Introduction
Meniscal injury is a common traumatic injury in knee
and is second only to osteoarthritis. The meniscus is a complex
fibrocartilaginous tissue, which is essential in the knee joint for
shock absorption, load distribution, maintenance of stability and
protection of articular cartilage (1,2).
Meniscal injury may lead to long-term degenerative joint changes,
such as osteophyte formation, articular cartilage degeneration,
joint space narrowing and symptomatic osteoarthritis. Treatments of
meniscal injury in the knee joint include partial or total
meniscectomy, meniscus repair and allogeneic meniscus
transplantation. However, these treatments are not perfect and
often lead to cartilage degeneration, increase in pain and loss of
function (3,4).
Tissue engineering of the meniscus using stem cells
and polymer scaffolds may be an alternative option to treat
meniscal injury (5,6). Tissue engineering may offer the use
of a patient’s own cells with the exact shape and size to fit the
meniscal defect, which may be an ideal alternative for patients
undergoing meniscal injuries (7).
However, there have been several fundamental biological concerns
about this technique, including the cell source, the matrix
scaffold, bioreactor considerations and environmental conditions.
We believe that the two major problems related to meniscal tissue
engineering are cell source and meniscal scaffold.
Concerning cell source, meniscal chondrocytes,
mesenchymal cells and pluripotential fibroblasts have all been
identified as potential sources for the repair of meniscal tissue
(8–10). Compared to the above-mentioned cell
sources, myoblasts represent a more promising source for meniscal
engineering, as they are relatively abundant and easily accessible
with minimal donor site morbidity. At the same time, myoblasts
further promote the development of tissue engineering, as they have
a higher cell yield and more rapid proliferative ability during
in vitro expansion (11).
In regards to the scaffold, a variety of
biomaterials, either naturally derived or artificial, have been
investigated for the tissue-engineered meniscus. Because of the
poor biomechanical properties and the fast degradation of fibrin
and alginate, polymer scaffolds with a stable, biodegradable,
permeable pore network were introduced to support cell attachment
and proliferation and nutrient exchange, and to provide stability.
The most common of such polymers are polylactic acid (PLA),
polyglycolic acid (PGA) and poly(lactic-co-glycolide acid) (PLGA).
PGA has been widely used for anchoring stem cells to facilitate the
generation of the meniscus in vitro or to restore damaged
meniscus in vivo (12). The
suitable biocompatibility of the PGA scaffold to the seed cells has
been demonstrated. Moreover, in order to prepare scaffolds with
specific anatomical shape and to improve mechanical stability, a
certain amount of PLA solution was usually added during the
processing of the PGA scaffold. Such a type of PLGA composite has
been successfully used in healing meniscal defects using a tissue
engineering approach (8).
Thus, we investigated whether myoblasts repair full
thickness meniscal defects in canines with follow-up of long-term
outcomes. Autologous myoblasts were first expanded and induced with
cartilage-derived morphogenetic protein-2 (CDMP-2) and transforming
growth factor-β1 (TGF-β1) in vitro, seeded onto a PLGA
scaffold and then chondrogenically induced again. The cell-scaffold
complexes, after being induced in vitro for 14 days, were
implanted to treat critical-sized meniscal defects. Successful
repair was achieved at 12 weeks after transplantation, indicating
that autologous myoblasts, along with the PLGA scaffold, could be
potentially used for meniscal repair.
Materials and methods
Isolation, culture and induction of
myoblasts
Canine myoblasts were isolated and cultured as
previously described (13).
Primary cells were seeded on a culture dish at a density of
5×105 cells/cm2 in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco BRL, Grand Island, NY, USA) containing
10% fetal bovine serum (FBS; Gibco BRL), 300 μg/ml L-glutamine, 50
μg/ml vitamin C, 100 U/ml penicillin G, 100 μg/ml streptomycin and
0.25 μg/ml amphotericin B (all from Sigma, St. Louis, MO, USA).
After medium change, cultured myoblasts were either subjected to
chondrogenic induction, with the culture medium containing 50 ng/ml
CDMP-2 and 20 ng/ml TGF-β1 (Sigma). The myoblasts were cultured at
37°C in a humidified atmosphere of 95% air and 5% CO2.
