Introduction
High frequency hyperthermia is widely used in
various countries as an adjuvant therapy for advanced tumors,
including radiofrequency (RF) hyperthermia and microwave
hyperthermia (1). Under such
conditions, selective heating of the tumor is only possible when
heat dissipation by blood flow in the normal tissue is much greater
than that in the tumor tissues. Although both of these techniques
are effective, currently RF is the preferred technique and the one
most widely practiced, as RF is non-invasive and can produce
localized deep heating (2). Yet RF
hyperthermia techniques apply energy in an unfocused manner, and
energy is delivered to both the tumor and normal tissues (3). The most serious shortcomings of RF
hyperthermia in clinical use include no tissue-specific heating due
to the indistinct border between the heating area and the
non-heating area, over-heating in fat tissues and the requirement
for a high output of power (1,000–2,000 W) (4).
In 1997 Jordan et al discovered that a
nanoscaled magnetic fluid (MF) could be absorbed with much higher
power in an alternating magnetic field, and used to treat diseases
(5). This method is known as
‘magnetic fluid hyperthermia’ (MFH). Compared to other available
hyperthermia modalities, MF, suspensions consisting of magnetic
particles, is delivered to the tumor. An alternating magnetic field
is then used to heat the particles and the corresponding tumor,
thereby ablating it. In this way, focused heating of the particles
is obtained in the regions where the static field is dominated by
the alternating magnetic field (6–8).
In order to reduce the limitations of conventional
RF thermotherapy and improve therapeutic anticancer activity, in
this study the heating effects of magnetic nanoparticles were
induced by radiofrequency capacitive field (RCF) with low power
(0–200 W) and its treatment feasibility was investigated using
Wistar rats bearing subcutaneous tumors.
Materials and methods
Regents and instruments
Fe3O4 MF was provided by the
Institute of Medical Physics and Biomedical Engineering of Tsinghua
University and characterized by a transmission electron microscope
(TEM) (Hitachi H-600 instrument; Hitachi Corp., Tokyo, Japan). RCFs
were produced by the Erbtherm1100 P hyperthermia system (27.12 MHz,
0–200 W, 11.0 m wavelength, Italy). RCF power was regulated by
changing the output power of the system. Temperatures were measured
by an IT-24P-Tiny thermocouple thermometer and 24 gauge
polyurethane coated wire with polyester insulated thermocouple bead
(Physitemp, US), and recorded dynamically by a
temperature-recording instrument with 4 channels (Beijing Kunlun
Tianchen Instrument Science and Technology Co., China).
Rats
Wistar rats (male, 4–5 weeks old) were purchased
from the Institute of Dongchuan Animal Experimental Center, Central
South University. The animal experiments were approved by the
regional animal ethics committee and the rats were treated in
accordance with the international animal ethics guidelines.
Walker-256 transplanted subcutaneous tumor models
were established by implanting Walker-256 cells into the right
thigh according to the previous literature (9).
When tumor diameters reached 0.8–1.0 cm 8–9 days
following tumor implantation, 50 rats bearing subcutaneous tumors
were randomly divided into five groups: i) the pseudo-treatment
(PT) control group, ii) MF group: injection of MF without
hyperthermia, iii) pure hyperthermia (PH) group: received one
hyperthermia without injection of magnetic fluid, iv) magnetic
fluid hyperthermia 1 (MFH1) group: received a single intratumoral
injection of MF and one hyperthermia, v) magnetic fluid
hyperthermia 2 (MFH2) group: received a single intratumoral
injection of MF and two hyperthermias. Each group contained ten
rats.
Hyperthermia test
MF was directly injected into the tumors at the 3,
6, 9 and 12 o’clock points with a volume equal to half of the tumor
volume in the MF, MFH1 and MFH2 groups (10). The tumors were subjected to
irradiation for 30 min in the MFH1 group 24 h following injection
of MF. The tumors were subjected to irradiation for 30 min in the
MFH2 group 24 h following injection of MF and irradiated repeatedly
for 30 min 72 h following injection of MF. Similarly, tumors of the
PH group underwent one hyperthermia. The Erbtherm1100 P
hyperthermia system was applied to produce RCF, and the distance
between the upper and lower electrodes placed on opposite sides of
the right tumor region were 30 mm. RCF parameters were carefully
adjusted while the maximal temperature of rectal tissue was not
above 40°C and the maximal temperature of the tumor core was
maintained at 50°C. Temperatures of different areas in rats were
detected by IT-24P-Tiny thermocouple thermometers inserted into
tumor cores, tumor rim, left leg tissue and rectal tissue of model
rats and were recorded dynamically by a temperature-recording
instrument with 4 channels.
