Introduction
Mitotic arrest deficiency 2 (MAD2) was the first
gene involved in the mitotic spindle checkpoint pathway to be
characterized (1,2). MAD2 localizes on kinetochores
following chromosome condensation and prior to anaphase (3), and plays a crucial role in the
transition from metaphase to anaphase by inhibiting the
anaphase-promoting complex/cyclosome (APC/C). This ensures that all
the chromosomes are correctly aligned at the metaphase plate prior
to daughter cell segregation (1,4).
Therefore, MAD2 is a key component of the mitotic spindle
checkpoint pathway, which plays an important role in preventing
loss or gain of chromosomes within cells (5). A compromised mitotic spindle
checkpoint results in an abnormal number of chromosomes, known as
chromosomal instability (CIN) (6).
CIN, characterized by an alteration in chromosome number, and
commonly detected as aneuploidy (7,8), has
been reported in the majority of types of human cancer. Although
the underlying molecular mechanisms have yet to be clarified, it is
notable that the overexpression of MAD2 in transgenic mice results
in CIN, and initiates carcinogenesis in a wide variety of tumors
(9). In fact, the overexpression
of MAD2 has also been observed in a variety of human cancers
(10–20). Certain studies have suggested a
correlation between the overexpression of MAD2 and a variety of
clinicopathological characteristics, such as histological grade
(differentiation), metastasis and prognosis (13–19).
However, reduced expression of MAD2 occurs in certain types of
human cancer (4,21) and is associated with in
vitro resistance to chemotherapy using microtubule-targeting
agents or DNA-damaging agents (22,23).
MAD2 expression has not been examined in uterine
cervical cancer; a form of cancer that, if advanced, is very
difficult to treat. Concurrent chemoradiotherapy (CCRT) is
recommended as the standard treatment for locally advanced uterine
cervical cancer, such as International Federation of Gynecology and
Obstetrics (FIGO) stages IIIa, IIIb and IVa (24,25);
however, the prognosis is poor (26,27).
Certain studies have reported the use of hysterectomy following
successful neoadjuvant chemotherapy (NAC) for locally advanced
uterine cervical cancer (28,29),
which may control genital bleeding or improve hydronephrosis.
However, the prognosis worsens if NAC is unsuccessful, since
hysterectomy cannot then be performed and, consequently, the
treatment strategy has to be changed from surgery to radiation
therapy, resulting in a crucial delay (30,31).
Thus, it is important to identify prognostic factors in patients
with locally advanced cervical cancer that predict whether NAC is
likely to be successful (32–35).
Therefore, the aim of the present study was to
examine the correlation between MAD2 expression and the efficacy of
NAC for locally advanced uterine cervical cancer.
Patients and methods
Patients and samples
We reviewed 53 cases of locally advanced uterine
cervical cancer [stage IIIa and IIIb (FIGO)] initially treated at
Osaka City University Medical School Hospital, Japan, from 1995 to
2008 in patients that were under 70 years old. Tumor samples were
obtained by biopsy prior to NAC. The cases were divided into two
groups: one group in which NAC was effective, surgery was possible
and radiation therapy was performed (NAC+OP+R group; n=33), and
another group in which NAC was ineffective and, therefore,
radiation therapy alone was performed (NAC+R group; n=20) (Table I). Moreover, the cases were further
divided into a complete/ partial remission (CR+PR) group and a
stable/progressive disease (SD+PD) group according to the measured
effects of NAC (Table II). Written
informed consent was obtained from all patients prior to
immunohistochemical examination and this study was approved by the
Ethics Committee of Osaka City University (IRB No.2202).
 | Table I.Characteristics of the patients in
the NAC+OP+R and NAC+R groups. |
Table I.
Characteristics of the patients in
the NAC+OP+R and NAC+R groups.
| NAC+OP+R group | NAC+R group | P-value |
|---|
| No. of cases | 33 | 20 | |
| Age | | | a0.023 |
| Mean±SD | 46.2±13.2 | 54.2±9.8 | |
| Range | 22–69 | 37–67 | |
| FIGO stage | | | b0.432 |
| IIIa | 1 | 0 | |
| IIIb | 32 | 20 | |
| Histology | | | b0.220 |
| SCC | 29 | 18 | |
| A | 4 | 1 | |
| AS | 0 | 0 | |
| Others | 0 | 1 | |
 | Table II.Characteristics of the patients in
the CR+PR and SD+PD groups. |
Table II.