The medium was replaced completely every third day, to wash out all
non-adherent cells. Cells were subcultured at a density of
1.0×104 cells/cm2 and treated with 0.25%
trypsin plus 0.02% EDTA (Gibco BRL) when they reached 80%
confluence.
Immunocytochemistry assay of collagen
II
To determine the in vitro chondrogenic
induction effect, induced myoblasts were examined for type II
collagen expression with immunocytochemical staining. Briefly
stated, cells were incubated at 37°C for 1 h with mouse
anti-collagen II monoclonal antibody (IgG1; BD Biosciences
Clontech, Franklin Lakes, NJ, USA) diluted in phosphate-buffered
saline (PBS; 1:200), followed by incubation with 1:100 diluted
horseradish peroxidase (HRP)-conjugated anti-mouse antibody (Dako,
Carpinteria, CA, USA) for 30 min, and color development was carried
out using diaminobenzidine tetrahydrochloride (DAB). Normal menisci
served as positive controls.
Analysis of mRNA for extracellular
matrices and collagen with reverse transcriptase-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from passage 3-induced
myoblasts and normal menisci, and RT-PCR was performed to detect
pro-collagen I, II and aggrecan mRNA expression. The purity and
amount of isolated RNA were assessed by spectrophotometric
measurement at 260 and 280 nm. Total RNA was reverse-transcribed to
cDNA at 50°C for 50 min in a volume of 20 μl containing 1 μl of 10
mM dNTP mix, 1 μl of 10 mM dithiothreitol, 1 μl of 50 μM oligo
(dT), 4 μl of 5X first strand buffer, 1 μl of RNase OUT and 200 U
of Superscript III (RNase H-free RT; Gibco BRL). After terminating
the reaction at 70°C for 15 min, 2 units of RNase H were added to
the reaction mixture, followed by incubation at 37°C for 20 min to
remove the RNA. The cDNA was diluted 1:500 and then amplified in 50
μl of a PCR mixture containing 5 μl of Taq buffer, 4 μl of
MgCl2, 4 μl of 10 mM dNTP mix, 1.25 units of Taq
polymerase and primer sets. PCR was performed in a minicycler,
including an initial denaturation at 94°C for 2 min, followed by 35
cycles of denaturation at 94°C for 1 min, annealing at 54°C (type I
collagen) and 56°C (type II collagen and aggrecan) for 1 min, and
extension at 72°C for 1 min. The final cycle included 5 min of
extension. The PCR products were analyzed by electrophoresis in 2%
agarose gels and stained with ethidium bromide. The mRNAs analyzed
were collagen I (681 bp), collagen II (447 bp), aggrecan (321 bp)
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (211 bp).
Prime sequences for GAPDH, aggrecan, collagen I and collagen II
were as follows: GAPDH, sense 5′CCTCTATGCCAACACAGTGC3′, antisense
5′GTACTCCTGCTTGCTGATCC3′; aggrecan, sense
5′TAGAGAAGAAGAGGGGTTAGG3′, antisense 5′AGCACTAGGAGCCAGGGTTAT3′;
collagen I, sense 5′ATGCCCAAGACTACCAGTGG3′, antisense 5′TCC
TGGAAGCTCTTCTCAGT3′; collagen II, sense 5′-TTT
CCCAGGTCAAGATGGTC-3′, antisense 5′-CTTCAGCAC CTGTCTCACCA-3′.
Preparation of the PLGA scaffold and cell
seeding
A non-woven copolymer scaffold of L-lactide and
glycolide (90/10; PLGA) in the form of fibers was generously
provided by the Shanghai Ju Rui Biomaterials Company Inc., China.
The scaffolds were cuboid, with a width of 2.5 mm, a height of 2.5
mm and a length of 7 mm. The pore sizes of the non-woven fibers
were on average 75 μm, the pore volume accounts for 97% of the
total volume and the filament diameter was 13 μm. The PLGA
constructs were treated using the low-pressure plasma technique at
the end of the production process. A partially ionized gas reacted
with the surface of the scaffolds and formed reactive particles.
Before cell seeding, the scaffolds were immersed in DMEM/F12 medium
containing 10% (v/v) FBS for 12 h to enhance cell adhesion onto the
scaffold.
