Introduction
Bone metastasis occurs in approximately 80% of
patients with advanced breast cancer. Twenty to eighty-five percent
of all cancer patients show bone metastasis during the clinical
course, regardless of cancer type. Sixty-five to seventy-five
percent of these suffer from intolerable pain (1–3).
Since the goal of treatment in advanced cancer patients is to
prolong survival and improve their quality of life (QOL), pain
relief is crucial. In clinical practice, the World Health
Organization (WHO) 3-Step Pain Removal Ladder System, in which
non-steroidal anti-inflammatory drugs (NSAIDs), weak opioids and
strong opioids are administered for pain relief using a multistep
system, is commonly employed.
Clinical studies of bisphosphonates have been
conducted in breast and prostate cancer patients, in whom the
incidence of bone metastasis is reportedly high. These studies
demonstrated that bisphosphonates decreased the incidence of
skeletal-related events associated with radiotherapy or surgery for
bone metastasis-related fracture or bone pain. In addition, these
agents relieve bone pain, maintaining the QOL of patients with bone
metastasis, and therefore they are standard agents for the
management of bone metastasis in clinical practice.
In 2006, zoledronic acid was approved in Japan for
the treatment of bone metastasis from other solid cancers, in
addition to that from breast cancer and multiple myeloma. A
pre-clinical study showed that bisphosphonates exhibited a variety
of antitumor effects, such as the prevention of bone metastasis,
induction of cancer cell apoptosis, antineovascularization actions
and induction of γ/δ cells. Gnant et al reported that
combination therapy with hormone and bisphosphonate (zoledronate)
preparations as post-operative adjuvant therapy for breast cancer
in pre-menopausal women prolonged the relapse-free survival time
compared to hormonal therapy alone (4,5).
Many studies are currently being conducted to confirm the
possibility that bisphosphonates contribute to prolonged
survival.
In 2007, an oral radiation agent containing
strontium-89 (Sr-89) was approved as a commercially available new
drug for the treatment of bone metastasis in Japan. Sr-89 is
recognized as an agent that relieves radiation-induced pain, as
demonstrated in cases of external irradiation. This agent reaches
metastatic bone sites throughout the whole body when intravenously
administered as a single dose, and is therefore often employed to
treat multiple bone metastases (Table
I). However, an adverse effect of Sr-89 is that it induces bone
marrow suppression, and the guidelines established in the US
(6) and Europe (7) emphasize that physicians must
carefully consider the use of chemotherapeutic agents and external
irradiation in relation to the development and treatment of
metastatic bone pain. However, there are no restrictions regarding
combinations, such as with endocrine therapy, analgesic agents or
adjuvant analgesic agents (e.g., bisphosphonates).
 | Table I.Efficacy studies involving
single-dosage treatment with Sr-89 chloride.a |
Table I.
Efficacy studies involving
single-dosage treatment with Sr-89 chloride.a
| Author | Year | No. of
patients | Cancer type | Radiation
dosage | Response rate |
|---|
| Buchali | 1988 | 41 | Prostate | 3×75 MBq | 37 |
| Robinson | 1989 | 128 | Prostate,
breast | 40 microCi/kg | 80–89 |
| Lewington | 1991 | 26 | Prostate | 150 MBq | 67 |
| Laing | 1991 | 83 | Prostate | 1.5–3.0 MBq | 75 |
| Haesner | 1992 | 200 | Prostate | 3x37, 3x75 MBq | 59 |
| Quilty | 1994 | 123 | Prostate | 200 MBq | 65–70 |
| Pons | 1997 | 76 | Prostate,
breast | 148 MBq | 89–92 |
| Baziotis | 1998 | 64 | Breast | 2 MBq/kg | 81 |
| Kasalicky | 1998 | 118 | Prostate,
breast | 148 MBq | 96 |
| Fuster | 2000 | 40 | Breast | 148 MBq | 92 |
| Kraeber-Bodere | 2000 | 94 | Prostate | 150 MBq | 60 |
| Dafemou | 2001 | 527 | Prostate | 148 MBq | 60 |
| Turner | 2001 | 93 | Prostate | 150 MBq | 63 |
Few studies have reported the combination of
bisphosphonates and Sr-89. Storto et al (8) reported that combination therapy with
these two agents more effectively relieved pain than a single agent
alone (Fig. 1). However, in their
administration schedule, the simultaneous administration of the two
agents was not employed. Sr-89 was gradually administered after a
6-month pre-treatment period with zoledronic acid alone, that is,
Storto et al did not examine Sr-89 administration under
continuous therapy with zoledronic acid. In a previous clinical
trial involving Sr-89 in Japan (9), the effects of the combination therapy
of this radiopharmaceutical with zoledronic acid on treatment
efficacy was not considered. Thus, the efficacy and safety of
simultaneous combination therapy with Sr-89 and zoledronic acid in
Japanese patients remain to be verified.