Characteristics of the patients in
the CR+PR and SD+PD groups.
| CR+PR group | SD+PD group | P-value |
|---|
| No. of cases | 41 | 12 | |
| Age | | | a0.076 |
| Mean±SD | 47.5±12.8 | 54.8±10.0 | |
| Range | 22–69 | 38–67 | |
| FIGO stage | | | b0.584 |
| IIIa | 1 | 0 | |
| IIIb | 40 | 12 | |
| Histology | | | b0.252 |
| SCC | 37 | 10 | |
| A | 4 | 1 | |
| AS | 0 | 0 | |
| Others | 0 | 1 | |
| Main therapy | | | b<0.00001 |
| NAC+OP+R | 32 | 0 | |
| NAC+R | 9 | 12 | |
Balloon-occluded arterial infusion
chemotherapy (BOAI) for NAC
Pelvic angiography was performed under local
anesthesia using Seldinger’s technique (36) to localize the tumor and feeder
vessels. The procedure involves the insertion of a
balloon-wedge-single-pressure catheter (5 F, 80 cm in length;
Dispomedica, Hamburg, Germany) into each femoral artery, which is
then passed into the internal iliac artery. The balloon catheters
are then advanced until they reach the vicinity of the feeder
vessel (usually the uterine artery), where the balloon is inflated
to interrupt the local blood flow. Cis-diamminedichloro-platinum
(CDDP) is then slowly infused intra-arterially through the two
catheters over a period of 30 min (36). In the present study, the two
ovarian arteries were blocked after the first round of BOAI to
increase the intratumor concentration of CDDP. BOAI was performed
three times to shrink the tumor. Adequate hydration was ensured
prior to and following CDDP administration, and antiemetics and
diuretics were used as appropriate. CDDP was administered at doses
of 50, 75 or 100 mg/m2, depending on the patient’s age
and renal function. The efficacy of CDDP arterial infusion therapy
was evaluated by cytology, histology, serum tumor marker levels and
MRI, prior to the initiation of CDDP treatment. The results were
then compared with those obtained following the completion of each
arterial infusion. MRI was used to estimate tumor regression by
measuring its size in two dimensions (37,38).
Tumor tissue was obtained from all patients who had undergone punch
biopsy or surgery.
Immunohistochemical analysis
The expression of MAD2 was examined in
paraffin-embedded sections using an anti-MAD2 antibody and the
avidin-biotin peroxidase complex method. Paraffin sections (4-μm
thick) were de-paraffinized and immersed in 3% hydrogen peroxidase
in methanol to block endogenous peroxidase activity. An antigen
retrieval procedure was then performed by immersing the slides in
10 mM citrate buffer (pH 6.0) and heating in an autoclave at 110°C
for 20 min. The sections were then washed in PBS. The protocol
supplied with the Dako LSAB 2 peroxidase kit (Dako, Kyoto, Japan)
was followed. Sections were incubated with the primary antibody
(monoclonal rabbit anti-human MAD2; 1:100; ProteinTech Group,
Chicago, USA) for 2 h at room temperature. Sections were then
rinsed with PBS for 15 min and incubated for 10 min with the
secondary antibody (biotinylated goat anti-mouse and rabbit
immunoglobulin G; Dako). The sections were then incubated with the
streptavidin-peroxidase complex and 3,3′-diaminobenzidine was used
as the chromogen. Finally, the sections were counterstained with
Mayer’s hematoxylin. The specificity of the immunohistochemical
reactions was checked by omitting the primary antibody.
Quantitative analysis of MAD2 expression was based on the scoring
method of Sinicrope et al (39). The mean percentage of positive
tumor cells was determined in five separate areas (at x400
magnification) and assigned to one of the following categories: 0,
<5%; 1, 5–25%; 2, 25–50%; 3, 50–75%; or 4, >75%. The
intensity of immunostaining was scored as follows: 1+, weak; 2+,
moderate; or 3+, intense. For each specimen, the percentage of
positive tumor cells was multiplied by the staining intensity to
produce a weighted score.