Detection of thermotherapeutic
effect
Computed tomography (CT) scanning was performed to
document the intratumoral distribution of magnetic nanoparticles
one day after the first and second hyperthermia, respectively.
Tumor volumes were measured weekly according to the
literature (11). Each tumor was
measured with a sliding caliper to obtain a maximal diameter (a)
and a minimal diameter (b), and tumor volume was calculated using
the following formula: volume = a x b x b/2.
The tumor volume inhibition ratio was calculated as
(1 - mean tumor volume of the experimental group/mean tumor volume
of the control group) x 100%. All rats were sacrificed for
histopathological examination after six weeks. Rat survival time
was examined according to the literature (12).
Statistical analysis
The Kaplan-Meier method was carried out to plot
animal survival time. Values were expressed as the means ± standard
deviation (SD).
The data were analyzed by SPSS 13.0 statistical
software. Heating rates were compared using the Chi-square
(χ2) test between the two groups. Differences in the
results were considered to indicate statistical significance when
P<0.05.
Results
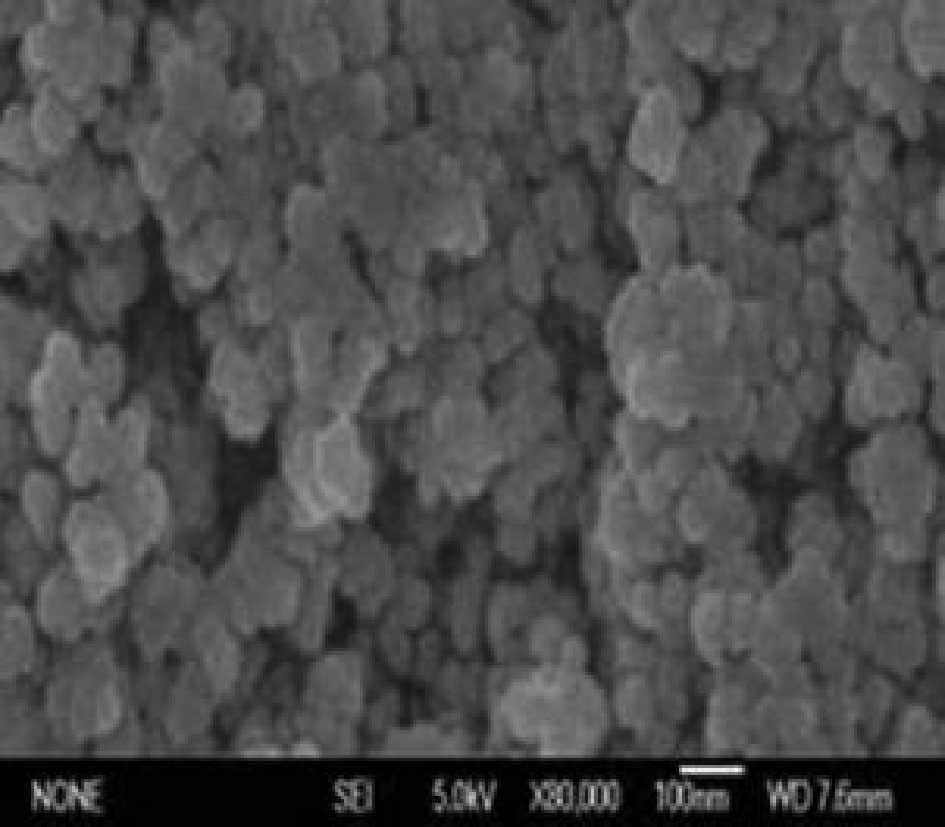
Characterization and detection of MF
The concentration of Fe3O4 in
the MF was 32.93 mg/ml. The TEM result indicates that magnetic
particles were approximately spherical, approximately 10 nm
diameter on average, round and had strong magnetism, as shown in
Fig. 1.
