Introduction
Neoadjuvant chemotherapy is now the preferred
approach for treating patients with inflammatory breast cancer and/
or locally advanced breast carcinoma (LABC) (1,2).
There are multiple advantages to this approach, including the
downstaging of an inoperable cancer to an operable one, an increase
in the availability of breast conservation for those patients
otherwise required to undergo a mastectomy, and provision of in
vivo testing of the efficacy of reduction in the primary tumor
volume during treatment, a surrogate marker for a reduction in
micrometastatic disease (3,4).
Another advantage with the neoadjuvant approach is the short
observation time for response to therapy when compared to adjuvant
chemotherapy (5). Finally,
neoadjuvant chemotherapy allows for the genetic profiling of tumors
prior to treatment coupled with the subsequent assessment of the
responsiveness of a particular chemotherapy regimen, thereby
providing the potential for individualized therapy for patients
with LABC (6). Despite these
advantages when compared with adjuvant chemotherapy, neoadjuvant
chemotherapy has not demonstrated significant survival advantages
(4,7–11);
regardless of treatment, the majority of patients with LABC usually
succumb to these diseases.
Thymidine kinase (TK) is an enzyme in the pyrimidine
salvage pathway and catalyzes the phosphorylation of thymidine
monophosphate (12). There are two
forms of TK: A cytosolic (TK1) and a mitochondrial form (TK2)
(13). The level of TK1 is very
low in non-proliferating cells but increases dramatically at late
G1 to late S-phase/early G2 phase during the cell cycle in
proliferating cells and tumor cells. This makes TK1 a noteworthy
marker for cell proliferation and tumor growth. In patients with
malignancies, >95% of the TK1 activity in serum (S-TK1) is
derived from malignant cells (14). Thus, S-TK1 should be a good marker
for tumor cell proliferation. S-TK1 activity has been used to
monitor the extent of tumor metastasis and prognosis in patients
with acute leukemia, chronic leukemia, Hodgkin’s and non-Hodgkin’s
disease, bladder carcinoma, and cervical carcinoma (15–21).
Yet, no study to date has examined the significance of S-TK1 as a
prognostic indicator for LABC patients who have undergone
neoadjuvant chemotherapy. Therefore, we sought to determine whether
a high S-TK1 level in cancer specimens retrieved from LABC patients
receiving neoadjuvant chemotherapy is an independent predictor of
poor outcome.
Materials and methods
Patients
A total of 48 patients with LABC were recruited.
None of the patients had inflammatory breast cancer. Treatment and
surveillance protocols were standardized to ensure study
homogeneity. Compliance with treatment and surveillance protocol
was 95 and 99%, respectively. All patients underwent standard
treatment protocol for neoadjuvant chemotherapy. The majority of
patients received four cycles of cyclophosphamide and epirubicin,
followed by four cycles of docetaxel. Surgical treatment consisted
of either a modified radical mastectomy or breast conservation
therapy (BCT) (lumpectomy with tumor-free margin, axillary node
dissection, and breast irradiation). Adjuvant axillary irradiation,
systemic chemotherapy, and anti-estrogen therapy were offered and
administered as indicated according to the current standard of
care. Surveillance protocol consisted of a history and physical
examination every 3 months during the first year, every 6 months
during the second year and annually thereafter. Annual chest X-ray,
mammogram, complete blood count, and liver function test were
obtained. Any additional radiological and/or histological
evaluation was performed based on the patient’s examination and
history. Clinical data were accrued and recorded prospectively and
included age at diagnosis, comorbid conditions, stage of disease,
treatment protocol, surveillance protocol compliance, and study
endpoints. Study primary endpoints were cancer recurrence and
cancer-related death. Serum samples were obtained from the patients
at the following time points: prior to neoadjuvant chemotherapy,
after each cycle of neoadjuvant chemotherapy, after surgery, after
each cycle of adjuvant chemotherapy, and 3, 6, 12 and 24 months
post-adjuvant chemotherapy. For logistical reasons, 48 patients
were analyzed at the time point before neoadjuvant chemotherapy,
before surgery, before adjuvant chemotherapy and 3 months after
adjuvant chemotherapy.