Chondrogenically induced myoblasts at passage 3
(1.5×107 in 0.3 ml) were harvested and dropped onto PLGA
scaffolds to form cell-scaffold constructs and the constructs were
cultured at 37°C in a humidified atmosphere of 5% CO2
for 5 h, which allowed complete adhesion of myoblasts to the
scaffold. The cell-PLGA constructs in inductive media were
subsequently cultured in vitro for 14 days. Medium was
changed three times a week. As an experimental control, scaffolds
without myoblasts were cultured for the same time periods.
Surgical procedure for meniscal
defect
Six-month-old male Beagle canines were used in this
study, and were obtained from the Agricultural Institute of
Shanghai Jiaotong University, China. Animal care and experimental
procedures were in accordance with the guidelines of the
Administrative Panel on Laboratory Animal Care of China. The Beagle
canines were anaesthetized. The model of canine meniscal defect was
as follows: a defect of 2.5 mm in width, 2.5 mm in thickness near
the capsule and 1.5 mm in thickness near the cavity, the entire
length of the transverse diameter without reservation of the
basement at the anterior horn of the meniscus 0.5 cm distant from
the anterior edge was made, which involved the red-red, red-white
and white-white areas of the meniscus (Fig. 1). The size of the resected portion
was measured to ensure uniformity. In the first experiment, PLGA
scaffolds were implanted in 6 canine right knees. Defects were also
created in the contralateral knees and left empty to serve as
controls. In the second experiment, meniscal defects in 12 animals
were repaired with cell-loaded, pre-cultured PLGA scaffolds. Empty
PLGA scaffolds were placed in the menisci of the contralateral
knees. All animals were sacrificed at 12 weeks. Six additional
canines without any surgical treatment to the knee joints were used
for the histological analysis of normal meniscal architecture. The
wound sites were injected with Marcaine after closure to minimize
post-operative pain. All canines recovered quickly from the
surgeries and none required any further pain medication.
Post-operatively, the animals were allowed free movement without
use of any type of immobilization.
Gross assessment of the meniscal
repair
Canines with surgical implants were euthanized for
tissue harvest. After exposure of the knee joint, the macroscopic
morphology of the meniscus was evaluated with a stereomicroscope
and photographed.
Histological and immunohistochemical
analyses
Twelve weeks after implantation, the implants were
retrieved and analyzed histologically and immunohistochemically.
For histological analyses, specimens were fixed in 10% (v/v)
buffered formalin, dehydrated with a series of graded alcohol and
embedded in paraffin. Tissue sections (4-μm thick) were stained
with H&E for morphological analysis.
Expression of collagen type I and II was detected
using monoclonal antibodies (Dako). Briefly stated, after
deparaffinization, sections were predigested with trypsin at 37°C
for 30 min to facilitate antibody access. Endogenous peroxidase was
quenched by the treatment of 0.3% H2O2 in
methanol at room temperature for 30 min, and non-specific antibody
binding was blocked by incubation of the sections in a 10% normal
goat serum at 37°C for 30 min. Mouse anti-canine collagen type I
and II diluted 1:100 in 0.01 M PBS (pH 7.4) were applied as a
primary antibody at 4°C, overnight. Sections were then incubated
with the secondary antibody, rabbit anti-mouse immunoglobulin
(Dako) for 60 min followed by mouse PAP kit (Dako). Collagen type I
and II were visualized by the reactions with 0.05% DAB containing
0.01% H2O2.
In addition to the qualitative assessment of all the
menisci, the thickness of the repair tissue was measured on images
of H&E-stained histological sections of menisci with a
computerized image analysis system. The mean thickness of the
repair zone was assessed and compared to the mean thickness of the
normal menisci, harvested and processed in the same manner from 6
untreated canines.
Statistical analysis
The presence of type II collagen in the repair
tissue of each meniscus of the different groups was noted and
statistically compared using the Fisher’s exact test. The thickness
of the repair tissue was measured and the thickness in the
different groups was statistically compared using two-tailed
t-tests. For all evaluations, the level of statistical significance
was set at a probability value of <0.05.