Magnetic nanoparticles remained stable in the
injected location without widespread delivery to other organs. A
relative uniformity and homogeneity in intratumoral distribution of
magnetic nanoparticles locally in targeted tumor tissues were
demonstrated by CT and CT three-dimensional imaging (Fig. 2), so repeatable hyperthermias may
be possible without repeatable injection of MF, and magnetic
nanoparticles were not found in the other organs or tissues.
Diminished deposits of injected magnetic
nanoparticles detected by CT imaging indicates that the local tumor
underwent necrosis or ulceration and broke away following the
second hyperthermia treatment in the MFH2 group (Fig. 2). If further thermotherapy is
required, magnetic nanoparticles could be injected repeatedly.
MFH induced by RCF
Due to the intratumor injection of
Fe3O4 magnetic nanoparticles and exposure to
RCF, the temperatures of the tumor cores and rims rapidly reached
the desired temperature (∼50°C) for the treatment the tumor within
5 to 10 mins in the MFH1 and MFH2 groups, and were then maintained
at a relatively constant level of 46 to 50°C by manually adjusting
the output power (70–130 W). Temperatures of normal tissue in the
left leg and rectum were raised slowly and were all below 40°C
(Fig. 3). There was no statistical
temperature difference between left leg tissue and rectal tissue
(P=1.3); however, there was a statistical temperature difference
between the other tissues (P=0.01). Whereas in the PH group, under
the same RCF, temperatures of the four areas slowly reached 40°C
within 15 to 20 min, similarly, and then were maintained at a
relatively constant level of 40°C by manually adjusting the output
power to 70–150 W (Fig. 3). The
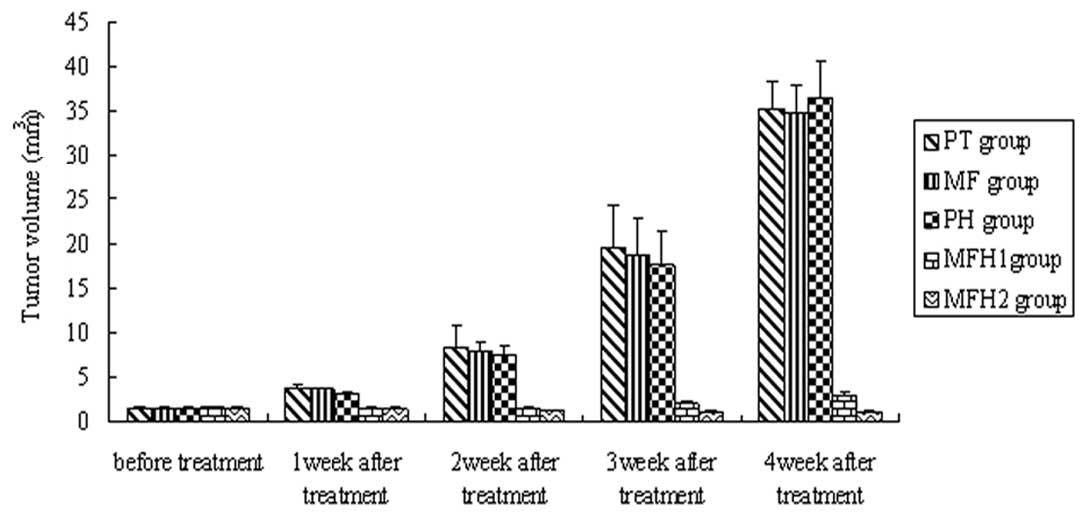
tumor volume in the MFH1 and MFH2 groups decreased (Fig. 4).
Effects of MFH on tumor growth
Tumor growth in the MFH1 and MFH2 groups was
inhibited and the boundaries of coagulated areas were clear.
Compared to the histopathological findings in these groups,
thermocoagulation areas were unclear in the PT, MF and PH groups.