The characteristics of the patients are shown in
Table I. During the follow-up, a
total of 11 patients developed distant recurrence, while 7 patients
developed loco-regional recurrence within 3 years of surgery. One
patient developed distant recurrence after 2 years of surgery. The
tumors were scored for patient age, ER and nodal status. The blood
sera were stored at −80°C. Sera from 15 healthy individuals were
used as negative controls. At the time of analysis, the sera were
thawed and immediately assayed for S-TK1. Informed consent was
obtained from all patients, and the study was approved by the
Committee on Research Ethics at Shenzhen University Hospital,
China.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Number | Percent (%) |
|---|
| Mean age | 49±8.5 years | |
| Median
follow-up | 28 months | |
| Surgical
procedure | | |
| BCT | 2 | 4 |
| MRM | 46 | 96 |
| Post-neoadjuvant T
stage | | |
| T0 (pCR) | 3 | 6 |
| T1 | 5 | 10 |
| T2 | 24 | 50 |
| T3 | 14 | 29 |
| T4 | 2 | 5 |
| Nodal status | | |
| N0 | 13 | 27 |
| N1 | 14 | 29 |
| N2 | 12 | 25 |
| N3 | 9 | 19 |
| Receptor
status | | |
|
ER+ | 28 | 58 |
|
PR+ | 24 | 50 |
|
HER-2+ | 16 | 33 |
Assay for S-TK1
The concentration of S-TK1 was measured by enhanced
chemiluminescence (ECL) dot blot assay provided by Sino-Swed
Molecular Bio-Medicine Research Institute, Shenzhen, China.
Briefly, 3 μl of serum sample was applied to a nitrocellulose
membrane, in duplicate. The sera were probed with and without
anti-TK1 chicken immunoglobin Y (IgY) antibody, the latter were
used as negative controls. Sera from 13 healthy individuals were
also used as negative controls. We also used anti-TK1 mouse
immunoglobin G (IgG) monoclonal antibodies, with identical results.
The ECL-treated membranes were exposed to X-ray films, taking into
account the variation in S-TK1 concentration of the samples. The
intensities of the spots on the films were determined using a
GS-700 Imaging Densitometer (Bio-Rad, USA). The area of the spots
were equally defined by integration computer program of the GS-700
Imaging Densitometer. From the three different concentrations of
TK1, a standard curve was created, permitting calculation of S-TK1,
as pmol/l (pM). The accuracy of the assay was 4–6%. The sensitivity
varied from 0.75 to 1.0, depending on the type of malignancy, and
the specificity was found to be 1.0 at a cut-off value of 2 pM.
Fig. 1 shows an example of the
dot-blot and western blot analyses.
Assay for HER-2 expression
A positive HER-2 status was defined as a value ≥2,
using FISH method.
Estrogen and progesterone receptor
status
Estrogen receptor (ER) and progesterone receptor
(PR) status was determined using immunohistochemical methods.
Activity >10% was considered positive.
Statistical analysis
Statistical analyses were performed using SPSS
software. Level of S-TK1, tumor size, tumor grade, nodal status,
HER-2, ER and PR statuses were correlated using the samples t-test,
Chi-square test and Spearman rank correlation. Survival analysis
was performed using the Kaplan-Meier method, and the log-rank test
was used to compare the curves, and Cox proportional hazard
regression models were applied for multivariate analysis. Risk
ratios and 95% confidence intervals (CI) were calculated from the
model. A P-value ≤0.05 was considered statistically
significant.
