Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies worldwide, accounting for approximately 6% of
all human carcinomas and 1 million deaths annually, with an
estimated number of new cases of over 500,000/year (1). Clinical and experimental evidence
suggests a link between infection with hepatitis C virus (HCV)
and/or hepatitis B virus (HBV), chronic hepatitis (CH) and
cirrhosis, as well as the progression of HCC. Liver cirrhosis is
observed in up to 90% of patients with HCC, and HCV is the
causative factor in 80% and HBV in 10% of cases in Japan (2–5). In
the United States, almost 4 million individuals are infected with
HCV each year which progresses to chronic hepatitis C, which could
potentially progress to liver cirrhosis. The results are often
liver failure or HCC. Chronic hepatitis C is the nation's leading
cause of HCC, and according to the American Liver Foundation, is
also the leading reason for liver transplantation. In Japan, HCV
and/or HBV-based hepatitis and cirrhosis are also serious problems
since they progress to HCC at a ratio of 5 to 7% per year (4,5).
These findings strongly suggest the existence of a link between
hepatocarcinogenesis and HCV/HBV infection and chronic liver
inflammation.
Various therapies are currently in use for HCC.
These include surgical resection, percutaneous ethanol injection
(PEI), systemic or arterial chemotherapy using either single or
combination drugs, transcatheter arterial chemoembolization (TACE),
hormonal therapy and selective radiotherapy. However, the prognosis
of patients with HCC remains poor, as they often develop
intrahepatic and/or multicentric tumor recurrence, at a rate of
20–40% within 1 year, and ~80% within 5 years of therapy even when
curative treatment is applied (6–9).
Liver transplantation offers the best prognosis for patients with
small HCC, although its use is limited due to the scarcity of donor
organs. Therefore, an effective therapeutic strategy against HCC is
required.
In a previous study, we reported that proteasome
activator 28γ (PA28γ) directly enhances the degradation of the HCV
core protein and plays a key role in the genesis of hepatic
steatosis and HCC in HCV core protein transgenic mice (10). Furthermore, the above events were
not observed in PA28γ-knockout mice. The present study is an
extension of our previous study and was designed to assess the
utility of PA28γ expression as a biological marker for HCV-related
human liver disease and HCC. The findings showed the presence of
high levels of nuclear PA28γ in multistep hepatocarcinogenesis and
HCC invasion, suggesting that selective inhibitors of nuclear PA28γ
may be useful in the prevention and/or treatment of this
disease.
Materials and methods
Tissue samples
The study protocol was approved by the Human Ethics
Review Committee of Osaka University, and a signed consent form was
obtained from each subject for the use of tissue samples for
medical research. Tissue samples were obtained from 51 patients
with liver tumors, who underwent hepatectomy at the Department of
Gastroenterological Surgery, Osaka University Hospital. All
patients had HCV infection (28 patients) and some had HCV plus HBV
infection (18 patients), but none had only HBV infection. The mean
post-treatment follow-up period was 6.2±2.5 years ± standard
deviation (SD). The excised hepatic tissue samples were examined
immunohistochemically for PA28γ expression, including 46 paired
HCCs. Non-tumor tissues were also examined, which comprised 15
CH-based livers (5 chronic active hepatitis and 10 chronic inactive
hepatitis) and 31 cirrhotic livers. Prior to hepatectomy for HCC,
10 patients were treated with transarterial embolization (TAE). In
these cases, histopathological examination showed complete hepatic
necrosis. Histologically normal livers were also obtained from
patients negative for hepatic viral infections who had liver
metastasis secondary to colorectal cancer.
For immunohistochemistry, the tissue samples were
fixed in 10% neutral buffered formalin, processed through graded
ethanol and embedded in paraffin. The samples were frozen
immediately in liquid nitrogen and stored at −80°C for subsequent
analysis by reverse transcription-polymerase chain reaction
(RT-PCR).
Histopathological examination
Tissue sections (4 μm thick) were deparaffinized in
xylene, rehydrated and stained with hematoxylin and eosin solution.
Separation of the tissues into non-tumor and tumor tissues was
determined by a pathologist (K.W.) who was blinded to the clinical
background. For non-tumor tissues, the presence of inflammation or
cirrhotic nodules was examined. Tumor tissues were examined for the
following characteristics: cell differentiation (well, moderate,
poorly differentiated), number of tumors, capsular formation,
septal formation, capsular invasion, portal vein tumor thrombus
formation and hepatic vein invasion.
Preparation of anti-human PA28γ
antibody
Chicken anti-human PA28γ antibody was prepared by
immunization using the synthetic peptides of residues from 75 to
88, SHDGLDGPTYKKRR, of human PA28γ. The antibody was purified by
affinity chromatography using beads conjugated with the antigen
peptide.
Immunohistochemistry and evaluation of
PA28γ immunostaining
Formalin-fixed tissues were embedded in paraffin
according to the standard procedures. For immunohistochemistry,
formalin-fixed tissue sections were boiled in Target Retrieval
Solution (Dako, Glostrup, Denmark) and then treated with 3%
H2O2. The activated sections were washed
twice with phosphate-buffered saline (PBS), blocked with PBS
containing 5% bovine serum albumin, and incubated overnight with
the purified chicken antibody to PA28γ, followed by incubation with
horseradish peroxidase-conjugated anti-chicken IgG antibody (ICN,
Biomedicals, Inc., Aurora, OH, USA) as a secondary antibody.