In this study, Sr-89 was administered to breast
cancer patients with painful bone metastasis who had continuously
received zoledronic acid in our hospital and to those previously
treated with an initial dose of zoledronic acid in order to
evaluate the efficacy and safety of combination therapy with Sr-89
and zoledronic acid.
Materials and methods
The subjects were 16 breast cancer patients with
painful bone metastasis in whom Sr-89 was added to zoledronic acid
continuous therapy, or was added after an initial administration of
zoledronic acid at the Department of Radiology in our hospital,
between March 2007 and February 2011. We intravenously administered
Sr-89 at a dose of 2 MBq/kg to a maximum dose of 141 MBq per
person. Written informed consent was obtained from the patients
before the study.
To assess treatment efficacy, patients who did not
require an analgesic agent after Sr-89 administration because of
the complete relief of pain were regarded as having achieved a
complete response (CR). Those in whom the doses of analgesic
agents, such as opioids and NSAIDs, could be decreased were
regarded as having achieved a partial response (PR). Those in whom
there were no changes in the doses of analgesic agents were
regarded as showing no change (NC). Those in whom the doses of
analgesic agents were increased were regarded as non-responders
(NRs). After Sr-89 administration, bremsstrahlung imaging in which
Sr-89 β-ray damping radiation was visualized, which reflected the
distribution of Sr-89, was performed to investigate the
distribution of Sr-89 and compare it with 99mTc
accumulation on bone scintigraphy.
To evaluate safety, we examined bone marrow toxicity
and confirmed the minimum leukocyte and platelet counts during the
2 months after administration. The results were evaluated according
to the Common Term Criteria for Adverse Events (v. 3.0).
Results
The characteristics of the 16 breast cancer patients
with painful bone metastasis in this study are presented in
Table II. CR was achieved in 5
patients, PR was observed in 9 and NC in 2 patients. In this study,
pain relief was achieved in 14 of the 16 patients, indicating the
efficacy of this combination. These effects appeared from 1 week to
1 month after administration.
 | Table II.Patients with painful bone metastasis
from breast cancer (n=16). |
Table II.
Patients with painful bone metastasis
from breast cancer (n=16).
| Case no. | Age (years) | ZOL periods
(months)a | Patient relief | WBC (x1,000) | Blood platelets
(x10,000) | Radiation
history |
Pre-/during/post-therapy | Survival period
(months)b | Metastatic organs
(except bone) |
|---|
| 1 | 50 | 10 | CR | 7.1→5.0 | 22.4→18.3 | + | PTX-CBDCA/AI,
H/ | 2 M death | Lung |
| 2 | 48 | 46 | PR | 5.0→ | 19.1→22.7 | + | TAM, PTX-H,
VNB-H// | 10 M | Lung, liver |
| 3 | 59 | 20 | PR | 3.8→5.7 | 20.1→19.4 | + | /AI/ | 10 M death | ONJ |
| 4 | 51 | 62 | NC | 4.1→2.8 | 18.6→20.0 | + | ECT, CEF,
T/AI/VNB | 24 M death | Lung, liver |
| 5 | 57 | 34 | PR | 3.5→1.9 | 15.5→18.5 | + | XC,
DTX/AI/VNB | 20 M death | Liver |
| 6 | 60 | 10 | PR | 6.9→7.4 | 20.0→24.7 | + | EC-HT/H,
AI/X | 7 M death | Contra-breast,
liver, adrenal |
| 7 | 66 | 0 | CR | 7.9→7.5 | 19.5→15.1 | - | /AI/ | 2 M death | Lung, liver, lymph
node |
| 8 | 50 | 8 | PR | 3.3→ | 23.3→ | - |
EC-wPTX/TOR/ | 5 M death | Liver, ovary, lymph
node |
| 9 | 53 | 12 | PR | 6.1→2.3 | 22.9→12.7 | - |
/X/GEM-VNB | 2 M death | Liver |
| 10 | 75 | 1 | PR | 8.6→ | 29.6→22.7 | - | AI | 12 M death | Bile pleural
effusion |
| 11 | 49 | 1 | CR | 5.2→2.4 | 46.0→20.9 | - |
/GEM+VNB | 5 M | Liver |
| 12 | 52 | 49 | PR | 4.5→3.1 | 21.6→17.3 | - | AI | 5 M | None |
| 13 | 47 | 2 | NC | 4.0→3.7 | 31.2→13.7 | - |
AI/GEM+VNB | 4 M | Liver, pancreas,
bile pleural effusion |
| 14 | 46 | 0 | CR | 5.3→2.9 | 20.0→14.5 | - | TAM | 3 M | None |
| 15 | 39 | 0 | CR | 7.7→5.8 | 47.7→25.7 | - | TAM+LH-RH
agonist | 2 M | Lung, pleural
effusion |
| 16 | 72 | 38 | PR | 5.3→5.0 | 16.3→15.0 | - |
AI/AI+XC/TOR/AI+5FU | 2 M | Lung |
With respect to safety, neither grade 3 or higher
leukopenia nor thrombopenia was observed; the tolerance to adverse
events was favorable.