Results
Forty-eight patients were investigated
for this study
The mean age at diagnosis was 49 years, and the mean
follow-up was 28 months. Due to the advanced nature of the disease,
the majority of patients (46 patients) underwent a modified radical
mastectomy. There were 22 patients who developed recurrent disease,
of which 18 patients (81.8%) had distant disease. The median
disease-free survival (DFS) and overall survival (OS) were 38
months for each. Note that the concentration of S-TK1 was observed
in varying degrees in breast cancer specimens. Based on our
previous study (20), we used 2.0
as our cut-off value. Patients were distributed into two groups: a
low S-TK1 group (<2.0 pM, n=19 patients) and a high S-TK1 group
(≥2.0 pM, n=29 patients).
The breakdown of patients as grouped by tumor size
(T stage) and nodal status (N) following neoadjuvant chemotherapy
is shown in Table I. The T stage
distribution was as follows: T0 lesions (n=3), T1 lesions (n=5), T2
lesions (n=24), T3 lesions (n=14), and T4 lesions (n=2). There were
35 node-positive patients and 13 node-negative patients. The N
stage distribution was as follows: N0=13 patients, N1=14 patients,
N2=12 patients and N3=9 patients.
To assess the robustness of our sample set, we
evaluated outcome with known traditional prognostic markers. Among
traditional clinicopathologic factors, nodal status was the
strongest predictor of outcome; patients who had a high number of
positive pathological nodes after neoadjuvant chemotherapy had a
worse DFS (P=0.006) and OS (P=0.036) than those who had none or
minimal nodal disease. These results indicate that our sample size
was adequate.
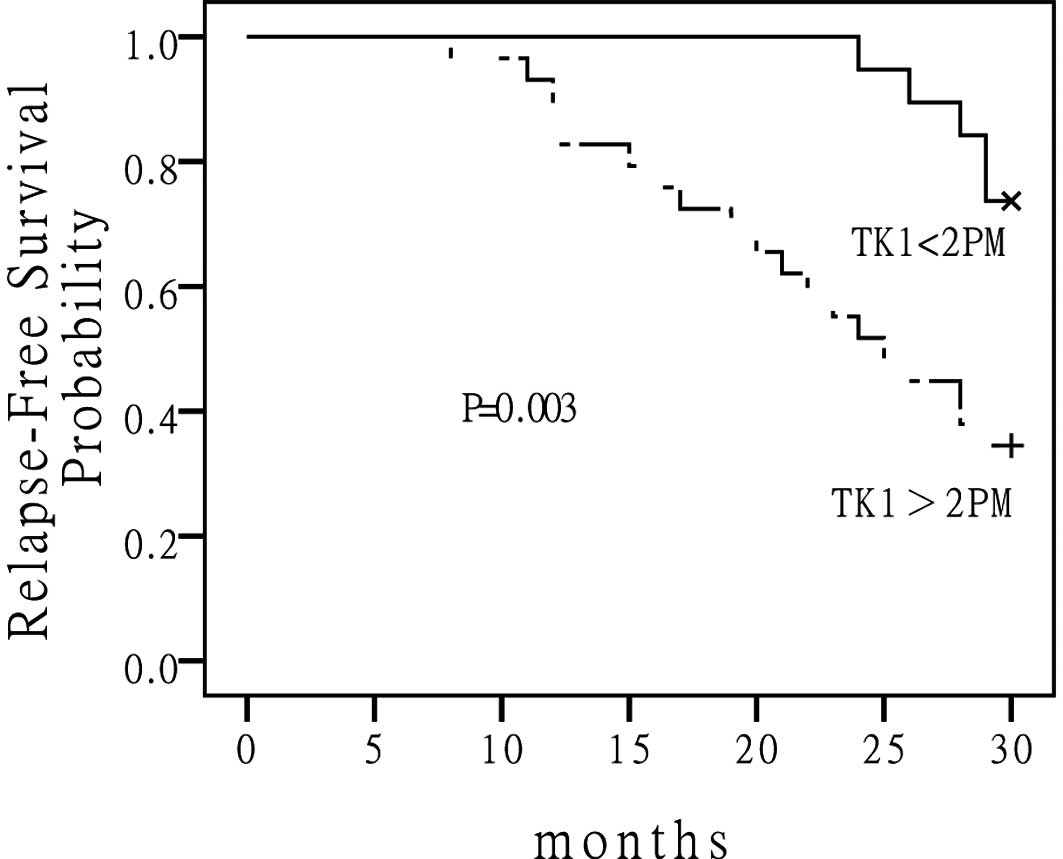
Note that patients who had elevated S-TK1 levels in
their serum had a higher rate of recurrence and cancer-related
death when compared to those who had low S-TK1 levels. The 5-year
DFS for the low S-TK1 group versus the high S-TK1 group were 56 and
21%, respectively (P=0.003) (Fig.