In the MFH2 group, tumors of two model rats completely disappeared
and there was no local recurrence for two months, and the tumor
growth rates of the other eight model rats were markedly decreased.
In the MFH1 group, the tumors of the model rats did not completely
disappear. The tumor complete disappearance rate of the MFH2 group
was 20% and showed more effective inhibition than the MFH1 group.
Compared with the PT, MF and PH groups, tumor volumes of the MFH1
and MFH2 groups began to reduce from the first week after
hyperthermia and the reduction was maintained until the fourth week
after hyperthermia (P= 0.02, Fig.
4). Tumor volume inhibition ratios in the MFH1 and MFH2 groups
were 85.21 and 91.57%, respectively, significantly higher than
those observed in the PH, MF and PT groups (P= 0.01). There was no
statistical difference in tumor volume among the PT, MF and PH
groups (P=1.2), and there was a statistical difference in tumor
volume between the MFH1 and MFH2 group (P=0.01).
Tumor volume inhibition ratios in the MFH1 group
were 32.46 (first week), 50.62 (second week), 48.86 (third week)
and 39.35% (fourth week) following thermotherapy; tumor volume
inhibition ratios in the MFH2 group were 40.82 (first week), 72.32
(second week), 74.41 (third week) and 73.26% (fourth week),
respectively. Tumor inhibition in the MFH2 group was more effective
than that of the MFH1 group.
Kaplan-Meier survival time analysis showed that the
survival time of the MFH2 group (51.62±2.28 days) was longer than
that of the MFH1 group (43.10±1.57 days), and also longer than that
of the PH group (31.82±1.76 days), the MF group (32.50±1.85 days)
and the PT group (32.15±1.25 days) (Fig. 5).
Pathological changes after
thermotherapy
Histological examination revealed that a large
amount of black iron was deposited in the tumor cells in the MF,
MFH1 and MFH2 groups. The morphological features of tumor tissues
in the MFH2 group were observed under a light-microscope 24 h after
the two hyperthermia treatments, and included a detached or
carbonated epidermis or degeneration and large patchy necrosis of
tumor tissue. Entire tumor structures were completely destroyed and
replaced by marked hemorrhage, large necrotic areas and red-dyed
remnant without structure, or cavity, and some nuclei shrank, split
and dissolved. MF deposition could be observed inside or outside of
the necrosis region.
In the MFH 1 group, part of the tumor tissue became
escharotic, and tumor volumes in the rats decreased following
hyperthermia. On the second week after hyperthermia, the tumor
began to grow and the tumor growth rate was slower than that of the
PT, MF and PH groups. The degree of destroyed tumor tissue
morphological structures in the MFH1 group 24 h after one
hyperthermia treatment was lower than that in the MFH2 group. The
tumor cell volume reduced and tumor tissue structure still
survived. The coagulation necrosis area in the tumor tissue was
smaller. Apoptotic changes, including contracted chromatin and
deeply strained nucleus and apoptotic bodies, could be frequently
found. Normal morphological tumor cells were distributed with a
slice-shape in the border of the tumor tissue (Fig. 6).
Numerous black nanoparticles accumulated in the
stroma of tumors, with widespread tumor necrosis surrounding the
nanoparticles. Necrotic areas of the MFH2 group were larger than
those of the MFH1 group (Fig.
6).
Discussion
Heat therapies such as hyperthermia and
thermoablation are very promising approaches in the treatment of
cancer. RCF hyperthermia is a modality that produces deep heating
via conversion of electromagnetic energy to thermal energy. RF
ablation with high frequency and high power (1,000–2,000 W) is a
treatment for cancer that works by inserting a thin needle through
the skin into a tumor guided by CT or ultrasound. Electrical energy
is then delivered through a number of electrodes deployed through
the needle, causing a zone of thermal destruction that encompasses
the tumor (13).
RF ablation results in thermal injury as a
consequence of friction that is generated by agitation of ions and
is a commonly used technique for the treatment of localized tumors
in the liver, with increasing application in other organs, such as
the kidney, bone, lung, adrenal gland and prostate. Limitations of
current RF ablation technology include the requirement for invasive
needle placement, accuracy of image-guidance, tumor size limits,
operator dependence and collateral damage to non-tumor tissue and
adjacent structures (13).