Statistical analysis
Data were presented as the means ± standard
deviation. The Kaplan-Meier and log-rank tests were performed for
the prognostic analyses. Weighted scores were compared using the
Mann-Whitney U test. The Student’s t-test and χ2 test
were performed on a different set of data (age, FIGO stage,
histology and main therapy). StatView 5.0 (Abacus Concepts,
Berkley, CA, USA) was used for data analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
We reviewed 53 cases of locally advanced uterine
cervical cancer and divided them into two groups: NAC+OP+R (n=33)
and NAC+R (n=20). The mean age of the NAC+OP+R group was 46.2 years
(range, 22–69), and that of the NAC+R group was 54.2 years (range,
37–67). The cases in the NAC+OP+R group were classified as stage
IIIa (n=1) and stage IIIb (n=32), and the cases in the NAC+R group
were classified as stage IIIb (n=20). According to histological
type, the cases in the NAC+OP+R group were classified as squamous
cell carcinoma (n=29) and adenocarcinoma (n=4), and the cases in
the NAC+R group were classified as squamous cell carcinoma (n=18),
adenocarcinoma (n=1) and other (n=1; glassy cell carcinoma). There
was no significant difference between the two groups (Table I).
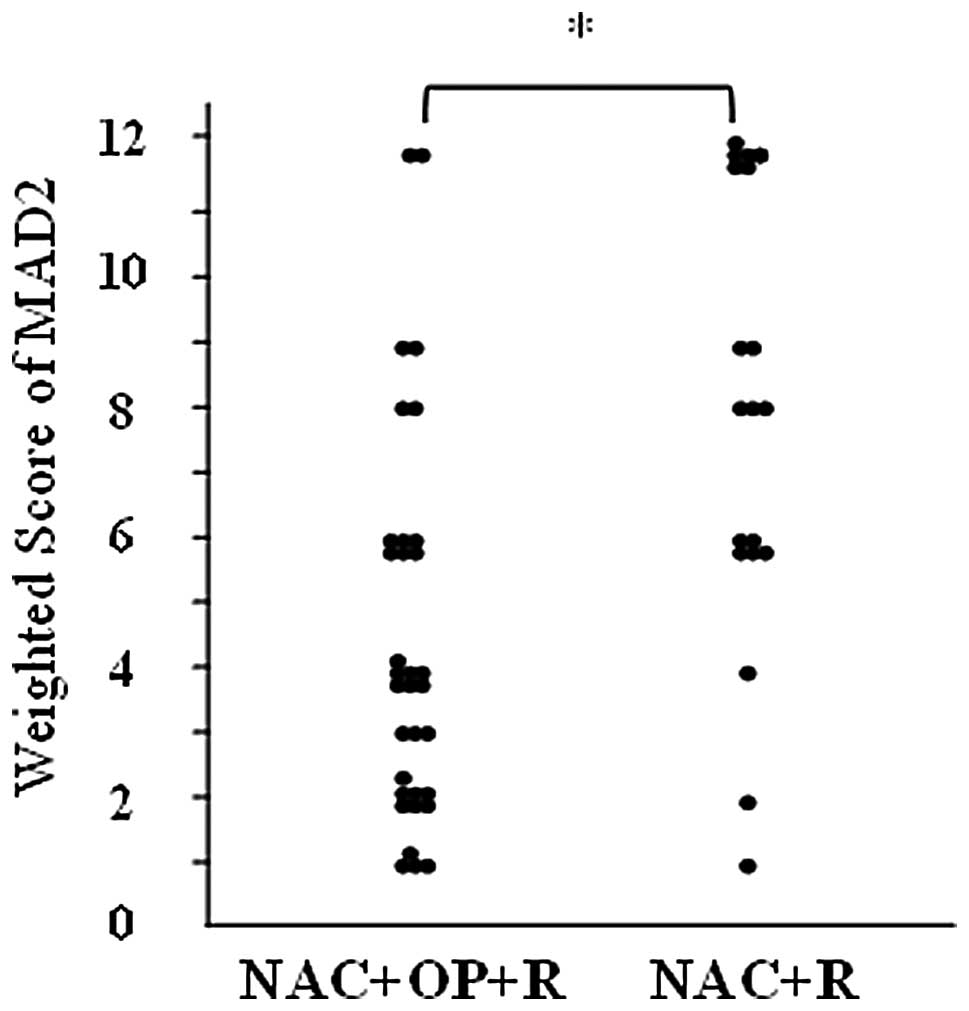
Expression of MAD2
MAD2 was expressed in the nuclei of the tumor cells
(Fig. 1). The weighted scores are
shown in Table III. The mean
weighted score for the NAC+OP+R group was 4.5, while that for the
NAC+R group was 8.2. MAD2 expression was significantly higher in
the NAC+R group compared to the NAC+OP+R group (P<0.001;
Fig. 2). The weighted scores were
classified as follows: 0, 1 and 2, low expression; 3, 4 and 6,
medium expression; and 8, 9 and 12, high expression. In the
NAC+OP+R group, 33.3% of the cases (n=11) showed low expression,
48.4% (n=16) medium expression and 18.2% (n=6) high expression. In
the NAC+R group, 10% of the cases (n=2) showed low expression, 30%
(n=6) showed medium expression and 60% (n=12) showed high
expression.