2). The median DFS had not been reached for the low S-TK1 group
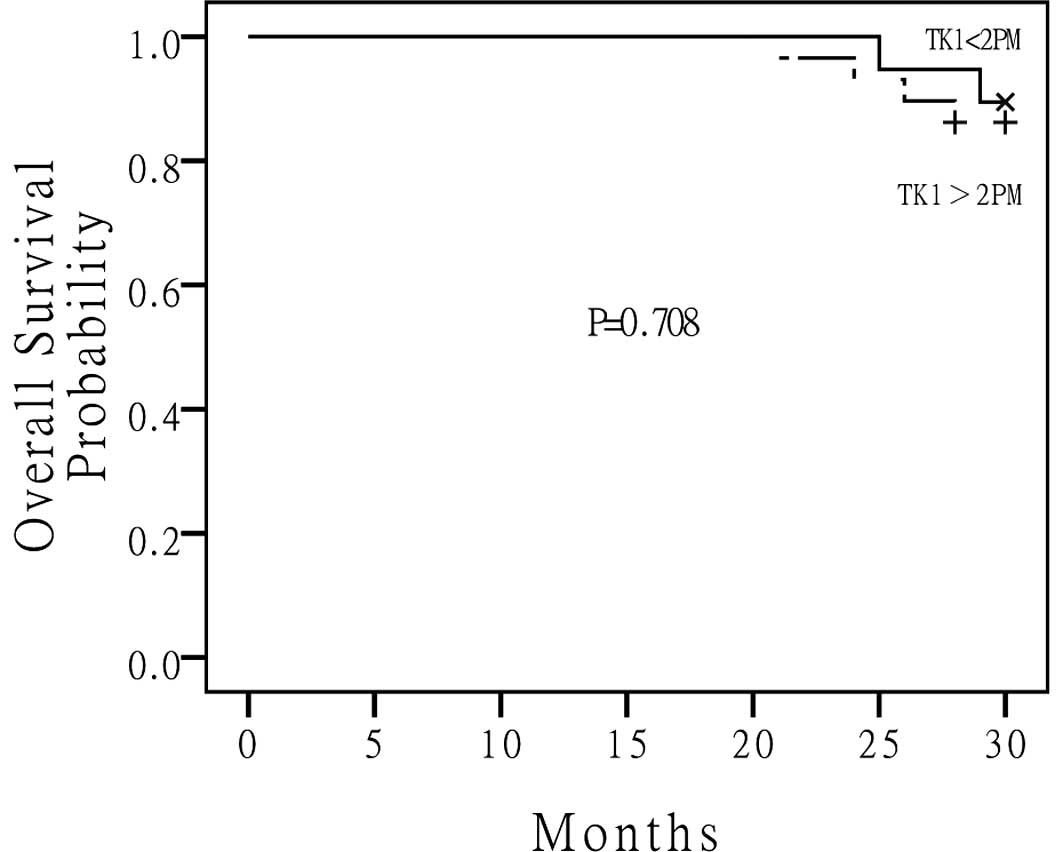
and was 23 months for the high S-TK1 group. The median OS for the
low S-TK1 group and high S-TK1 group had not been reached (Fig. 3). There were no statistically
significant differences between the two groups. Although a high
S-TK1 concentration appeared to be predictive of outcome, we
assessed whether S-TK1 was a covariant of nodal status. In other
words, was a high S-TK1 level a surrogate marker of advanced nodal
disease? There was no statistical correlation between the two
variables (P= 0.11), thus confirming that a high S-TK1
concentration was not a covariant of nodal status. Next, we
evaluated whether a high S-TK1 concentration was correlated with
any known clinicopathological factors such as tumor size, nodal
status, estrogen and progesterone receptor status, and HER-2
receptor status. There were no correlations between the degree of
S-TK1 concentration with tumor size (P=0.15), nodal status
(P=0.11), ER status (P=0.63), PR status (P=0.45), or HER-2 status
(P=0.89), thus suggesting that the S-TK1 concentration is an
independent predictor of outcome.