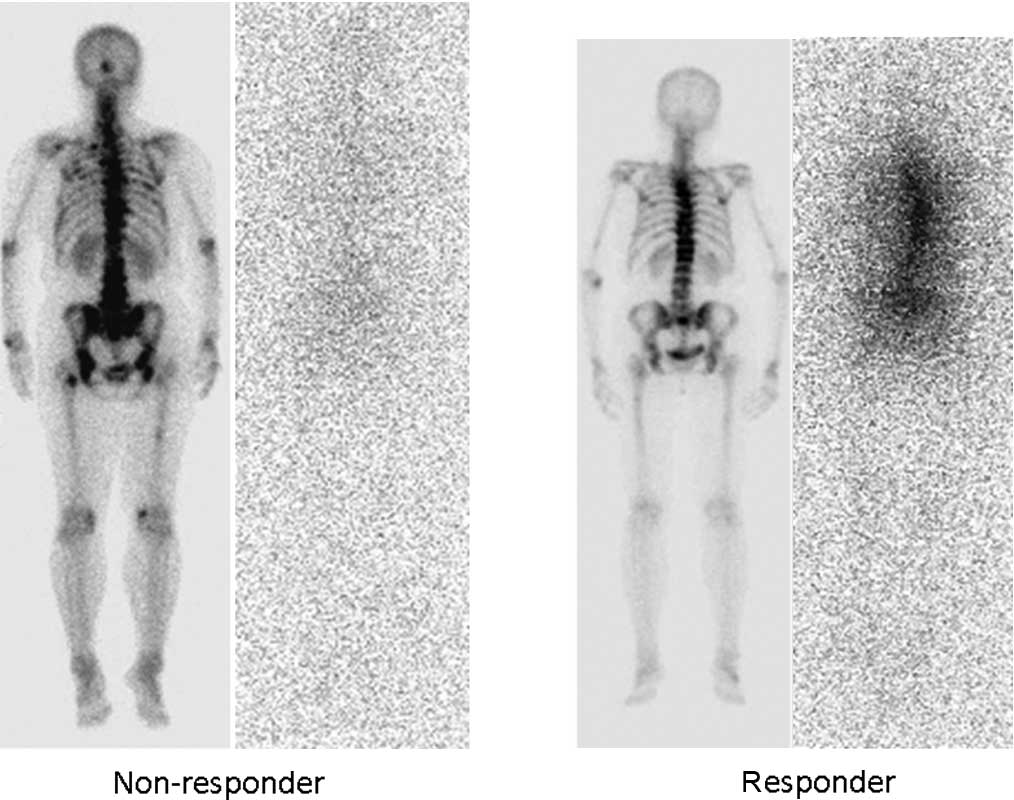
Bone scintigraphy and bremsstrahlung imaging were
performed. The accumulation of bremsstrahlung was consistent with
the 99mTc uptake on bone scintigraphy in responders.
However, only slight accumulation was noted in NRs (Fig. 2).
Discussion
We set out to investigate the efficacy and safety of
combination therapy with zoledronic acid and Sr-89 for painful bone
metastases from breast cancer.