Immunoreactive antigen was visualized with 3,3′-diaminobenzidine
substrate. The resulting sections were counterstained with
hematoxylin. Staining of endogenous PA28γ with the antibody was
identified in normal mouse liver sections but not in the liver
sections from PA28γ-deficient mice. Pre-immune purified antibody
did not react with any other antigen in these sections under the
experimental conditions.
For evaluation of PA28γ immunostaining, each section
was scored for nuclear and cytoplasmic staining using a scale from
0 to 2 where 0 represented negative or faint staining, 1
represented moderate staining, and 2 represented strong staining.
In general, the nuclei of the bile ducts faintly expressed PA28γ
(Fig. 1a). Thus, the staining
level was used as a nuclear inner control within the sample, which
was designated arbitrarily as intensity level 0. Also, slightly
higher expression was designated arbitrarily as intensity level 1
and clearly higher expression was designated arbitrarily as
intensity level 2. PA28γ expression was very faint or undetectable
in the vascular epithelia and nuclei (Fig. 1a), whereas the cytoplasm of bile
duct epithelial cells and nuclei devoid of significant inflammation
generally expressed faint levels of PA28γ (Fig. 1a). For semi-quantitative analysis,
the latter level of staining was used as a cytoplasmic inner
control within the sample, and designated arbitrarily as intensity
level 0. Furthermore, a slightly higher expression was designated
arbitrarily as intensity level 1 whereas clearly higher expression
was designated arbitrarily as intensity level 2. PA28γ expression
was generally heterogeneous in each sample. For assessment of
nuclear and cytoplasmic PA28γ, 4 high-power fields in each specimen
were selected at random, and staining was examined under high power
magnification. More than 1,000 cells were counted to determine the
labeling index, which represented the percentage of immunostained
cells relative to the total number of cells. The tissue samples
were also categorized as positive (levels 1 and 2) and negative
(level 0) for evaluation of the relationship between immunostaining
and various clinicopathological factors.
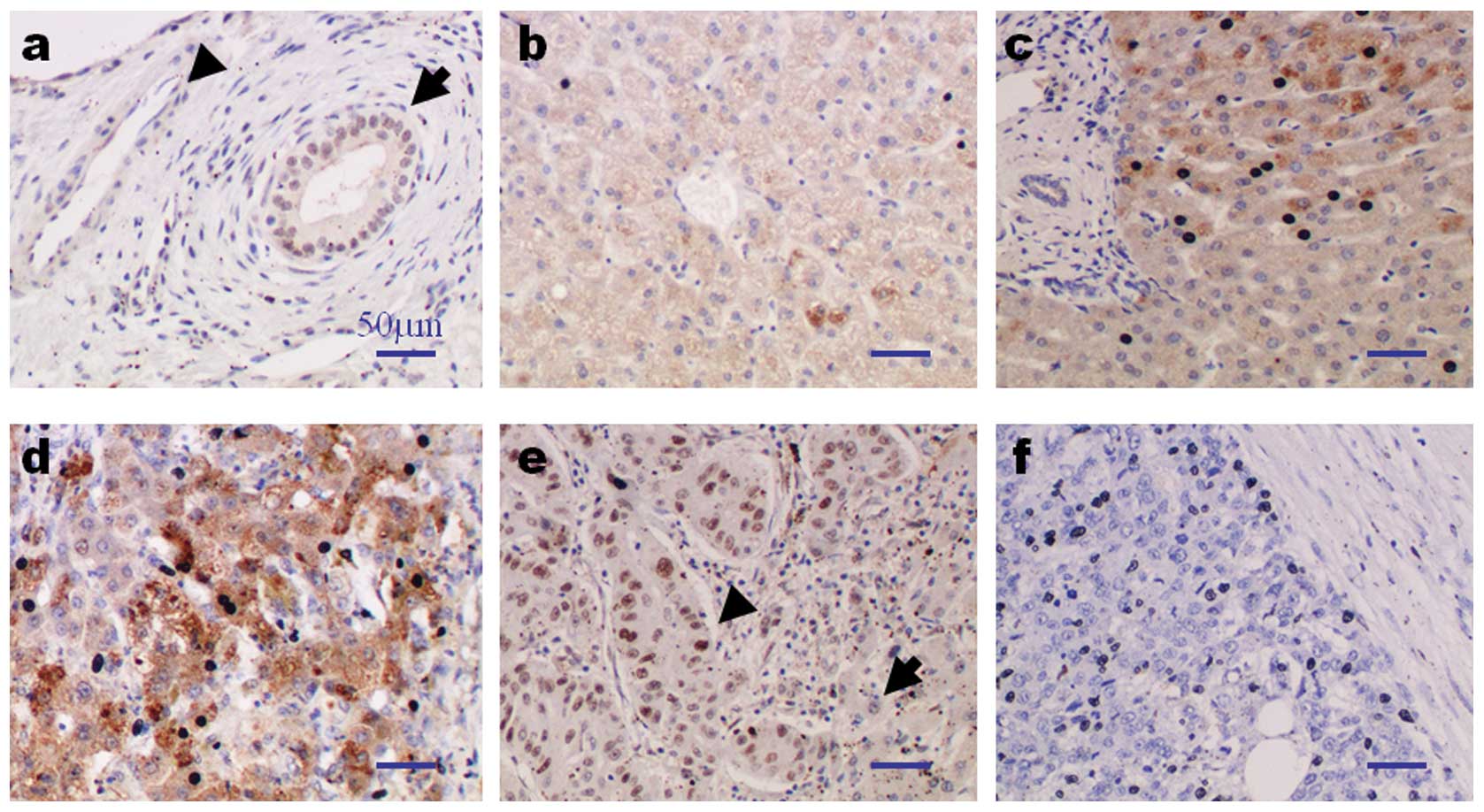 | Figure 1.Immunohistochemical staining for
PA28γ. (a–f) Representative samples for bile duct (inner control),
vascular epithelium and various liver pathologies; (a) bile duct
(arrow), vascular epithelium (arrowhead); (b) normal liver; (c)
chronic hepatitis; (d) cirrhotic liver; (e) HCC with high nuclear
PA28γ expression (arrowhead; left side) and non-tumor liver tissue
with low nuclear PA28γ expression (arrow; right side); (f) HCC with
low expression of nuclear PA28γ. Magnification, x200. (g)
High-power view of liver section shown in (d). Note the faint
staining of hepatocytes with high expression of nuclear PA28γ
(arrow; hepatocytes, level 0 and nucleus, level 2), moderate
staining of hepatocytes with high expression of nuclear PA28γ
(asterisk; hepatocyte, level 1 and nucleus, level 2) and strong
staining of hepatocytes with low expression of nuclear PA28γ
(arrowhead; hepatocyte, level 2 and nucleus, level 0).