Finally, to further strengthen our hypothesis that a
high S-TK1 concentration in serum following neoadjuvant
chemotherapy is a noval independent prognostic indicator of poor
cancer outcome in patients who have LABC, we performed a Cox
regression analysis to compare the relative risks of cancer
recurrence (Table II) and
cancer-related death (Table III)
between S-TK1 and known clinicopathological factors. Note that for
both DFS and cancer-related death, S-TK1 overexpression
out-performed nodal status as a predictor of outcome. Patients
whose serum had a high S-TK1 concentration following neoadjuvant
chemotherapy had a higher risk of cancer recurrence compared with
patients whose S-TK1 concentration was low (P=0.003). In
comparison, patients who had evidence of nodal disease had a higher
risk of cancer recurrence compared with patients who had no
evidence of nodal disease (P=0.002).
 | Table II.TK-1 and cancer recurrence (Cox
regression analysis). |
Table II.
TK-1 and cancer recurrence (Cox
regression analysis).
| Score | Significance |
|---|
| High S-TK1 | 8.633 | P=0.003 |
| Tumor size | 2.839 | P=0.158 |
| Estrogen
receptor | 1.6 | P=0.206 |
| Progesterone
receptor | 3.126 | P=0.077 |
| Nodal status | 9.282 | P=0.002 |
| Her-2 | 1.030 | P=0.310 |
 | Table III.TK-1 and cancer death (Cox regression
analysis). |
Table III.
TK-1 and cancer death (Cox regression
analysis).
| Score | Significance |
|---|
| High S-TK1 | 0.140 | P=0.708 |
| Tumor size | 2.011 | P=0.156 |
| Estrogen
receptor | 0.230 | P=0.632 |
| Progesterone
receptor | 0.556 | P=0.456 |
| Nodal status | 4.099 | P=0.043 |
| Her-2 | 0.019 | P=0.891 |
Discussion
Patients with locally advanced breast cancer (LABC)
are at risk of cancer recurrence and death. Neoadjuvant
chemotherapy has become the mainstay treatment. Even with this
approach, the 5-year survival rates remain disappointingly low,
ranging between 20 and 55% (22–24).
Irrespective of postoperative chemotherapy, outcome remains dismal
due to the spread of metastases (24). Apart from nodal status and a
complete pathological response (pCR), there are virtually no
additional prognostic factors available, either clinicopathological
or molecular biological, that can assist in identifying subgroups
of patients at a heightened risk of cancer recurrence and death.
Furthermore, if one were to consider that only 8 to 20% of all LABC
patients achieve pCR following neoadjuvant chemotherapy, then the
prognostic indication of the majority of LABC patients depends
solely on nodal status. An additional discriminating factor
independent of nodal status would greatly assist clinicians in
identifying subgroups of high risk patients to be targeted for
either more intensive and/or novel targeted therapy.
In general, prognostic factors are those that
predict patient outcome regardless of the treatment administered,
while predictive factors indicate responsiveness to a specific
treatment (25). Past studies have
yielded highly variable results on the utility of predictive
factors to predict response to neoadjuvant chemotherapy (26–29).
