF-1α in
skeletal developmentAnn New York Acid Sci11923223262010
|
|
12.
|
K AmemiyaY KanekoT InouePulp cell
responses during hypoxia and reoxygenation in vitroEur J Oral
Sci1113238200310.1034/j.1600-0722.2003.00047.x12887399
|
|
13.
|
H AmemiyaK MatsuzakaT InoueCellular
responses of rat periodontal ligament cells under hypoxia and
re-oxygenation conditions in vitroJ Periodontal
Res43322327200810.1111/j.1600-0765.2007.01032.x18086167
|
|
14.
|
H SomiyaK MatsuzakaT InoueMolecular and
morphological analyse of the osteogenic activity of rat cultured
periosteum cells in vivo and in vitroOral Med
Pathol141928200910.3353/omp.14.19
|
|
15.
|
S SenzuiK MatsuzakaT InoueResponses of
immature dental pulp cells to hypoxic stimulationOral Med
Pathol14107111201010.3353/omp.14.107
|
|
16.
|
TS NowakSynthesis of a stress protein
following transient ischemia in the gerbilJ
Neurochem4516351641198510.1111/j.1471-4159.1985.tb07236.x4045468
|
|
17.
|
MF PittengerM AlastairR
MarshakMultilineage potential of adult human mesenchymal stem
cellsScience284143147199910.1126/science.284.5411.14310102814
|
|
18.
|
J RingeI LeinhaseS StichHuman mastoid
periosteum-derived stem cells: promising candidates for skeletal
tissue engineeringJ Tissue Eng Regen
Med2136146200810.1002/term.7518383554
|
|
19.
|
C PierreBone marrow mesenchymal stem
cells: historical overview and conceptsHum Gene
Ther2110451056201010.1089/hum.2010.11520565251
|
|
20.
|
Y SakaguchiI SekiyaT MunetaComparison of
human stem cells derived from various mesenchymal tissuesArthritis
Rheum5225212529200510.1002/art.2121216052568
|
|
21.
|
H KrzysztofM WlodarskiNormal and
heterotopic periosteumClin Orthop Relat Res2412652771989
|
|
22.
|
E JeroenPL FrankSpecies specificity of
ectopic bone formation using periosteum-derived mesenchymal
progenitor cellsTissue
Eng1222032213200610.1089/ten.2006.12.220316968161
|
|
23.
|
F WeiR CrawfordY XiaoStructural and
cellular differences between metaphyseal and disphyseal periosteum
in different aged ratsBone428189200810.1016/j.bone.2007.08.048
|
|
24.
|
F WeiR StefanY XiaoStructural and cellular
features in metaphyseal and disphyseal periosteum of osteoporotic
ratsJ Mol Histol108189201020232237
|
|
25.
|
J BeauS RobertC PeterHypoxia-amplified
proliferation of human dental pulp cellsJ Dent Res258188232009
|
|
26.
|
Y UenoC KitamuraT NishiharaRe-oxygenation
improves hypoxia-induced pulp cell arrestJ Dent
Res85824828200610.1177/15440591060850090916931865
|
|
27.
|
S KanekoK TakamatsuN ShinozakiIndividual
tissue culture system in a disposable capsule with hypoxic
atmosphereAnn Cancer Res Therap16811200810.4993/acrt.16.8
|
|
28.
|
D JanL DennisHV OosterwyckTowards a
quantitative understanding of oxygen tension and cell density
evolution in fibrin hydrogelsBiomaterials931122010
|
|
29.
|
H JiaweiC DamianJL KentOxygen tension
differentially influences osteogenic differentiation of human
adipose stem cells in 2D and 3D culturesJ Cell
Biochem1108796201020213746
|
|
30.
|
M HiraoJ HashimotoH YoshikawaOxygen
tension is an important mediator of the transformation of
osteoblasts to osteocytesJ Bone Miner
Metab25266276200710.1007/s00774-007-0765-917704991
|
|
31.
|
Y YoshidaK TakahashiS YamanakaHypoxia
enhances the generation of induced pluripotent stem cellsCell Stem
Cell8237241200910.1016/j.stem.2009.08.00119716359
|
|
32.