Magnification, x400. No staining was observed when the primary
antibody was substituted by non-immunized rabbit IgG or TBS (data
not shown). PA28γ, proteasome activator 28γ; HCC, hepatocellular
carcinoma; IgG, immunoglobulin G; TBS, Tris-buffered saline. |
Semi-quantitative RT-PCR
RNA extraction was carried out with TRIzol reagent
using the single-step method, and the cDNA was generated with avian
myeloblastosis virus reverse transcriptase (Promega, Madison, WI,
USA), as described previously (11). Sterol regulatory element binding
protein-1c (SREBP-1c) mRNA expression was analyzed
semi-quantitatively using the multiplex RT-PCR method. In this
assay, the housekeeping gene, porphobilinogen deaminase (PBGD), was
used as the internal control. This gene is favored over β-actin or
glyceraldehyde-3-phosphate dehydrogenase as a reference gene for
competitive PCR amplification as the presence of pseudogenes for
the latter housekeeping genes may produce false-positive signals
from genomic DNA contamination (12,13).
In addition, in order to minimize possible inter-PCR differences,
PCR was performed with SREBP-1c and PBGD primers in an identical
tube, under unsaturated conditions. PCR was performed in a 25-μl
reaction mixture containing 1 μl of the cDNA template, 1X
Perkin-Elmer PCR buffer, 1.5 mM MgCl2, 0.8 mM
deoxynucleotide triphosphates, 0.8 μM of each primer for SREBP-1c
and 80 nM PBGD, and 1 unit of TaqDNA polymerase (AmpliTaq Gold;
Roche Molecular Systems, Inc.). The PCR primers used for the
detection of SREBP-1c and PBGD cDNAs were synthesized as described
previously (14,15). The conditions for multiplex PCR
were one cycle of denaturation at 95°C for 12 min, followed by 40
cycles at 95°C for 1 min, 62°C for 1 min and 72°C for 1 min, and a
final extension at 72°C for 10 min. The electrophoresed PCR
products were scanned by densitometry, and the relative value of
the SREBP-1c band relative to that of PBGD was calculated for each
sample.
Statistical analysis
Data were expressed as the means ± SD. The
Chi-square test and Fisher's exact probability test, or the
log-rank test, were used to examine the association between PA28γ
expression and the clinicopathological parameters or prognosis. A
P-value of <0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
the StatView-J-5.0 program (SAS Institute, Cary, NC, USA).
Results
Immunohistochemical analysis of
PA28γ
Immunohistochemical assays were performed on a
series of 46 paired HCCs and their matched non-tumor tissues, and 5
normal livers. The labeling index of nuclear PA28γ showed a wide
spectrum and increased from low in the normal livers to strong in
the cirrhotic livers (Fig. 1b–d).
Specifically, the nuclear PA28γ labeling index was generally low in
the normal liver tissues, but was moderate-strong in HCV-related
liver tissues. The nuclear labeling index was markedly higher in
the majority of cirrhotic liver tissues. Fig. 2 summarizes the above results and
the analysis of cytoplasmic expression of PA28γ. The difference in
the PA28γ-nuclear labeling index between normal and cirrhotic
livers was significant (P<0.0001) as was that between CH and
cirrhosis (P<0.0001) (Fig. 2A).
Also, the difference in the proportion of the PA28γ-cytoplasmic
expression labeling index between normal and cirrhotic livers was
significant (P<0.05) (Fig. 2B).
The mean labeling indexes of nuclear PA28γ expression was 42% in
both HCC and HCV-related livers.