A previous study of 89 patients with LABC found that the recurrence
score (RS) developed by Paik et al (30) was positively associated with the
likelihood of a pCR (P=0.05) following neoadjuvant paclitaxel and
doxorubicin. These findings suggest that the greatest benefits of
chemotherapy are reserved for those LABC patients at the greatest
risk of developing recurrence (27). Similarly, Hess et al
(27) reported the utility of
using a 30-probe genomic profile to identify patients who achieve
pCR in response to paclitaxel, fluorouracil, doxorubicin, and
cyclophosphamide neoadjuvant regimens. However, Sorlie et al
(28), using a large-scale gene
expression profile on 81 tumors of patients with LABC, were unable
to demonstrate a convincing evidence that could reliably predict
response to neoadjuvant regimens. Similarly, Tiezzi et al
(22) evaluated 60 patients who
received neoadjuvant docetaxel and epirubicin and found that there
were no reliable molecular markers that could predict response to
therapy. Finally, Piega et al (29) utilized a microarray-based
comparative genomic hybridization technique using 44 cancer
specimens and were unable to establish a correlation between DNA
copy number changes and clinical response to doxorubicin and
cyclophosphamide neoadjuvant chemotherapy.
While studies concerning predictive molecular
markers for LABC are few, publications on prognostic molecular
markers are even fewer. Most have concentrated on more traditional
clinicopathologic prognostic features such as extent of nodal
diseases, presence of inflammatory breast cancer, or poor
pathologic response to neoadjuvant chemotherapy (31,32).
Our study is unique in that we identified a molecular marker that
is able to prognosticate outcome for patients with LABC.
The concentration of S-TK1 has been used as a
serological tumor marker, particularly in leukemia and lymphoma and
in breast cancer patients. We recently demonstrated its clinical
utility in patients with HER-2-negative tumors, mainly in primary
tumors indicating a poor outcome, independent of HER-2 status,
ER/PR status, and nodal status (33). As an extension of this study, we
evaluated the prognostic significance of S-TK1 in patients with
LABC who received neoadjuvant chemotherapy. We found that, among
patients with LABC, those who had a high S-TK1 concentration
following neoadjuvant chemotherapy exhibited a poorer survival
outcome than those who had a low S-TK1 concentration. Apart from
predicting a significantly higher relative risk for cancer
recurrence (P=0.003), the concentration of S-TK1 also appeared to
be a predictor of cancer-related death. Although a significant
difference was not achieved between the high and low S-TK1
concentration in predicting cancer-related death (P=0.708), we
proposed that if the period of follow-up had been prolonged,
perhaps a significant outcome may have been achieved. These
findings have tremendous importance as, to our knowledge, this
report is the first to demonstrate that a single molecular marker
is a predictor of outcome independent of nodal status, a factor
that long has been held to be the strongest prognostic indicator of
cancer outcome.
The clinical significance of this finding is that
the activity of S-TK1 can be used as a molecular prognostic marker
in addition to nodal status and pCR since it appears to be
independent of HER-2 status, ER/PR status, tumor size, and nodal
status. The lack of a correlation between S-TK1 concentration and
HER-2 status remains an important observation. These findings
appear to contradict a recent preclinical report that linked HER-2
expression to S-TK1 activity. It is plausible that although S-TK1
activity may be influenced by HER-2, there may be other stronger
factors that control S-TK1 activity.
Although our dataset had only 48 patients, we
believe that results from this dataset are reliable since we were
able to verify that outcome was dependent on nodal status, a
well-established prognosticator. We found that nodal status
significantly influenced both disease-free survival and overall
survival in these patients. Furthermore, comparable to other,
larger series, our overall 5-year survival rates and the percentage
of patients who had pCR were similar to theirs. Finally, our
results, although retrospective, were based on a prospective
database.