 | Table III.Weighted scores for the NAC+OP+R and
NAC+R groups. |
Table III.
Weighted scores for the NAC+OP+R and
NAC+R groups.
| No. of cases
|
|---|
| Weighted score | NAC+OP+R group | NAC+R group |
|---|
| 0 | 0 | | 0 | |
| 1 | 4 | 11 (36.3%) | 1 | 2 (9.5%) |
| 2 | 7 | | 1 | |
| 3 | 3 | | 0 | |
| 4 | 7 | 16 (48.4%) | 1 | 6 (28.5%) |
| 6 | 6 | | 5 | |
| 8 | 2 | | 3 | |
| 9 | 2 | 6 (15.1%) | 2 | 12 (61.9%) |
| 12 | 2 | | 7 | |
| Total | | 33 | | 20 |
| Weighted score
(mean) | | 4.5 | | 8.2 |
Correlation between expression of MAD2
and effects of NAC
All cases (n=53) were classified into two groups
according to the measured effects of NAC: CR+PR (n=41) and SD+PD
(n=12) (Table II). The mean
weighted score for the CR+PR group was 4.9, while that for the
SD+PD group was 9.3 (Table IV).
MAD2 expression was significantly higher in the SD+PD group than in
the CR+PR group (P<0.01; Fig.
3).
 | Table IV.Weighted scores for the CR+PR and
SD+PD groups. |
Table IV.
Weighted scores for the CR+PR and
SD+PD groups.
| No. of cases
|
|---|
| Weighted-score | CR+PR group | SD+PD group |
|---|
| 0 | 0 | | 0 | |
| 1 | 4 | 11 (26.8%) | 1 | 2 (16.7%) |
| 2 | 7 | | 1 | |
| 3 | 3 | | 0 | |
| 4 | 8 | 22 (53.7%) | 0 | 0 (0%) |
| 6 | 11 | | 0 | |
| 8 | 3 | | 2 | |
| 9 | 3 | 8 (19.5%) | 1 | 10 (83.3%) |
| 12 | 2 | | 7 | |
| Total | | 41 | | 12 |
| Weighted
score(mean) | | 4.9 | | 9.3 |
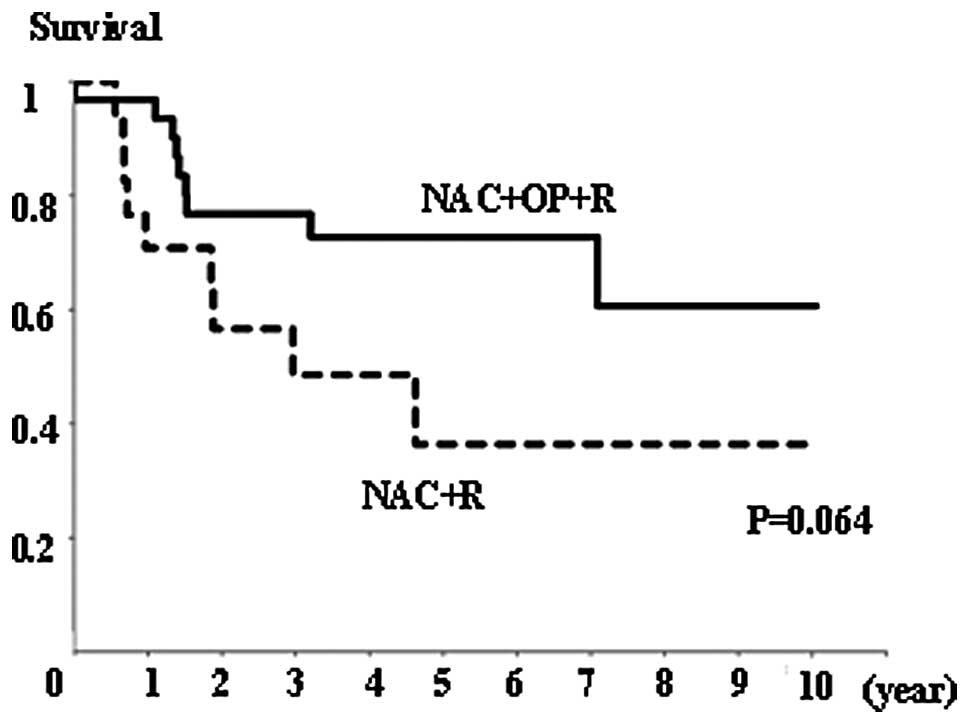