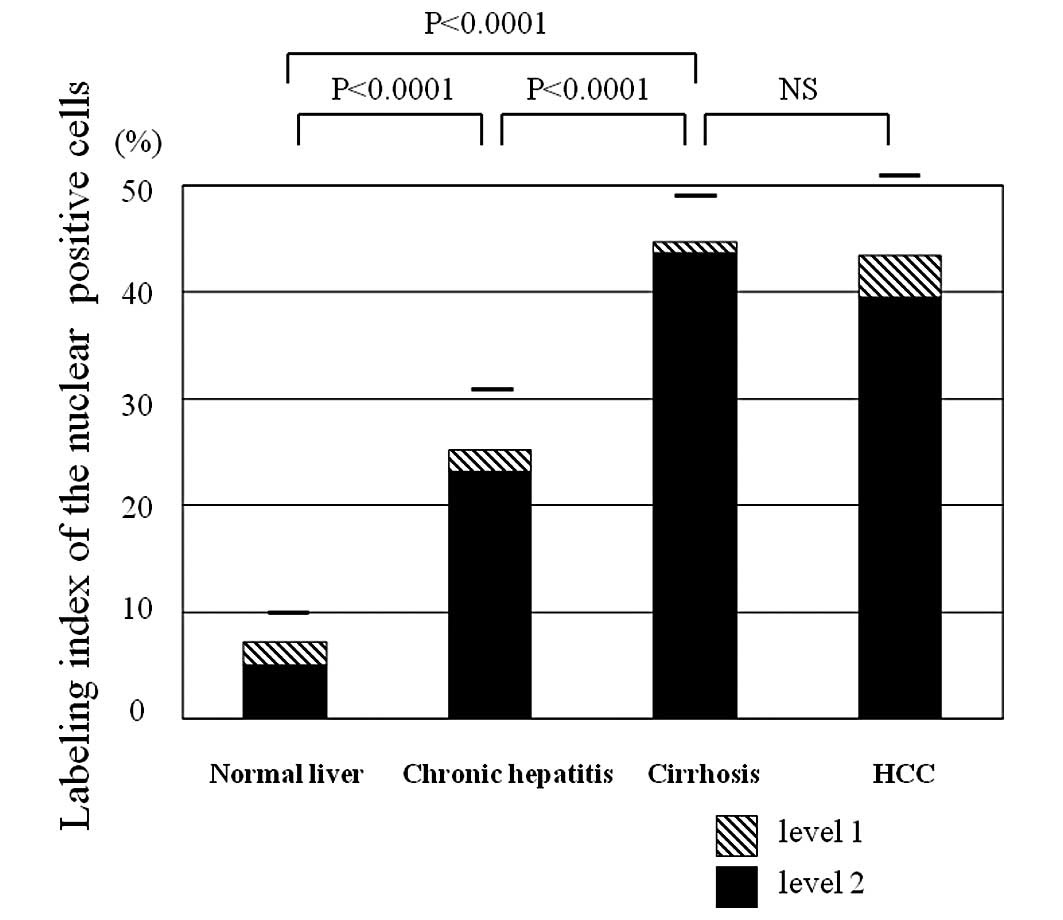 | Figure 2.(A) Nuclear PA28γ expression in
multistep hepatocarcinogenesis. The labeling index increased in a
stepwise manner with the severity of liver damage and
carcinogenesis. Quantitative analysis showed that 25, 10 and 1% of
cells of the normal liver, CH and cirrhosis, respectively, were
moderately positive (level 1). In HCCs, 10% of cells were evaluated
as moderately positive (level 1). (B) Cytoplasmic PA28γ expression
in multistep hepatocarcinogenesis. The expression increased
slightly in a stepwise manner. Quantitative analysis showed that
80, 68 and 50% of cells of the normal liver, CH and cirrhosis,
respectively, were moderately positive (level 1). In HCCs, 82% of
cells were evaluated as moderately positive (level 1). PA28γ,
proteasome activator 28γ; CH, chronic hepatitis; HCC,
hepatocellular carcinoma. NS, not significant. |
To evaluate the relationship between
immunohistochemical staining and various clinicopathological
factors, we divided the samples into nuclear PA28γ high index
(≥42%) and low index (<42%) groups. The labeling index was low
in half of the examined HCC cases (50%; 18/36) and markedly high in
the other half (50%; 18/36) (Table
I). The labeling index was low in 30% (14/46) of HCV-related
cases and markedly higher in the remaining 70% (32/46) (Table II). The samples were also divided
into 2 groups according to the labeling index of cytoplasmic
staining. The mean PA28γ-labeling index of the HCC and HCV-related
cases was 58 and 80%, respectively. The labeling index was low in
47% (17/36) and high in 53% (19/36) of the HCC cases. The
respective values for HCV-related cases were 28% (13/46) and 72%
(33/46). All cut-off values used were according to the mean
labeling index.
 | Table I.Correlation between nuclear PA28γ
expression and various clinicopathological parameters in patients
with HCC. |
Table I.