When evaluating the efficacy of this combination
with respect to changes in the doses of analgesic agents, 14 (88%)
of the 16 patients responded to this therapy. Storto et al
(8) examined patients with bone
metastasis from prostate or breast cancer, and reported that 96% of
patients after 6 months of therapy with zoledronic acid responded
to Sr-89 upon sequential administration, whereas the response rate
was 82% in patients treated with Sr-89 alone. Furthermore, Finlay
et al (10) in their
systematic review indicated that the response rate for Sr-89 alone
was 76%. The response rate of 88% under the combination therapy
with zoledronic acid obtained in this study may not be
significantly different to that reported by Storto et
al.
Furthermore, bremsstrahlung imaging was performed
with β-rays from Sr-89. As Sr-89 does not radiate γ-rays, it is
usually difficult to visualize. However, bremsstrahlung radiation
from β-rays partially facilitates the visualization of Sr-89
distribution, although the clarity is less marked than that of bone
scintigraphy (11). As Sr-89
accumulation can be directly confirmed, we added this procedure to
the efficacy assessment in this study. In breast cancer patients,
Baziotis et al (12)
reported a correlation between bremsstrahlung imaging findings
after Sr-89 administration in breast cancer patients with bone
metastasis and bone scintigraphy findings. In the present study,
responders showed Sr-89 accumulation, which was consistent with the
accumulation sites seen on bone scintigraphy. However, among NRs
there was no Sr-89 accumulation, suggesting an association between
efficacy and Sr-89 accumulation. According to Storto et al
(8), combination therapy with
zoledronic acid and Sr-89 may increase Sr-89 accumulation. This may
contribute to the combination-related enhancement of the effects.
In the present study, combination therapy with zoledronic acid and
Sr-89 was also effective, but 1 patient showed only low-level Sr-89
accumulation under treatment with zoledronic acid, which had been
administered over 62 months. It is known that zoledronic acid
reduces osteolytic actions through osteoclast suppression. This may
partially increase osteopoietic actions and promote Sr-89 uptake.
However, the effect of long-term therapy with zoledronic acid on
bone metabolism and Sr-89 accumulation remains to be clarified.
Further investigation is required.
Concerning safety, bone marrow suppression related
to Sr-89 administration under treatment with zoledronic acid was
observed as grade 2 or lower leukopenia or thrombopenia. There were
no grade 3 or higher adverse events. Storto et al reported
that in a combination therapy (zoledronic acid + Sr-89) group, bone
marrow suppression was slightly more marked than in an Sr-89 group.
However, no patient required treatment. In the present study, bone
marrow suppression associated with combination therapy with
zoledronic acid and Sr-89 was tolerable, similar to that described
by Storto et al (8).
In a clinical trial of Sr-89 in Japan, bone marrow
suppression resulting in leukopenia and thrombopenia gradually
appeared after Sr-89 administration and reached a peak 8 weeks
after administration, before gradually returning to the baseline.
Therefore, caution is required regarding combination therapy with
chemotherapeutic agents, since bone marrow suppression may be
enhanced. In established guidelines in Europe and the United
States, it is recommended that combination therapy should be
avoided for 3 months after Sr-89 administration. However, with
respect to the combination therapy of Sr-89 and chemotherapeutic
agents, few studies have examined the relationship between the
timing of administration and tolerance. Most studies regarding
Sr-89 involved patients who had received chemotherapy before Sr-89
administration, presenting it as a background factor. Akerley et
al (13), Tu et al
(14) and Sciuto et al
(15) reported simultaneous
combination therapy with Sr-89 and chemotherapeutic agents in
patients with prostate cancer and observed tolerance. Furthermore,
Tu et al (16) and Porfini
et al (17) investigated
whether or not chemotherapy can be performed after Sr-89
administration in prostate cancer patients, and concluded that
chemotherapy was possible. To the best of our knowledge, no study
of this combination in breast cancer patients alone has been
reported in the literature.
In this study, we also reviewed chemotherapeutic
regimens combined before and after Sr-89 administration.
Chemotherapy with taxans or binorerbin, which may cause bone marrow
suppression, was performed before Sr-89 administration in 7 of 16
patients treated with Sr-89: 2 patients during and 7 patients after
administration. Bone marrow suppression was tolerable, suggesting
that simultaneous combination therapy with chemotherapy before or
after Sr-89 administration, or combination therapy early after
administration is possible in patients with bone metastasis from
breast cancer.