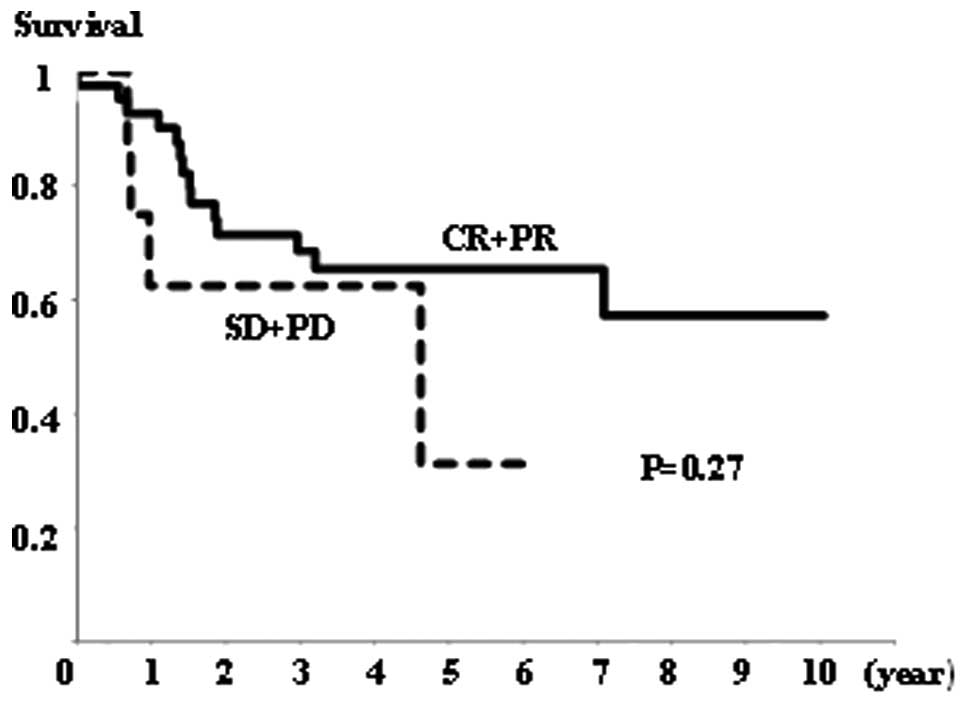
Survival
There was no significant difference in overall
survival rates between the NAC+OP+R and NAC+R groups (Fig. 4), or between the CR+PR and SD+PD
groups (Fig. 5). However, the
NAC+R group tended to show a worse prognosis than the NAC+OP+R
group (P=0.064; Fig. 4). Regarding
the extent of MAD2 expression, there was no significant difference
in overall survival between any of the groups (data not shown).
Discussion
The results of the present study show that the
overexpression of MAD2 correlates with ineffective NAC for locally
advanced uterine cervical cancer. MAD2 expression was significantly
higher in the SD+PD and NAC+R groups. (Figs. 2 and 3). In addition, our results agree with
those presented in previous studies showing that prognosis is worse
when NAC is unsuccessful (30,31).
In general, CCRT is recommended as the standard
treatment for locally advanced uterine cervical cancer, such as
FIGO stages IIIa, IIIb and IVa (24,25);
however, the prognosis following treatment is not good (26,27).
Certain studies have reported on the usefulness of hysterectomy
following successful NAC for locally advanced uterine cervical
cancer (28,29), which may improve prognosis and
control genital bleeding and/or improve hydronephrosis. However,
hysterectomy after NAC is not recommended at present, since the
prognosis becomes worse if NAC is not successful (30,31).