In non-invasive RCF hyperthermia, the major limiting
factor is the inability of the electric field to focus on the
tumor, so that all the tissues penetrated by the electric field are
heated (14). RCF output power was
significantly correlated with intra-tumor temperature, and it could
be used as a parameter to assess efficacy of hyperthermia for the
whole tumor region.
In general, malignant cells are more sensitive to
heat in the range 41–45°C than normal cells. In addition, the
majority of clinically apparent tumors have blood perfusion rates
less than 20% of those of the surrounding normal tissue, meaning
that they may be preferentially heated. The minimum temperature for
therapeutic benefit was above 42°C. Above 46°C the time for cell
killing becomes quite short and the different sensitivity of
malignant and benign cells disappears; above 50°C all cells are
killed very quickly (15). In the
capacitive heating technique, the current spread can also cause
excessive surface heating by the output of high power, so it is
impossible for a therapeutic temperature of 46–50°C to be used. In
our study, the temperature of the tumor was not above 42°C in the
FH group, and there was no significant difference in therapeutic
benefit in the FH group, compared with the PT and MF groups.
Routine medical use of RF is 13.56 MHz (22 m
wavelength), 27.12 MHz (11 m wavelength) and 40.68 MHz (7.5 m
wavelength). The frequency of 27.12 MHz is most commonly used in
the non-invasive method, as the use of higher frequencies results
in a decreased depth of penetration. RCF with 27.12 MHz and 0–200 W
RF electromagnetic waves as a source of heat produces deep heating
via conversion of electromagnetic energy to thermal energy.
Oscillation of high-frequency electrical and magnetic fields
produces movement of ions, rotation of polar molecules and
distortion of non-polar molecules, with resultant heat generation.
However, the heating effect of MF under RCF with 27.12 MHz and
0–200 W for treating tumors has been not reported (16,17).
In this study, the desired temperature (50°C) of MF
induced by non-invasive RCF for cancer hyperthermia was obtained,
and the temperature of the target tumor area with magnetic
particles rapidly reached 50°C within 5 min at a power of 150 W and
the tumor could undergo necrosis. Compared to surrounding tissue,
limited higher density imaging of MF deposits in the tumor was
observed clearly by CT scanning (Fig.
2). Compared with the PT, MF and PH groups, large areas of
necrosis and a marked inhibitory effect on tumor growth were found
in the MFH1 and MFH2 groups, which indicates that MFH had a
significant therapeutic effect on tumors in model rats, and
thermotherapy in the MFH2 group was the best therapeutic agent
among all the groups tested. In contrast to conventional
hyperthermic techniques, hyperthermia using magnetic nanoparticles
under RCF with only a low power of 70–150 W enhanced the
temperature to reach the target of 50°C without any substantial
damage to the surrounding tissue. The three-dimensional thermal
analysis could be developed in further studies.
The results obtained by CT showed that there was no
evidence of injury to other organs in rats by using magnetic
nanoparticles. Nanosized Fe3O4 magnetic
nanoparticles are a new kind of biomaterial without cytotoxic
effects (Fig. 2).
The use of MF provides an impetus for developing a
non-invasive manner for RCF. MFH promises to be a viable
alternative in the treatment of localized cancerous tumors. MFH for
tumor therapy could improve regional control and decrease the risk
of complications, as it has good power absorption capabilities in a
high-frequency alternating electromagnetic field.
In conclusion, following injection of MF in rat
tumors and exposure to RCF with a low power (150 W), the tumor core
and tumor rim can both rapidly reach the desired temperature
(∼50°C) within 5 to 10 mins and maintain a relatively stable-state
intratumoral temperature for tumor treatment. Tumor volumes of the
MFH1 and MFH2 group were smaller than those of the PT, MF and PH
groups. MFH induced by RCF shows a marked inhibitory effect on
tumor growth in model rats and may be a potential and promising
method with better heat localization and focusing abilities for
treating tumors.