In Sr-89 administration, bone marrow function (as
indicated by blood count values) must be maintained. When
chemotherapy has been administered before Sr-89, Sr-89 treatment
should be conducted after confirming recovery of blood count
values. When administering chemotherapy after Sr-89 administration,
physicians should confirm the reduction of Sr-89-related bone
marrow suppression or recovery and tolerance to chemotherapeutic
agents.
In this study, 8 of the 16 patients had undergone
external irradiation. The mean and maximum tissue tracks of β-rays
emitted from Sr-89 were 2.4 and 8 mm, respectively. Even if β-rays
accumulate in vertebral bone metastatic sites, they may not reach
the intramedullary space. Therefore, when additional external
irradiation is not possible owing to the total dose reaching the
tolerance level, Sr-89 therapy remains possible. However,
combination therapy with external irradiation and Sr-89 should be
carefully performed, since it may promote bone marrow suppression
as an adverse effect. In the guidelines established in Europe and
the US, it is stated that combination therapy with half-body
irradiation within 2–3 months should be avoided, whereas the
combination of topical irradiation and Sr-89 is possible. In the
present study, all subjects had also undergone topical external
irradiation. Nevertheless, the results confirmed the efficacy and
safety of Sr-89 therapy.
In the present study, the survival time after Sr-89
administration varied among the 14 responders (14/16), from 2 to 23
months or more (all responders succumbed to their primary disease).
Several studies indicated that Sr-89 was effective in patients when
administered in the early stage as an efficacy-predicting factor,
whereas Sr-89 was less effective in the terminal stage and caused
marked adverse effects (18–22).
Therefore, when life expectancy is estimated to be extremely short
(1 month or less), Sr-89 administration is not indicated. On the
other hand, pain-removing effects may also be achieved in advanced
cancer patients. Although it is difficult to accurately predict the
effects of Sr-89 before treatment (23,24),
we cannot deny its usefulness in terminal cancer patients. The
results of this study showed the efficacy of Sr-89 regardless of
life expectancy (2–23 months). When Sr-89 is indicated, early
treatment should be positively considered. Even in terminal cancer
patients, Sr-89 therapy may be a treatment option.
In conclusion, in this study pain-relief effects
were achieved in 14 of 16 patients, suggesting the efficacy of
combination therapy with zoledronic acid and Sr-89. Furthermore,
there were no serious adverse events related to this therapy, and
drug tolerance was favorable.
This combination therapy can be combined with
endocrine and molecule-targeting therapies. In certain patients,
combination therapy with chemotherapeutic agents was also possible.
This combination therapy was safe and effective in patients with a
history of external irradiation. As the use of Sr-89 may relieve
pain and improve QOL, treatment with Sr-89 at the early stage, when
its effects are more potent, should be considered in the future,
and a large-scale clinical study should be conducted.
Acknowledgements
The authors are indebted to Mr.
Roderick J. Turner, Assistant Professor Edward F. Barroga and
Professor J. Patrick Barron, Chairman of the Department of
International Medical Communications at Tokyo Medical University,
for their review of the English manuscript.
References
|
1.
|
D TongL GillickFR HendricksonThe
palliation of symptomatic osseous metastases: final results of the
study by the Radiation Therapy Oncology
GroupCancer50893899198210.1002/1097-0142(19820901)50:5%3C893::AID-CNCR2820500515%3E3.0.CO;2-Y6178497
|
|
2.
|
RE ColemanRD RubensThe clinical course of
bone metastases from breast cancerBr J
Cancer556166198710.1038/bjc.1987.133814476
|
|
3.
|
N KohnoTreatment of breast cancer with
bone metastasis: bisphosphonate treatment – current and futureInt J
Oncol1318232008
|
|
4.
|
M GnantB MlineritschW SchippingerEndocrine
therapy plus zoledronic acid in premenopausal breast cancerN Engl J
Med360679691200910.1056/NEJMoa080628519213681
|
|
5.
|
M GnantB MlineritschH StoegerAdjuvant
endocrine therapy plus zoledronic acid in premenopausal women with
early-stage breast cancer: 62-month follow-up from the ABCSG-12
randomised trialLancet
Oncol12631641201110.1016/S1470-2045(11)70122-X21641868
|
|
6.