|
K HanadaLA SolchagaJ BrianBMP-2 induction
and TGF-β1 modulation of rat periosteum cell chondrogenesisJ Cell
Biochem812842942001
|
|
33.
|
J PieterE FrankRK VonckenA novel in vivo
model to study endochondral bone formation; HIF-1α activation and
BMP expressionBone40409418200716979964
|
|
34.
|
M ImaiH IshibashiK ShirasunaInhibition of
vascular endothelial growth factor in oral cancer cells by HIF-1
decoyJ Jpn Stomatol5422282005
|
|
35.
|
W ChaoSR GillbertL ClemensActivation of
the hypoxia-inducible factor-1α pathway accelerates bone
regenerationProc Natl Acad Sci USA156866912008
|
|
36.
|
M AkiyamaM NakamuraBone regeneration and
neovascularization processes in a pellet culture system for
periosteal cellsCell
Transplant18443452200910.3727/09636890978880982019622231
|
|
37.
|
C RyanRK RiddleL ThomasRole of
hypoxia-inducible factor-1α in angiogenic-osteogenic couplingJ Mol
Med875835902009
|
|
38.
|
M NaganoK KimuraO OhnedaHypoxia responsive
mesenchymal stem cells derived from human umbilical cord blood are
effective for bone repairStem Cells
Dev1911951209201010.1089/scd.2009.044720345248
|
|
39.
|
J HwangJ WengW KuoHypoxia-induced
compensatory effects as related to shh and HIF1α in ischemia embryo
rat heartMol Cell Biochem31112151220200818228117
|
|
40.
|
T KomoriH YagiS NomuraTarget disruption of
Cbfa1 result in a complete lack of bone formation owing to
maturation arrest of
osteoblastsCell89755764199710.1016/S0092-8674(00)80258-59182763
|
|
41.
|
F OttoAP ThornellT CromptonCbfa1, a
candidate gene for cleidocranial dysplasia syndrome, is essential
for osteoblast differentiation and bone
developmentCell89765771199710.1016/S0092-8674(00)80259-79182764
|
|
42.
|
H KobayashiY GaoK KomoriMultilineage
differentiation of Cbfa1 deficient calvial cells in vitroBiochem
Biophys Res Commun273630636200010.1006/bbrc.2000.298110873656
|
|
43.
|
W LiuS ToyosawaT FuruichiOverexpression of
Cbfa1 in osteoblasts inhibits osteoblast maturation and causes
osteopenia with multiple fracturesJ Cell
Biol15387100200111581292
|
|
44.
|
Q WangC HuangX ZhangExpression of
endogenous BMP-2 in periosteal progenitor cells is essential for
bone healingBone1019201021056707
|
|
45.
|
K HoriuchiN AmizukaA KudoIdenitification
and characterization of a novel protein, periostin, with restricted
expression to periosteum and periodontal ligament and increased
expression by transforming growth factor βJ Bone Miner
Res1412391249199910404027
|
|
46.
|
L GillanD MateiB ChangPeriostin secreted
by epithelial ovarian carcinoma is a ligand for alpha-V/beta3 and
alpha-V/beta5 integrins and promotes cell motilityCanc
Res6253585364200212235007
|
|
47.
|
Y KudoI OgawaT TakataPeriostin promotes
invasion and anchorage-independent growth in the metastatic prosess
of head and neck cancerCanc
Res1469286935200610.1158/0008-5472.CAN-05-454016849536
|
|
48.
|
R ShaoS BaoT DangAcquired expression of
periostin by human breast cancers promotes tumor angiogenesis
through up-regulation of vascular endothelial growth factor
receptor 2 expressionMol Cell
Biol2439924003200410.1128/MCB.24.9.3992-4003.2004
|
|
49.
|
M ShimazakiK NakamuraA KudoPeriostin is
essential for cardiac healing after acute myocardial infarctionJ
Exp Med205295303200810.1084/jem.2007129718208976
|
|
50.
|
W TsengS YangC TangHypoxia induces BMP-2
expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblastsJ
Cell Physiol223810818201020232298
|