Although we are highly encouraged by these results,
we are nevertheless cautious not to overstate their importance. The
prognostic significance of S-TK1 for patients with LABC who have
undergone neoadjuvant chemotherapy should be validated either by a
future prospective clinical trial or by an independent
database.
References
|
1.
|
GN HortobagyiComptehensive management of
locally advanced breast
cancerCancer6613871391199010.1002/1097-0142(19900915)66:14+%3C1387::AID-CNCR2820661414%3E3.0.CO;2-I2205369
|
|
2.
|
GN HortobagyiFC AmesAU BuzdarManagement of
stage III primary breast cancer with primary chemotherapy, surgery,
and radiation
therapyCancer6225072516198810.1002/1097-0142(19881215)62:12%3C2507::AID-CNCR2820621210%3E3.0.CO;2-D3056604
|
|
3.
|
B FisherN GunduzEA SafferInfluence of the
interval between primary tumor removal and chemotherapy on kinetics
and growth of metastasesCancer Res431488149219836831397
|
|
4.
|
B FisherJ BryantN WolmarkEffect of
preoperative chemotherapy on the outcome of women with operable
breast cancerJ Clin Oncol162672268519989704717
|
|
5.
|
S GobleHD BearEmerging role of taxanes in
adjuvant and neoadjuvant therapy for breast cancer: the potential
and the questionsSurg Clin North
Am83943971200310.1016/S0039-6109(03)00071-912875604
|
|
6.
|
JC ChangEC WootenA TsimelzonGene
expression profiling for the prediction of therapeutic response to
docetaxel in patients with breast
cancerLancet362362369200310.1016/S0140-6736(03)14023-812907009
|
|
7.
|
SM SchollJY PiergaB AssselainBreast tumor
response to primary chemotherapy predicts local and distant control
as well as survivalEur J
Cancer31A19691975199510.1016/0959-8049(95)00454-88562150
|
|
8.
|
VF SemiglazovEE TopuzovJL BavliPrimary
chemotherapy and radiotherapy compared with primary radiotherapy
alone in stage IIb-IIIa breast cancerAnn Ocol559159519947993833
|
|
9.
|
A MakrisTJ PowlesM DowsettPrediction of
response to neoadjuvant chemoendocrine therapy in primary breast
carcinomasClin Cancer Res359360019979815725
|
|
10.
|
L MauriacG MacGroganA AvrilNeoadjuvant
chemotherapy for operable breast carcinoma larger than 3 cm: a
unicentre randomized trial with a 124-month median follow-up.
Institute Bergonie Bordeaux Groupe SeinAnn
Oncol104752199910.1023/A:1008337009350
|
|
11.
|
JA van der HageCJ van der VeldeJP
JulienPreoperative chemotherapy in primary operable breast cancer:
results from the European Organization for Research and Treatment
of Cancer trial 10902J Clin Oncol19422442372000
|
|
12.
|
CM HuZF ChangMitotic control of dTTP pool:
a necessary or coincidence?J Biomed
Sci14491497200710.1007/s11373-007-9175-117525869
|
|
13.
|
AS Al-MadhounW TjarksS ErikssonThe role of
thymidine kinase in the activation of pyrimidine nucleoside
analoguesMini Rev Med Chem4341350200415134537
|
|
14.
|
C WuR YangJ ZhouProduction and
characterization of a novel chicken IgY antibody raised against
C-terminal peptide from human thymidine kinase 1J Immunol
Methods277157169200310.1016/S0022-1759(03)00062-012799048
|
|
15.
|
A GowdaJC ByrdUse of prognostic factors in
risk stratification at diagnosis and time of treatment of patients
with chronic lymphocytic leukemiaCurr Opin
Hematol13266272200616755224
|
|
16.
|
BR MadewellSerum thymidine kinase
activity: an alternative to histologic markers of cellular
proliferation in canine lymphomaJ Vet Intern
Med18595596200415515571
|
|
17.