Results
Morphological change and expression of
cartilage-specific genes by RT-PCR
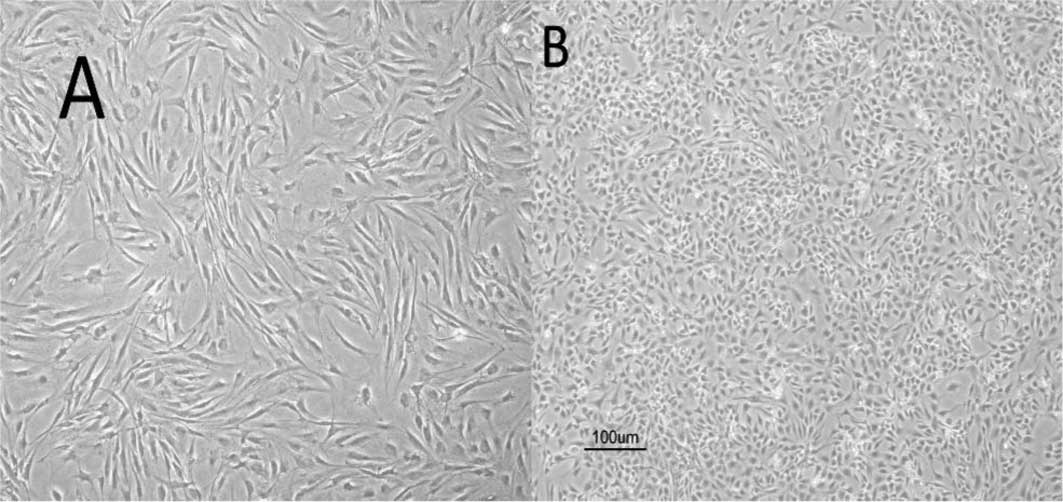
As shown in Fig.
2A, myoblasts induced with CDMP-2 and TGF-β1 underwent a
morphological change after chondrogenic induction, approaching the
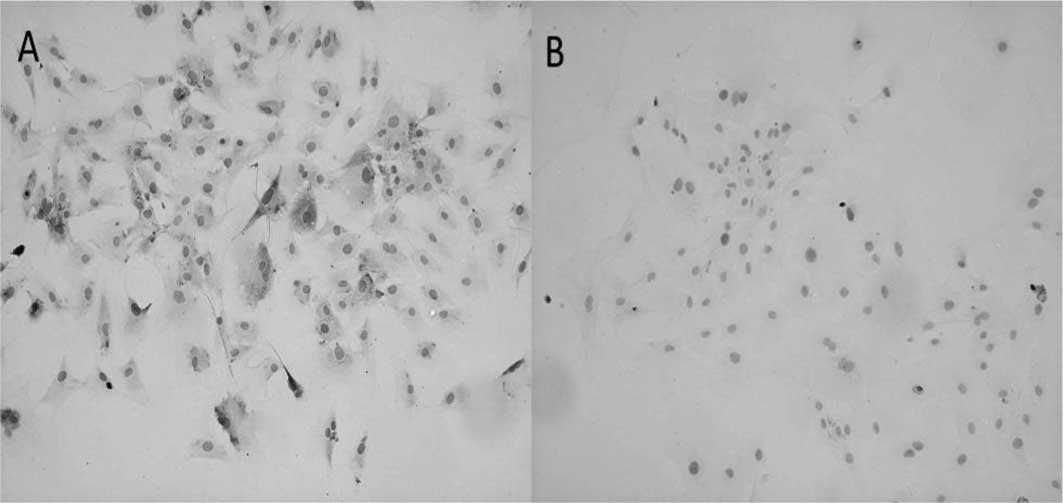
shape of native chondrocytes. In addition, the induced cells showed
significantly enhanced collagen I and II expression (Fig. 3) and gene levels (p<0.05;
Fig. 4) compared to control cells.
Furthermore, aggrecan gene expression was also significantly
enhanced in chondrogenically induced cells (p<0.05; Fig. 4).
Gross morphology of the meniscal
repair
In each knee that had an empty meniscal defect, only
a muted healing response, consisting of a thin, fibrous-like band
next to the surrounding tissue, was detected; however, most of the
defect remained unfilled. The appearance of additional meniscal
degeneration, such as a longitudinal tear in the anterior horn of
the meniscus, was noted in 4 of the 6 empty-defect animals
(Fig. 5A). Compared to the repair
in these empty defects, the repair from the empty PLGA scaffolds
exhibited more complete defect filling: macroscopically, good
integration of the repair tissue with the anterior meniscal horns
was noted in 4 of 6 animals after 12 weeks. However, there were
surface irregularities in the anterior meniscal horns in all
animals with implants (Fig. 5B).