Correlation between nuclear PA28γ
expression and various clinicopathological parameters in patients
with HCC.
| n | PA28γ
| P-value |
|---|
| Low (<42%) | High (≥42%) |
|---|
| Age (years) | | | | |
| ≥60 | 15 | 7 | 8 | |
| <60 | 21 | 11 | 10 | NS |
| Gender | | | | |
| Male | 21 | 10 | 11 | |
| Female | 15 | 8 | 7 | NS |
| Tumor size | | | | |
| ≤2 cm | 8 | 4 | 4 | |
| >2 cm | 28 | 14 | 14 | NS |
| Histological
type | | | | |
| Well/moderately
differentiated | 5 | 2 | 3 | |
| Poorly
differentiated | 31 | 16 | 15 | NS |
| Hepatic vein
invasion | | | | |
| Yes | 6 | 2 | 4 | |
| No | 30 | 16 | 14 | NS |
| Portal vein tumor
thrombus | | | | |
| Yes | 5 | 2 | 3 | |
| No | 31 | 16 | 15 | NS |
| Number of
tumors | | | | |
| Multiplea | 3 | 1 | 2 | |
| Solitary | 33 | 17 | 16 | NS |
| Septum
formation | | | | |
| Yes | 15 | 8 | 7 | |
| No | 21 | 10 | 11 | NS |
| Capsular
formation | | | | |
| Yes | 14 | 6 | 8 | |
| No | 22 | 12 | 10 | NS |
| Capsular
invasion | | | | |
| Yes | 8 | 1 | 7 | |
| No | 6 | 5 | 1 | 0.026 |
 | Table II.Correlation between nuclear PA28γ
expression and various clinicopathological parameters in non-tumor
liver tissues. |
Table II.
Correlation between nuclear PA28γ
expression and various clinicopathological parameters in non-tumor
liver tissues.
| n | PA28γ
| P-value |
|---|
| Low (<42%) | High (≥42%) |
|---|
| Age (years) | | | | |
| ≥60 | 22 | 5 | 17 | |
| <60 | 24 | 9 | 15 | NS |
| Gender | | | | |
| Male | 27 | 6 | 21 | |
| Female | 19 | 8 | 11 | NS |
| HCV | 28 | 9 | 19 | |
| HBV | 0 | | | |
| HCV plus HBV | 18 | 5 | 13 | NS |
| Inflammatory status
(HAI score) | | | | |
| Absent-mild
(0–3) | 22 | 12 | 10 | |
| Moderate-severe
(>4) | 24 | 2 | 22 | 0.0007 |
| Degree of fibrosis
(HAI score) | | | | |
| Absent-moderate
(0–2) | 12 | 11 | 1 | |
| Severe-cirrhosis
(>3) | 34 | 3 | 31 | <0.0001 |
Correlation between nuclear PA28γ
expression and clinicopathological parameters
We examined the correlation between PA28γ nuclear
expression analyzed in 36 HCCs (10 samples with complete necrosis
by TAE were excluded from this analysis) and various
clinicopathological features (Table
I). The cases were divided into two groups based on the
labeling index of nuclear expression of PA28γ, using a cut-off mean
value of 42%. There was a significant difference in PA28γ
expression based on capsular invasion (Table I). We also analyzed the
relationship between nuclear PA28γ expression in non-tumor tissues
(15 CH and 31 cirrhosis) and disease-free survival, as the
pathologic status of non-tumor tissues has been shown to correlate
with the relapse of HCC (16–18).
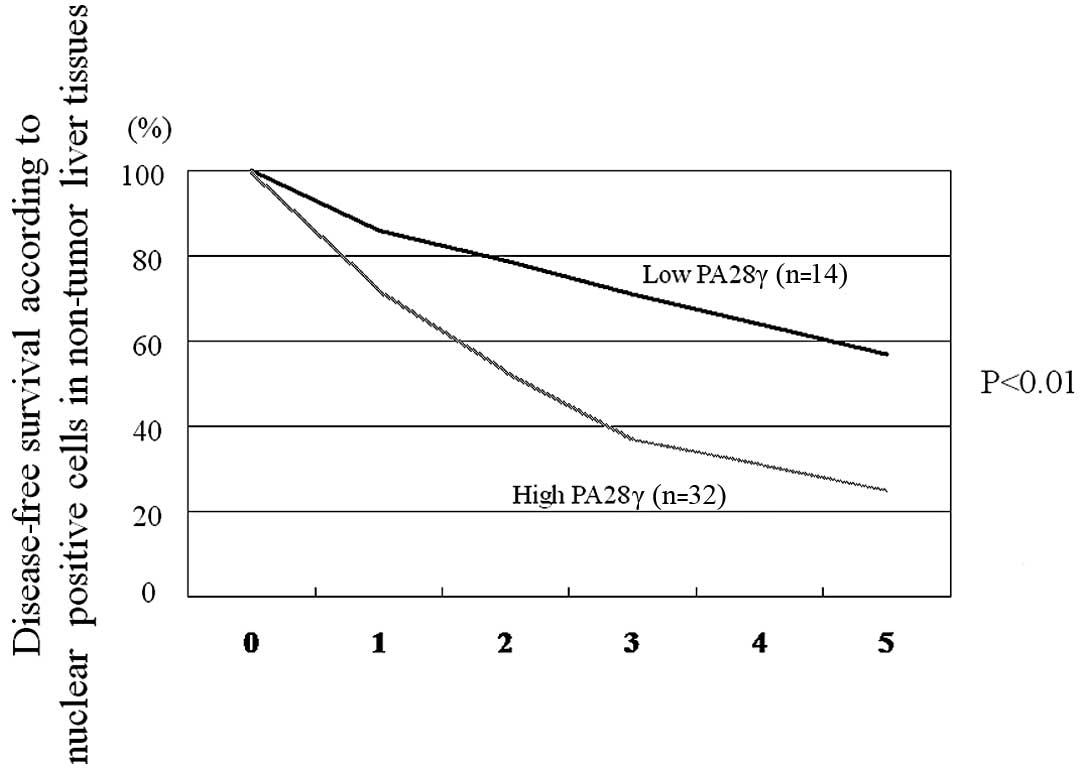
The disease-free survival, but not overall survival (P=0.052), was
significantly different between high and low nuclear PA28γ
expressors (P<0.01) (Fig. 3).