|
RF DiR GiustolisiS LernerRetrospective
study of the prognostic role of serum thymidine kinase level in CLL
patients with active disease treated with fludarabineAnn
Oncol12621625200110.1023/A:101113882559311432619
|
|
18.
|
MK SchwartzEnzymes as prognostic markers
and therapeutic indicators in patients with cancerClin Chim
Acta2067782199210.1016/0009-8981(92)90008-E1572080
|
|
19.
|
KL O’NeillF ZhangH LiThymidine kinase1 – a
prognostic and diagnostic indicator in ALL and AML
patientsLeukemia215605632007
|
|
20.
|
J ZhangQ JiaS ZouThymidine kinase 1: a
proliferation marker for determining prognosis and monitoring the
surgical outcome of primary bladder carcinoma patientsOncol
Rep15455461200616391869
|
|
21.
|
R FujiwakiK HataM MoriyamaClinical value
of thymidine kinase in patients with cervical
carcinomaOncology614754200110.1159/00005535211474248
|
|
22.
|
D TiezziJ AndrateA Ribeiro-SilvaHER-2,
p53, p21 and hormonal receptor protein expression as predictive
factors of response and prognosis in locally advanced breast cancer
treated with neoadjuvant docetaxel plus epirubicin combinationBMC
Cancer736200710.1186/1471-2407-7-36
|
|
23.
|
S SingletaryM McNeeseG
HortobagyiFeasibility of breast-conservation surgery after
induction chemotherapy for locally advanced breast
carcinomaCancer6928492852199210.1002/1097-0142(19920601)69:11%3C2849::AID-CNCR2820691134%3E3.0.CO;2-P1571916
|
|
24.
|
M AlassasQ ChuG BurtonNeoadjuvant
chemotherapy in stage III breast cancerAm
Surgeon71487492200516044927
|
|
25.
|
C IsaccsV StearnsDF HayesNew prognostic
factors for breast cancer recurrenceSemin
Oncol285367200110.1053/sonc.2000.2074211254867
|
|
26.
|
L GianniM ZambettiK ClarkGene expression
profiles in paraffin-embedded core biopsy tissue predict response
to chemotherapy in women with locally advanced breast cancerJ Clin
Oncol2372657277200510.1200/JCO.2005.02.081816145055
|
|
27.
|
K HessK AndersonW SymmansPharmacogenomic
predictor of sensitivity to preoperative chemotherapy with
paclitaxel and fluorouracil, doxorubicin and cyclophosphamide in
breast cancerJ Clin
Oncol2442364244200610.1200/JCO.2006.05.686116896004
|
|
28.
|
T SorlieC PerouC FanGene expression
profiles do not consistently predict the clinical treatment
response in locally advanced breast cancerMol Cancer
Ther529142918200610.1158/1535-7163.MCT-06-012617121939
|
|
29.
|
J PiegaJ Reis-FilhoS
CleatorMicro-array-based comparative genomic hybridization of
breast cancer patients receiving neoadjuvant chemotherapyBr J
Cancer96341351200710.1038/sj.bjc.6603483
|
|
30.
|
S PaikS ShakG TangA multigene assay to
predict recurrence of tamoxifen-treated, node-negative breast
cancerN Engl J Med35128172826200410.1056/NEJMoa04158815591335
|
|
31.
|
T PalangieV MosseriJ MihuraPrognostic
factors in inflammatory breast cancer and therapeutic
implicationsEur J
Cancer30A921927199410.1016/0959-8049(94)90115-57946584
|
|
32.
|
A HonkoopP van DiestJ de JongPrognostic
role of clinical, pathological and biological characteristics in
patients with locally advanced breast cancerBr J
Cancer77621626199810.1038/bjc.1998.999484820
|
|
33.
|
N HolmK ByrnesB LiElevated levels of
chemokine receptor CXCR4 in HER-2 negative breast cancer specimens
predict recurrenceJ Surg
Res1415359200710.1016/j.jss.2007.03.01517574038
|