Meniscal repair using the pre-cultured, cell-loaded implants
resulted in near-complete filling of the defects (Fig. 5C). Integration of the implant with
the retained anterior horn of the meniscus was noted in all animals
at 12 weeks. In comparison, in the contralateral knee with an empty
composite scaffold, only 2 of the 12 animals demonstrated good
integration at 12 weeks, consistent with the results of the first
experiment.
Histological and immunohistochemical
assessment of the meniscal repair
In the empty meniscal defects, only a thin band of
fibrous tissue was found in the anterior meniscal horns (Fig. 6A). This stunted repair tissue
consisted of non-metachromatic tissue with fibroblastic cells and
high cellularity. No type II collagen expression was detected by
immunohistochemistry (Fig. 7A).
The repair tissue in the meniscal defects treated by the
implantation of empty PLGA scaffolds also demonstrated
predominantly non-metachromatic, fibrous tissue that did not
contain type II collagen (Fig.
7B). In the repair tissue produced after the implantation of
pre-cultured cell-scaffolds, meniscus-like fibrocartilage with
hyaline cartilage-like areas was noted in 9 of the 12 animals
(Fig. 7C). The contralateral
knees, with an empty scaffold implant, had a fibrocartilage
phenotype in only 2 of the 12 animals. The difference in the
fibrocartilage regeneration between the cell-loaded scaffold and
the empty scaffold was statistically significant (p<0.03,
Fisher’s exact test).
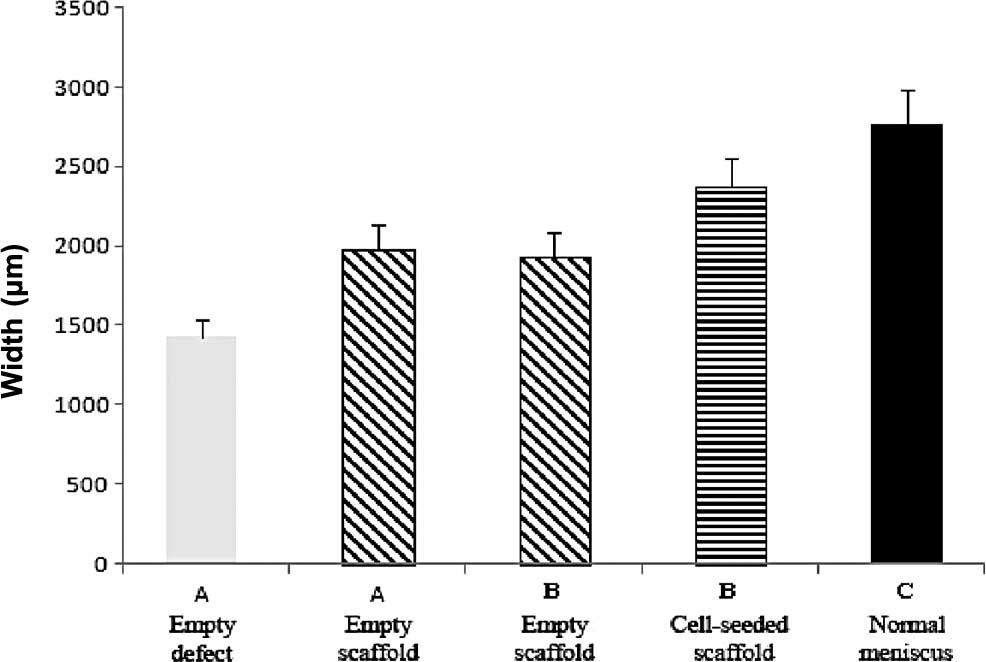
Thickness of the repair tissue
The mean thickness of the repaired meniscal regions
for all groups was determined and compared to the equivalent
measurements from normal menisci. The repair tissue produced with
empty PLGA scaffolds had a mean thickness of 1,975 μm (Fig. 8). This repair tissue was
significantly thicker (p<0.05) compared to that of the empty
defects, which had a mean thickness of 1,428 μm. The mean thickness
of the repair tissue in the meniscal defects that contained
cell-scaffold composites was 2,367 μm; significantly thicker
(p<0.004) than the repair tissue of the comparable contralateral
empty scaffold group, which had a mean thickness of 1,937 μm.