Acknowledgements
The authors thank Lin-Yun Zhao,
Shao-Wen Wang and Xiao-Dong Zhang of the Institute of Engineering
and Physics at Tsinghua University for valuable discussion on the
preparation of magnetic fluid suspensions. This study was supported
by Projects 30571779 and 10775085 of the National Natural Science
Foundation of China and project (2009SK3171) of the Hunan
Provincial Science and Technology Department.
References
|
1.
|
RW HabashR BansalD KrewskiHT
AlhafidThermal therapy, part 1: an introduction to thermal
therapyCrit Rev Biomed
Engl34459489200610.1615/CritRevBiomedEng.v34.i6.2017725479
|
|
2.
|
PR StaufferEvolving technology for thermal
therapy of cancerInt J
Hyperthermia21731744200510.1080/0265673050033186816338856
|
|
3.
|
B HildebrandtB RauJ GellermannStandards
and perspectives in locoregional hyperthermiaWien Med
Wochenschr154148158200410.1007/s10354-004-0050-715182041
|
|
4.
|
ED HagerH DziamborD HöhmannD GallenbeckM
StephanC PopaDeep hyperthermia with radiofrequencies in patients
with liver metastases from colorectal cancerAnticancer
Res1934033408199910629627
|
|
5.
|
A JordanR ScholzP WustEffects of magnetic
fluid hyperthermia (MFH) on C3H mammary carcinoma in vivoInt J
Hyperthermia13587605199710.3109/026567397090235599421741
|
|
6.
|
M LatorreC RinaldiApplications of magnetic
nanoparticles in medicine: magnetic fluid hyperthermiaP R Health
Sci J28227238200919715115
|
|
7.
|
P CherukuriES GlazerSA CurleyTargeted
hyperthermia using metal nanoparticlesAdv Drug Deliv
Rev62339345201010.1016/j.addr.2009.11.00619909777
|
|
8.
|
MM ShenoiNB ShahRJ GriffinGM VercellottiJC
BischofNanoparticle preconditioning for enhanced thermal therapies
in cancerNanomedicine6545563201110.2217/nnm.10.15321542691
|
|
9.
|
X LuoL HuC SunL XiongY MiAn experimental
study of energy controllable steep pulse in the treatment of rat
with subcutaneous transplantive tumorSheng Wu Yi Xue Gong Cheng Xue
Za Zhi24492495200717713246
|
|
10.
|
FY ChengCH SuYS YangCharacterization of
aqueous dispersions of Fe(3)O(4) nanoparticles and their biomedical
applicationsBiomaterials26729738200510.1016/j.biomaterials.2004.03.01615350777
|
|
11.
|
A JordanR ScholzU GneveckowDescription and
characterization of the novel hyperthermia and
thermoablation-system MFH 300F for clinical magnetic fluid
hyperthermiaMed Phys1014441451200415259647
|
|
12.
|
CA CamargoME da SilvaRA da SilvaGZ JustoMC
Gomes-MarcondesH AoyamaInhibition of tumor growth by quercetin with
increase of survival and prevention of cachexia in Walker 256
tumor-bearing ratsBiochem Biophys Res
Commun406638642201110.1016/j.bbrc.2011.02.11121362404
|
|
13.
|
VL FlandersDA GervaisAblation of liver
metastases: current statusJ Vasc Interv
Radiol21S214222201010.1016/j.jvir.2010.01.04620656231
|
|
14.
|
ES GlazerSA CurleyRadiofrequency
field-induced thermal cytotoxicity in cancer cells treated with
fluorescent
nanoparticlesCancer11632853293201010.1002/cncr.2513520564640
|
|
15.
|
J CardinalJR KluneE ChoryG JeyabalanJS
KanziusM NalesnikDA GellerNoninvasive radiofrequency ablation of
cancer targeted by gold
nanoparticlesSurgery144125132200810.1016/j.surg.2008.03.03618656617
|
|
16.
|
R BanerjeeY KatsenovichL LagosM McIintoshX
ZhangCZ LiNanomedicine: magnetic nanoparticles and their biomedical
applicationsCurr Med
Chem1731203141201010.2174/09298671079195976520629620
|
|
17.
|
M JohannsenU GneveckowK TaymoorianThermal
therapy of prostate cancer using magnetic nanoparticlesActas Urol
Esp31660667200717896563
|