In addition, PA28γ expression in non-tumor tissues correlated
closely with active inflammation and fibrosis (Table II).
In univariate analysis, PA28γ expression in
non-tumor liver tissues, portal vein tumor thrombus, inflammatory
status and degree of fibrosis in the non-cancerous liver tissue
were significant factors for disease-free survival. These variables
were subsequently entered into multivariate analysis. The results
identified nuclear PA28γ expression level [95% confidence interval
(CI), 1.82–3.22; P<0.01], portal vein tumor thrombus (95% CI,
1.33–6.38; P=0.023), inflammatory status (95% CI, 2.11–3.58;
P=0.012) and degree of fibrosis (95% CI, 1.99–7.21; P<0.01) as
independent factors for disease-free survival (Table III).
 | Table III.Multivariate analysis of
clinicopathological factors for disease-free survival in patients
with HCC. |
Table III.
Multivariate analysis of
clinicopathological factors for disease-free survival in patients
with HCC.
| n | Relative risk | 95% confidence
interval | P-value |
|---|
| PA28γ | | | | |
| High | 32 | 2.67 | 1.82–3.22 | <0.01 |
| Low | 14 | | | |
| Portal vein tumor
thrombus | | | | |
| Yes | 5 | 2.21 | 1.33–6.38 | 0.023 |
| No | 31 | | | |
| Inflammatory status
(HAI score) | | | | |
| Absent-mild
(0–3) | 22 | 2.59 | 2.11–3.58 | 0.012 |
| Moderate-severe
(>4) | 24 | | | |
| Degree of fibrosis
(HAI score) | | | | |
| Absent-moderate
(0–2) | 12 | 2.68 | 1.99–7.21 | <0.01 |
| Severe-cirrhosis
(>3) | 34 | | | |
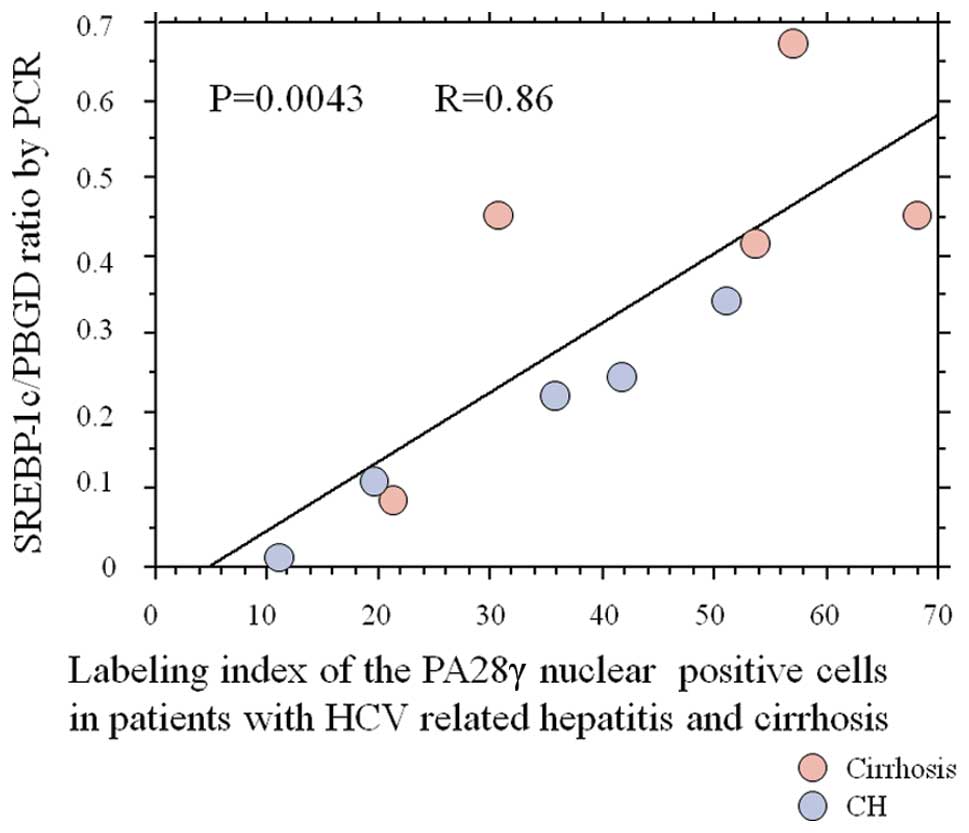
SREBP-1c expression
Five CH and five cirrhotic liver tissues were
selected to analyze the correlation between nuclear PA28γ
expression and SREBP-1c gene expression in non-tumor liver tissues.
Fig. 4 shows a clear correlation
between nuclear PA28γ expression and SREBP-1c gene expression.