Normal menisci had a mean thickness of 2,759 μm (Fig. 8). The cell-scaffold composite group
regenerated ∼90% of the thickness of the normal meniscus as
compared to 62% for the empty scaffold group. The results for the
empty scaffold groups of the two experiments were consistent and
not statistically different.
Discussion
In this study, the repair of a critical-size defect,
which involved the red-red, red-white and white-white areas of the
meniscus, was achieved in a canine model with the combination of a
PLGA scaffold and myoblasts. An injury model was created that
tested several important elements of successful tissue repair: the
size and shape of the resected meniscus; the formation of tissue
that has appropriate histological features; and the successful
integration of the repaired tissue with the remaining structure. To
achieve this, we completely resected the anterior horn of the
medial meniscus in the canine knee and replaced the resected
section with a biodegradable and biocompatible composite scaffold.
Two different tissue-engineering strategies were used in this
study: implantation of a composite scaffold alone and implantation
of the scaffold that was seeded with myoblasts and pre-cultured to
produce cartilaginous tissue.
In this study, we focused on myoblasts. Myoblasts,
which are adult stem cells and include skeletal muscle cell
precursors, have been reported to possess multipotential
mesenchymal stem cell activity and are capable of forming
chondrocytes, osteocytes and adipocytes as well as myocytes
(14,15). These reports suggest that a certain
degree of plasticity remains before terminal myoblastic
differentiation. Therefore, myoblasts may potentially prove very
useful for the development of new therapeutic approaches aimed at
the regeneration of damaged or diseased tissues. Myoblasts have
been considered a candidate cell source for tissue engineering
(16–18). Compared to other stem cell sources,
myoblasts represent a more promising source for cartilage
engineering, as they are relatively abundant and easily accessible
with minimal donor site morbidity (19,20).
At the same time, myoblasts further promote the development of
tissue engineering, as they have higher cell yield and more rapid
proliferation ability during in vitro expansion (21,22).
In this study, we also observed a chondrogenic response, with a
dose of 50 ng/ml CDMP-2 and 20 ng/ml TGF-β1 given continuously with
each medium change. Chondrogenic differentiation, analyzed on the
immunohistochemical and gene expression profiles, was observed for
the induced condition. This result suggests that key events
responsible for the commitment of myoblasts to the chondrogenic
lineage take place during the early initial period of cell growth
and proliferation.
PGA is one of the most commonly used synthetic
polymers in cartilage tissue engineering. To maintain its
dimensional stability and enhance its mechanical properties,
fibrous PGA meshes are always coated with solutions of PLA.
Evaporation of the solvent for PLA would thus result in PLGA
composites with specific shapes. The feasibility of using PLGA
composite as a scaffold to engineer cartilage tissue has been well
documented in a variety of studies (23,24).
It was also shown that adhesion and proliferation of chondrocytes
on PGA fibers was significantly suppressed with the increase in the
amount of PLA added. Therefore, the concentration of PLA solution
to be added has to be lowered, but sufficient to function as glue
to maintain the structural stability of the PGA 3-D scaffold. In
our study, 1.5% PLA in dichloromethane was used, as the further
lowering would result in an unstable configuration of the resultant
scaffold. It was observed by SEM that PLA of this concentration
wraps PGA fibers together and the shape of the scaffold is
maintained well when they are maintained in culture medium for as
long as 5 weeks (24).
One major advantage of using a PLGA scaffold for
meniscal tissue engineering is its suitable degradation rate which
matches the kinetics of new meniscal formation in vivo
(8). The complete degradation of a
non-woven PGA scaffold is reported to be accomplished over a period
of 2 months in vivo (25).
In the present study, no remnant of un-degraded PLGA fibers could
be observed histologically, either in the experimental or control
groups at 12 weeks post-implantation. Due to its fast degradation,
the PLGA scaffold was found to be able to accelerate chondrogenesis
of constructs prepared from dedifferentiated chondrocytes and PLGA,
as the accumulation of the deposited cartilage-specific
extracellular matrix, and expression of marker genes both in
vitro and in vivo were significantly enhanced compared
to those of constructs prepared from PGA. It was also proposed that
early degradation of PLGA fibers may render a positive effect on
chondrogenesis by leaving new spaces for cells to further fill in
and produce new intercellular matrix, which in turn may facilitate
the formation of more cell-matrix and cell-cell contact.