This may be due to a delay in curative treatment, or
cross-resistance to radiotherapy. Hence, it is crucial to identify
prognostic factors that predict the likely efficacy of NAC in
patients with locally advanced cervical cancer (32–35).
MAD2 is a key component of the mitotic spindle
checkpoint pathway, which, if compromised, results in CIN and
tumorigenesis. The overexpression of MAD2 has been shown to promote
aneuploidy, tumorigenesis and tumor progression in mice (19). The overexpression of MAD2 has also
been observed in a number of types of human cancer (10–20)
and appears to correlate with a variety of clinicopathological
characteristics, such as metastasis and prognosis (13–19).
Reduced expression of MAD2 has also been reported in certain human
cancers (4,21) and is associated with resistance to
chemotherapy using microtubule-targeting agents or DNA-damaging
agents (22,23).
The present study is the first to report a
correlation between MAD2 expression and locally advanced uterine
cervical cancer. The data show that MAD2 expression was
significantly higher in the NAC+R and SD+PD groups than in the
NAC+OP+R or SD+PD groups. This suggests that MAD2 expression is
capable of predicting the efficacy of NAC for locally advanced
uterine cervical cancer. We speculate that the overexpression of
MAD2 induces resistance to chemotherapy; however, other in
vitro studies have shown that the decreased expression of MAD2
mediates resistance to chemotherapy (22,23).
This discrepancy may be explained by the fact that the
overexpression of MAD2 appears to be involved in tumor progression,
which may be attributed to resistance to chemotherapy. Also, it is
difficult to compare the criteria used to judge whether MAD2
expression was high or low in each study. There are differences in
the degree of MAD2 expression between tumors and differences in the
measurement methods used (4,10–19,21).
In terms of overall survival rates, a number of
studies have indicated that the overexpression of MAD2 is a risk
factor for poor prognosis (13–19).
The data presented in the present study are inconsistent with this,
and show no correlation between MAD2 expression and prognosis (data
not shown).
As mentioned above, unsuccessful NAC for locally
advanced uterine cervical cancer leads to a worse prognosis.
Therefore, factors predicting the effectiveness of NAC will play a
critical role in trials of NAC for locally advanced uterine
cervical cancer. The results of the present study show that MAD2
expression correlates with resistance to cisplatin-based
chemotherapy and may be a strong predictor of the efficacy of
NAC.
Acknowledgements
We thank the gynecologists at Osaka
City University Medical School Hospital for their support. This
study was supported by the Osaka Medical Research Foundation for
Incurable Diseases.
References
|
1.
|
R LiAW MurrayFeedback control of mitosis
in budding
yeastCell66519531199110.1016/0092-8674(81)90015-51651172
|
|
2.
|
Y LiR BenezraIdentification of a human
mitotic checkpoint gene:
hsMAD2Science274246248199610.1126/science.274.5285.2468824189
|
|
3.
|
A Lopez-GironaB FurnariO MondesertP
RussellNuclear localization of Cdc25 is regulated by DNA damage and
a 14–3–3 proteinNature39717217519999923681
|
|
4.
|
X WangDY JinRW NgH FengYC WongAL CheungSW
TsaoSignificance of MAD2 expression to mitotic checkpoint control
in ovarian cancer cellsCancer Res6216621668200211912137
|
|
5.
|
T Orr-WeaverRA WeinbergA checkpoint on the
road to cancerNature392223224199810.1038/325209521314
|
|
6.
|
DS YoonRP WerstoW ZhouFJ ChrestES GarretTK
KwonE GabrielsonVariable levels of chromosomal instability and
mitotic spindle checkpoint defects in breast cancerAm J
Pathol161391397200210.1016/S0002-9440(10)64194-612163363
|
|
7.
|
C LengauerKW KinzlerB VogelsteinGenetic
instability in colorectal
cancersNature386623627199710.1038/386623a09121588
|
|
8.
|
C LengauerKW KinzlerB VogelsteinGenetic
instability in human
cancersNature396643649199810.1038/252929872311
|
|
9.
|
R SotilloE HernandoE Diaz-RodriguezJ
Teruya-FeldsteinC Cordon-CardoSW LoweR BenezraMad2 overexpression
promotes aneuploidy and tumorigenesis in miceCancer
Cell11923200710.1016/j.ccr.2006.10.01917189715
|
|
10.