|
Society of Nuclear Medicine Procedure
Guideline for Palliative Treatment of Painful Bone Metastases
ver.3.0 (http://interactive.snm.org/docs/pg_ch25_0403.pdf).
|
|
7.
|
EB SilbersteinAT Taylor JrEANMEANM
Procedure Guidelines for Treatment of Refractory Metastatic Bone
PainEur J Nucl
Med30BP7BP11200310.1007/s00259-002-1057-112723557
|
|
8.
|
G StortoM KlainG PaoneCombined therapy of
Sr-89 and zoledronic acid in patients with painful bone
metastasesBone393541200610.1016/j.bone.2005.12.00416434248
|
|
9.
|
M NishioM SanoY TamakiA multicenter study
to determine the efficacy and safety of strontium (89Sr) chloride
for palliation of painful bony metastases in cancer patientsNippon
Acta Radiologica65399410200516334394
|
|
10.
|
IG FinlayMD MasonM ShelleyRadioisotopes
for the palliation of metastatic bone cancer: a systematic
reviewLancet
Oncol6392400200510.1016/S1470-2045(05)70206-015925817
|
|
11.
|
M UchiyamaH NaritaM MakinoStrontium-89
therapy and imaging with bremsstrahlung in bone metastasesClin Nucl
Med22605609199710.1097/00003072-199709000-000059298293
|
|
12.
|
N BaziotisE YakoumakisA
ZissimopoulosStrontium-89 chloride in the treatment of bone
metastases from breast
cancerOncology55377381199810.1159/0000118819732212
|
|
13.
|
W AkerleyJ ButeraT WehbeA
multiinstitutional, concurrent chemoradiation trial of
strontium-89, estramustine, and vinblastine for hormone refractory
prostate carcinoma involving
boneCancer9416541660200210.1002/cncr.10437
|
|
14.
|
SM TuRE MillikanB MengistuBone-targeted
therapy for advanced androgen-independent carcinoma of the
prostate: a randomized phase II
trialLancet357336341200110.1016/S0140-6736(00)03639-411210994
|
|
15.
|
R SciutoA FestaS ReaEffects of low-dose
cisplatin on 89Sr therapy for painful bone metastases from prostate
cancer: a randomized clinical trialJ Nucl Med437986200211801708
|
|
16.
|
SM TuJ KimLC PagliaroTherapy tolerance in
selected patients with androgen-independent prostate cancer
following strontium-89 combined with chemotherapyJ Clin
Oncol2379047910200510.1200/JCO.2005.01.231016258090
|
|
17.
|
E PorfiriInitial feasibility and safety
results from a phase II/III clinical trial to evaluate docetaxel
(D) therapy in combination with zoledronic acid (ZA) ± strontium-89
(Sr89) in hormone-refractory prostate cancer patientsJ Clin
Oncol28S7abs 46772010
|
|
18.
|
AJ McEwanUse of radionuclides for the
palliation of bone metastasesSemin Radiat
Oncol10103114200010.1016/S1053-4296(00)80047-810727599
|
|
19.
|
AN SerafiniTherapy of metastatic bone
painJ Nucl Med42895906200111390554
|
|
20.
|
AH LaingDM AckeryRJ BaylyStrontium-89
chloride for pain palliation in prostatic skeletal malignancyBr J
Radiol64816822199110.1259/0007-1285-64-765-8161717094
|
|
21.
|
F Kraeber-BodéréL CampionC
RousseauTreatment of bone metastases of prostate cancer with
strontium-89 chloride: efficacy in relation to the degree of bone
involvementEur J Nucl Med2714871493200011083537
|
|
22.
|
PM WindsorPredictors of response to
strontium-89 (Metastron) in skeletal metastases from prostate
cancer: report of a single centre’s 10-year experienceClin Oncol (R
Coll Radiol)13219227200111527299
|
|
23.
|
EB SilbersteinC WilliamsStrontium-89
therapy for the pain of osseous metastasesJ Nucl
Med2634534819853920361
|
|
24.
|
EB SilbersteinTeletherapy and
radiopharmaceutical therapy of painful bone metastasesSemin Nucl
Med35152158200510.1053/j.semnuclmed.2004.11.00615765378
|
|
25.
|
MG LamJM de KlerkPP van RijkBA
ZonnenbergBone seeking radiopharmaceuticals for palliation of pain
in cancer patients with osseous metastasesAnticancer Agents Med
Chem.7381397200710.2174/18715200778105859617630915
|