The complete removal of the anterior horn of the
canine medial meniscus, without subsequent treatment, resulted in
only a muted healing response primarily composed of scar tissue
formation. No empty defects had complete or near-complete filling
with repair tissue, indicating that the defect created in our model
was a critical one. The implantation of the composite scaffold
without cells into the meniscal defect did produce better repair
compared to that in the empty defect controls. The thickness of the
repair tissue was significantly thicker after 12 weeks. The
histological and immunohistochemical analyses indicated that this
repair tissue was mainly fibrous and scar-like. Scaffolds loaded
with myoblasts and pre-cultured in chondrogenic medium prior to the
insertion into meniscal defects resulted in significantly better
meniscal defect filling and meniscal regeneration compared to the
repair after implantation of cell-free composites. The repair
tissue after myoblast-based repair revealed a more meniscus-like
appearance than that of the repair tissue of the controls with
cell-free composite scaffolds. Good integration of the repair
tissue with the host meniscus at the defect edges could be seen
macroscopically and ultrastructurally after meniscal repair with
myoblasts loaded implants. Results of histological and
immunohistochemical evaluation of the normal canine meniscus
indicated that the anterior horn of the medial meniscus contained
type II collagen. Thus, the presence of type II collagen in the
repair tissue was an important parameter to examine. Nine of the 12
menisci with pre-cultured implants had evidence of type II collagen
in the repair tissue, indicating retention of the type II
collagen-rich implant.
The cell-loaded and pre-cultured implants did not
completely restore the surface area and the tissue quality of the
normal meniscus. Therefore, modifications are necessary to improve
the meniscus implant. Also, long-term studies with functional
assessments of the repair tissue are important. One limitation of
the canine as a model for meniscus repair is that it does not have
a gait pattern close to that of humans. Therefore, the implantation
of the stem cell-loaded scaffolds in larger animal models is
necessary before clinical use. Despite these limitations, this
study demonstrates that regeneration of an important
musculoskeletal structure, such as the meniscus, can be achieved
using a scaffold and stem cell-based tissue engineering
approach.
Acknowledgements
This study was supported by the
Shanghai Natural Science Foundation, China (no. 09ZR1425500).
References
|
1.
|
AS VoloshinJ WoskShock absorption of
meniscectomized and painful knees: a comparative in vivo studyJ
Biomed Eng5157161198310.1016/0141-5425(83)90036-56687914
|
|
2.
|
AM AhmedDL BurkeA YuIn-vitro measurement
of static pressure distribution in synovial joints-part II:
retropatellar surfaceJ Biomech
Eng105226236198310.1115/1.31384106632824
|
|
3.
|
R HartM JanecekV SiskaB KuceraV
StipcákCorrelation of long-term clinical and radiological results
after meniscectomiesActa Chir Orthop Traumatol
Cech72304307200516316606
|
|
4.
|
RJ Van der WalBJ ThomassenER van
ArkelLong-term clinical outcome of open meniscal allograft
transplantationAm J Sports Med3721342139200919542303
|
|
5.
|
T YamasakiM DeieR ShinomiyaY YasunagaS
YanadaM OchiTransplantation of meniscus regenerated by tissue
engineering with a scaffold derived from a rat meniscus and
mesenchymal stromal cells derived from rat bone marrowArtif
Organs32519524200810.1111/j.1525-1594.2008.00580.x
|
|
6.
|
RJ WebberJL YorkJL VanderschildenAJ Hough
JrAn organ culture model for assaying wound repair of the
fibrocartilaginous knee joint meniscusAm J Sports
Med17393400198910.1177/0363546589017003142729490
|
|
7.
|
BM BakerAS NathanGR HuffmanRL MauckTissue
engineering with meniscus cells derived from surgical
debrisOsteoarthritis
Cartilage17336345200910.1016/j.joca.2008.08.00118848784
|
|
8.
|
SW KangSM SonJS LeeES LeeKY LeeSG ParkJH
ParkBS KimRegeneration of whole meniscus using meniscal cells and
polymer scaffolds in a rabbit total meniscectomy modelJ Biomed
Mater Res A78659671200610.1002/jbm.a.30579
|
|
9.