Discussion
The present study shows that non-tumor liver tissues
commonly express high levels of nuclear PA28γ protein relative to
those of carcinoma tissues. These results are contradictory to
those from other studies on other types of cancer, such as thyroid
carcinoma; the nuclear PA28γ level was higher in these tumors
compared to non-tumor tissues (19). While the exact reason for the
different results is not known at present, it is likely to be
related to the type of control tissue used in the present study;
the non-tumor tissues were mostly not normal, consisting of
HCV-infected CH or cirrhotic tissues. In support of this
conclusion, normal liver tissues from patients with metastatic
liver tumors from patients with colorectal carcinoma who were
negative for HCV/HBV showed low expression of nuclear PA28γ.
In non-neoplastic liver tissues, we found a wide
spectrum of nuclear PA28γ expression from normal liver to
cirrhosis. Our results also show that active inflammation with
hepatitis virus induces nuclear PA28γ in CH and cirrhotic livers
(Table II). This is reasonable
considering the fundamental action of nuclear PA28γ as a mediator
of inflammation. Another mechanism for the high induction of
nuclear PA28γ in cirrhosis might be related to the degradation of
the HCV core protein by PA28γ and its translocation from the
cytoplasm to the nucleus, based on the results of our previous
study (10). In fact, nuclear
PA28γ-expressing cells had no or faint-to-moderate cytoplasmic
PA28γ expression (Fig. 1c and g).
Furthermore, the nuclear overexpression could be due to the
relatively hypoxic microenvironment in the cirrhotic liver. In this
regard, we hypothesized that hypoxia might directly induce PA28γ,
which in turn enhances angiogenesis via the enhanced release of a
battery of angiogenic growth factors, such as vascular endothelial
growth factor (VEGF). Since the VEGF level is increased in
cirrhosis (20), it is possible
that nuclear PA28γ may improve the ischemic/hypoxic
microenvironment in the cirrhotic liver through upregulation of
angiogenesis. Although cirrhotic nodules occasionally show p53
mutation and increased telomerase activity (21,22),
cirrhosis is not considered a premalignant lesion. However, it is
apparent from a number of etiological studies that cirrhosis is a
strong risk factor for HCC. In this context, nuclear PA28γ
expression in cirrhosis might be a prerequisite for the genesis of
premalignant dysplastic nodules or early cancer.
From a clinical point of view, it is interesting to
note the correlation between high nuclear PA28γ expression in
non-tumor tissues and the relapse of HCC. The prognosis of HCC is
generally unfavorable. Although primary tumors are curatively
resected, 50–60% of patients develop relapse within 5 years. This
is due to either a newly established tumor from the remnant liver,
a process termed multicentric carcinogenesis, or recurrence of the
original tumor. One possible mechanism for a link between nuclear
PA28γ and disease relapse is that high expression of PA28γ in the
remnant liver may contribute to carcinogenesis. Nuclear PA28γ
expression highly correlated with the presence of active
inflammation (P<0.0001). Furthermore, active inflammation in
non-tumor tissues has been reported to be associated with relapse
of HCC (17,23,24).
In the present study, a clinicopathological survey
demonstrated a significant correlation between nuclear PA28γ
protein expression and capsular invasion of the cancer tissue. This
finding is in agreement with a recent study that showed increased
expression of PA28γ protein during cancer progression and its
correlation with PCNA labeling index (19). Thus, the results suggest the
possible involvement of PA28γ in HCC progression. Further studies
of larger population samples are required to confirm the clinical
significance of nuclear PA28γ in HCC. This is particularly
important, as the overall survival of patients with high nuclear
PA28γ expression was worse than that of those with low expression
level (P=0.052) (data not shown).
Also in our series, the labeling index of
cytoplasmic expression of PA28γ significantly increased from normal
liver to cirrhotic liver (Fig.
2b). Further extended studies are required to determine the
importance of cytoplasmic expression of PA28γ in HCC and
HCV-related liver.
In conclusion, the present study demonstrates a
close correlation between nuclear PA28γ expression in liver tissue
and the development and progression of HCC, as well as its possible
involvement in HCC relapse. Further studies are required to examine
the therapeutic benefits of the suppression of nuclear PA28γ
expression in HCV-related CH, cirrhosis or HCC.
Abbreviations:
|
CH,
|
chronic hepatitis;
|
|
HBV,
|
hepatitis B virus;
|
|
HCC,
|
hepatocellular carcinoma;
|
|
HCV,
|
hepatitis C virus;
|
|
PA,
|
proteasome activator;
|
|
PBGD,
|
porphobilinogen deaminase;
|
|
RT-PCR,
|
reverse transcription-polymerase chain
reaction
|
References
|
1.
|
G MontaltoM CervelloL GiannitrapaniF
DantonaA TerranovaLA CastagnettaEpidemiology, risk factors, and
natural history of hepatocellular carcinomaAnn NY Acad
Sci9631320200210.1111/j.1749-6632.2002.tb04090.x12095924
|
|
2.
|
K OkudaHepatocellular carcinoma: Recent
progressHepatology15948963199210.1002/hep.1840150532
|
|
3.
|
MC KewH PopperRelationship between
hepatocellular carcinoma and cirrhosisSemin Liver
Dis4136146198410.1055/s-2008-10406536087459
|
|
4.
|
K IkedaS SaitohI KoidaY AraseA TsubotaK
ChayamaH KumadaM KawanishiA multivariate analysis of risk factors
for hepatocellular carcinogenesis: A prospective observation of 795
patients with viral and alcoholic
cirrhosisHepatology184753199310.1002/hep.18401801097686879
|
|
5.