|
AA AlizadehMB EisenRE DavisC MaIS LossosA
RosenwaldJC BoldrickH SabetT TranX YuDistinct types of diffuse
large B-cell lymphoma identified by gene expression
profilingNature403503511200010.1038/3500050110676951
|
|
11.
|
X ChenST CheungS SoST FanC BarryJ
HigginsKM LaiJ JiS DudoitIO NgM Van De RijnD BotsteinPO Browngene
expression patterns in human liver cancersMol Biol
Cell1319291939200210.1091/mbc.02-02-0023.12058060
|
|
12.
|
ME GarberOG TroyanskayaK SchluensS
PetersenZ ThaeslerM Pacyna-GengelbachM Van de RijnGD RosenCM
PerouRI WhyteDiversity of gene expression in adenocarcinoma of the
lungProc Natl Acad Sci
USA981378413789200110.1073/pnas.24150079811707590
|
|
13.
|
GQ LiH LiHF ZhangMad2 and p53 expression
profiles in colorectal cancer and its clinical significanceWorld J
Gastroenterol919721975200312970887
|
|
14.
|
GQ LiHF ZhangMad2 and p27 expression
profiles in colorectal cancer and its clinical significanceWorld J
Gastroenterol1032183220200415457580
|
|
15.
|
SH ZhangAM XuXF ChenDH LiMP SunYJ
WangClinicopathologic significance of mitotic arrest defective
protein2 overexpression in hepatocellular carcinomaHum
Pathol3918271834200810.1016/j.humpath.2008.06.00318715617
|
|
16.
|
L WangF YinY DuW DuB ChenY ZhangK WuJ
DingJ LiuD FanMAD2 as a key component of mitotic checkpoint: A
probable prognostic factor for gastric cancerAm J Clin
Pathol131793801200910.1309/AJCPBMHHD0HFCY8W19461085
|
|
17.
|
K TanakaJ NishiokaK KatoA NakamuraT MouriC
MikiM KusunokiT NoboriMitotic checkpoint protein hsMAD2 as a marker
predicting liver metastasis of human gastric cancerJpn J Cancer
Res92952958200110.1111/j.1349-7006.2001.tb01186.x
|
|
18.
|
L YuWC GuoSH ZhaoJ TangJL ChenMitotic
arrest defective protein 2 expression abnormality and its
clinicopathologic significance in human
osteosarcomaAPMS118222229201010.1111/j.1600-0463.2009.02583.x20132188
|
|
19.
|
CW WuCW ChiTS HuangElevated level of
spindle checkprotein MAD2 correlates with cellular mitotic arrest,
but not with aneuploidy and clinicopathological characteristics in
gastric cancerWorld J gastroenterol1032403244200415484292
|
|
20.
|
R SotilloJM SchvartzmanND SocciR
BeneztraMad2-induced chromosome instability leads to lung tumor
relapse after oncogene
withdrawalNature464436440201010.1038/nature0880320173739
|
|
21.
|
X WangDY JinYC WongAL CheungAC ChunAK LoY
LiuSW TsaoCorrelation of defective mitotic checkpoint with
aberrantly reduced expression of MAD2 protein in nasopharyngeal
carcinoma
cellsCarcinogenesis1222932297200010.1093/carcin/21.12.229311133821
|
|
22.
|
MK FungHW CheungMT LingAL CheungYC WongX
WangRole of MEK/ERK pathway in the MAD2-mediated cisplatin
sensitivity in testicular germ cell tumour cellsBr J
Cancer95475484200610.1038/sj.bjc.660328416880791
|
|
23.
|
HW CheungAC ChunQ WangW DengL HuXY GuanJM
NichollsMT LingY Chuan WongSW TsaoDY JinX WangInactivation of human
MAD2B in nasopharyngeal carcinoma cells leads to chemosensitization
to DNA-damaging agentsCancer
Res6643574367200610.1158/0008-5472.CAN-05-360216618761
|
|
24.
|
Japan Society of Gynecologic
OncologyFormulation Committee of the Treatment Guidelines for
Cervical Cancer2007
|
|
25.
|
National Comprehensive Cancer NetworkNCCN
Clinical Practice Guidelines in Oncology - Cervical Cancer -
Version I2012
|
|
26.