|
K IshidaR KurodaM MiwaY TabataA HokugoT
KawamotoK SasakiM DoitaM KurosakaThe regenerative effects of
platelet-rich plasma on meniscal cells in vitro and its in vivo
application with biodegradable gelatin hydrogelTissue
Eng1311031112200710.1089/ten.2006.019317348798
|
|
10.
|
GM PerettiTJ GillJW XuMA RandolphKR
MorseDJ ZaleskeCell-based therapy for meniscal repair: a large
animal studyAm J Sports
Med32146158200410.1177/009539970325879014754738
|
|
11.
|
SH LuAH YangCF WeiHS ChiangMB
ChancellorMulti-potent differentiation of human purified
muscle-derived cells: potential for tissue regenerationBJU
Int10511741180200919712114
|
|
12.
|
G ZhouW LiuL CuiX WangT LiuY CaoRepair of
porcine articular osteochondral defects in non-weight-bearing areas
with autologous bone marrow stromal cellsTissue
Eng1232093221200610.1089/ten.2006.12.320917518635
|
|
13.
|
W ZhuY WangG QiuB ChenCharacterization of
the purification and primary culture of adult canine myoblasts in
vitroMol Med Rep3463468201021472263
|
|
14.
|
A AsakuraM KomakiM RudnickiMuscle
satellite cells are multipotential stem cells that exhibit
myogenic, osteogenic and adipogenic
differentiationDifferentiation68245253200110.1046/j.1432-0436.2001.680412.x11776477
|
|
15.
|
T MatsushitaN MatsuiH FujiokaS KuboR
KurodaM KurosakaS YoshiyaExpression of transcription factor sox9 in
rat L6 myoblastic cellsConnect Tissue
Res45164173200410.1080/0300820049051413015512770
|
|
16.
|
K GoldringT PartridgeD WattMuscle stem
cellsJ Pathol197457467200210.1002/path.115712115862
|
|
17.
|
CS DayC KasemkijwattanaJ MenetreySS Floyd
JrD BoothMS MorelandFH FuJ HuardMyoblast-mediated gene transfer to
the jointJ Orthop Res15894903199710.1002/jor.11001506169497816
|
|
18.
|
M KoningMC HarmsenMJ van LuynPM
WerkerCurrent opportunities and challenges in skeletal muscle
tissue engineeringJ Tissue Eng Regen
Med3407415200910.1002/term.19019575392
|
|
19.
|
D SinghV NayakA KumarProliferation of
myoblast skeletal cells on three-dimensional supermacroporous
cryogelsInt J Biol Sci6371381201010.7150/ijbs.6.37120617130
|
|
20.
|
A MarsanoSJ Millward-SadlerDM SalterA
AdesidaT HardinghamE TognanaE KonC Chiari-GrisarS NehrerM JakobI
MartinDifferential cartilaginous tissue formation by human synovial
membrane, fat pad, meniscus cells and articular
chondrocytesOsteoarthritis
Cartilage154858200710.1016/j.joca.2006.06.009
|
|
21.
|
SH LuAH YangCF WeiHS ChiangMB
ChancellorMulti-potent differentiation of human purified
muscle-derived cells: potential for tissue regenerationBJU
Int10511741180200919712114
|
|
22.
|
J Stern-StraeterG BranF RiedelA SauterK
HörmannUR GoesslerCharacterization of human myoblast cultures for
tissue engineeringInt J Mol Med2149562008
|
|
23.
|
JM MoranD PazzanoLJ
BonassarCharacterization of polylactic acid-poly-glycolic acid
composites for cartilage tissue engineeringTissue
Eng96370200310.1089/10763270376268754612625955
|
|
24.
|
L CuiY WuL CenH ZhouS YinG LiuW LiuY
CaoRepair of articular cartilage defect in non-weight-bearing areas
using adipose-derived stem cell-loaded polyglycolic acid
meshBiomaterials3026832693200910.1016/j.biomaterials.2009.01.04519217157
|
|
25.
|
J ZwingmannAT MehlhornN SudkampB StarkM
DaunerH SchmalChondrogenic differentiation of human articular
chondrocytes differs in biodegradable PGA/PLA scaffoldsTissue
Eng1323352343200710.1089/ten.2006.039317691868
|