|
Y ShiratoriS ShiinaM ImamuraCharacteristic
difference of hepatocellular carcinoma between hepatitis B- and C-
viral infection in
JapanHepatology2210271033199510.1002/hep.18402204037557847
|
|
6.
|
N NagasueM UchidaY MakinoIncidence and
factors associated with intrahepatic recurrence following resection
of hepatocellular carcinomaGastroenterology10548849419938392955
|
|
7.
|
K IkedaS SaitohA TsubotaY AraseK ChayamaH
KumadaG WatanabeM TsurumaruRisk factors for tumor recurrence and
prognosis after curative resection of hepatocellular
carcinomaCancer711925199310.1002/1097-0142(19930101)71:1%3C19::AID-CNCR2820710105%3E3.0.CO;2-I8380116
|
|
8.
|
M ShimadaK TakenakaT GionPrognosis of
recurrent hepatocellular carcinoma: a 10-year surgical experience
in JapanGastroenterology11172072619968780578
|
|
9.
|
T KumadaS NakanoI TakedaPatterns of
recurrence after initial treatment in patients with small
hepatocellular
carcinomaHepatology258792199710.1002/hep.5102501168985270
|
|
10.
|
K MoriishiR MochizukiK MoriyaCritical role
of PA28gamma in hepatitis C virus-associated steatogenesis and
hepatocarcinogenesisProc Natl Acad Sci
USA10416611666200710.1073/pnas.060731210417234812
|
|
11.
|
J MyersP MehtaAW HunterSA BernsteinPA
EricksonAutomated double-label immunohistochemistryJ Surg
Pathol11051131995
|
|
12.
|
S ChretienA DubartD BeaupainAlternative
transcription and splicing of the human porphobilinogen deaminase
gene result either in tissue-specific or in housekeeping
expressionProc Natl Acad Sci USA85610198810.1073/pnas.85.1.6
|
|
13.
|
S NagelM SchmidtC ThiedeD HuhnA
NeubauerQuantification of Bcr-Abl transcripts in chronic
myelogenous leukemia (CML) using standardized, internally
controlled, competitive differential PCR (CD-PCR)Nucleic Acids
Res2441024103199610.1093/nar/24.20.4102
|
|
14.
|
KH KimSP HongK KimMJ ParkKJ KimJ CheongHCV
core protein induces hepatic lipid accumulation by activating
SREBP1 and PPARgammaBiochem Biophys Res
Commun355883888200710.1016/j.bbrc.2007.02.04417331464
|
|
15.
|
J FinkeR FritzenP TernesW LangeG DolkenAn
improved strategy and a useful housekeeping gene for RNA analysis
from formalin-fixed, paraffin-embedded tissues by
PCRBiotechniques1444845319937681300
|
|
16.
|
Y SasakiS ImaokaM FujitaRegional therapy
in the management of intrahepatic recurrence after surgery for
hepatomaAnn
Surg.2064047200710.1097/00000658-198707000-000063038040
|
|
17.
|
S KoY NakajimaH KanehiroSignificant
influence of accompanying chronic hepatitis status on recurrence of
hepatocellular carcinoma after hepatectomyResult of multivariate
analysis. Ann Surg22459159519968916872
|
|
18.
|
Y SasakiS ImaokaS MasutaniI OhashiO
IshikawaH KoyamaT IwanagaInfluence of coexisting cirrhosis on
long-term prognosis after surgery in patients with hepatocellular
carcinomaSurgery1125152119921325673
|
|
19.
|
T OkamuraS TaniguchiT OhkuraAbnormally
high expression of proteasome activator-gamma in thyroid neoplasmJ
Clin Endocrinol
Metab8813741383200310.1210/jc.2002-02141312629132
|
|
20.
|
ON El-AssalA YamanoiY SodaClinical
significance of microvessel density and vascular endothelial growth
factor expression in hepatocellular carcinoma and surrounding
liver: possible involvement of vascular endothelial growth factor
in the angiogenesis of cirrhotic
liverHepatology2715541562199810.1002/hep.510270613
|
|
21.
|
J RaedleG OremekM TruschnowitschM LorenzWK
RothWF CasparyS ZeuzemClinical evaluation of autoantibodies to p53
protein in patients with chronic liver disease and hepatocellular
carcinomaEur J
Cancer3411981203199810.1016/S0959-8049(98)00056-29849479
|
|
22.
|
Y KishimotoG ShiotaY KamisakiLoss of the
tumor suppressor p53 gene at the liver cirrhosis stage in Japanese
patients with hepatocellular
carcinomaOncology54304310199710.1159/0002277089216855
|
|
23.
|
K TaraoS TakemiyaS TamaiRelationship
between the recurrence of hepatocellular carcinoma (HCC) and serum
alanine aminotransferase levels in hepatectomized patients with
hepatitis C virus-associated cirrhosis and
HCCCancer79688694199710.1002/(SICI)1097-0142(19970215)79:4%3C688::AID-CNCR5%3E3.0.CO;2-A
|
|
24.
|
S KoY NakajimaH KanehiroInfluence of
associated viral hepatitis status on recurrence of hepatocellular
carcinoma after hepatectomyWorld J
Surg2110821086199610.1007/s0026899001648798368
|