|
M MorrisPJ EifelJ LuPW GrigsbyC
LevenbackRE StevensM RotmanDM GershensonDG MutchPelvic radiation
with concurrent chemotherapy compared with pelvic and para-aortic
radiation for high-risk cervical cancerN Engl J
Med34011371143199910.1056/NEJM19990415340150110202164
|
|
27.
|
PJ EifelK WinterM MorrisC LevenbackPW
GrigsbyJ CooperM RotmanD GershensonDG MutchPelvic irradiation with
concurrent chemotherapy versus pelvic and para-aortic irradiation
for high-risk cervical cancer: an update of radiation therapy
oncology group trial (RTOG) 90-01J Clin
Oncol22872880200410.1200/JCO.2004.07.19714990643
|
|
28.
|
O IshikoT SumiT YasuiY MatsumotoN
KawamuraS OgitaT KaminoK NakamuraR YamadaBalloon-occluded arterial
infusion chemotherapy, simple total hysterectomy, and radiotherapy
as a useful combination-therapy for advanced cancer of the uterine
cervixOncol Rep71411442000
|
|
29.
|
JE SardiA GiaroliCE SananesN Gomez RuedaS
VighiM FerreiraML BastardasG PaniceresG Di PaolaRandomized trial
with neoadjuvant in stage IIIB squamous carcinoma cervix uteri: an
unexpected therapeutic managementInt J Gynecol
Cancer68593199610.1046/j.1525-1438.1996.06020085.x
|
|
30.
|
L SouhamiRA GilSE AllanPC CanaryCM
AraújoLH PintoTR SilveriaA randomized trial of chemotherapy
followed by pelvic radiation therapy in stage IIIB carcinoma of the
cervixJ Clin Oncol997097719911709686
|
|
31.
|
MH TattersallV LorvidhayaV VootipruxA
CheirsilpaF WongT AzharHP LeeSB KangA ManaloMS YenRandomized trial
of epirubicin and cisplatin chemotherapy followed by pelvic
radiation in locally advanced cervical cancer. Cervical Cancer
Study Group of the Asian Oceanian Clinical Oncology AssociationJ
Clin Oncol134444511995
|
|
32.
|
O IshikoT SumiT YasuiY MatsumotoS OgitaT
KaminouK NakamuraR YamadaTumor marker and MR imaging criteria for
evaluating the efficacy of cyclic balloon-occluded arterial
infusion for advanced cancer of the uterine cervixOncol
Rep4827830200010854552
|
|
33.
|
O IshikoT SumiH YoshidaS OgitaR
YamadaExpression of apoptosis regulatory proteins in advanced
cancer of the uterine cervix after cyclic balloon-occluded arterial
infusion chemotherapyInt J Oncol6115111552001
|
|
34.
|
H NobeyamaT SumiF MisugiE OkamotoK
HattoriY MatsumotoT YasuiK HondaK IwaiO IshikoAssociation of HPV
infection with prognosis after neoadjuvant chemotherapy in advanced
uterine cervical cancerInt J Mol Med1101105200415202023
|
|
35.
|
E OkamotoT SumiF MisugiH NobeyamaK
HattoriH YoshidaY MatsumotoT YasuiK HondaO IshikoExpression of
apoptosis-related proteins in advanced uterine cervical cancer
after balloon-occluded arterial infusion chemotherapy as an
indicator of the efficiency of this therapyInt J Mol
Med141472005
|
|
36.
|
K TsujiR YamadaM KawabataK MitsuzaneM
SatoM IwahashiS KitayamaR NakanoEffect of balloon occluded arterial
infusion of anticancer drugs on the prognosis of cervical cancer
treated with radiation therapyInt J Radiat Oncol Biol
Phys3213371345199510.1016/0360-3016(94)00651-Z7635773
|
|
37.
|
S SironiC BelloniG TaccagniA Del
MaschioInvasive cervical carcinoma: MR imaging after prospective
chemotherapyRadiology180719722199110.1148/radiology.180.3.18712831871283
|
|
38.
|
KH KimBH LeeYS DoSY ChinBG KimStage IIb
cervical carcinoma: MR evaluation of intraarterial
chemotherapyRadiology1926165199410.1148/radiology.192.1.82089678208967
|
|
39.
|
FA SinicropeSB RuanKR ClearyLC StephensJJ
LeeB LevinBcl-2 and p53 oncoprotein expression during colorectal
tumorigenesisCancer Res5523724119957812951
|