Introduction
With the projected increase in the elderly
population and the prevalence of age-related cardiovascular
disabilities worldwide, more and more elderly patients face the
problems of morbidity and mortality due to age-mediated
disabilities. Studies have found that the aging heart has a
diminished functional and adaptive reserve capacity, an increased
susceptibility to incur damage and a limited practical ability for
regeneration (1). Specific
age-related alterations in the myocardium cannot be directly
ascribed to the loss or reduced number of myocytes, coronary
atherosclerosis or vasospasm, scarring or other defined cardiac
abnormalities. The development of interstitial fibrosis may also
explain the altered cardiac function of the aged myocardium
(2), and aging of human atrial
myocardium is also accompanied by a decrease in nerve plexuses and
an increase in fibrosis (3).
However, the exact underlying mechanism is not yet fully
understood.
Different cardiac pathology appears to be caused by
a common fibrotic process. Recent studies indicate that dilated,
ischaemic and hypertrophic cardiomyopathies are all associated with
increased levels of transforming growth factor-β (TGF-β) 1, and
TGF-β1 has now been suggested to play a major role in valvular
disease and arrhythmia, particularly atrial fibrillation (4). Age-dependent changes, such as
increased activation of the renin-angiotensin-aldosterone system
(RAAS) (5) and an increase in
oxidative stress (6), can be
potent fibrogenic activators of TGF-β. After its extracellular
activation, TGF-β binds to the specific receptors TGF-β-R1 and -R2
mediating phosphorylation of transcription signaling molecules
(p-smads) and subsequent transcription of target genes (7).
Thrombospondin-1 (TSP-1) is the founding member of a
family of matricellular glycoproteins (8). While predominantly in vitro
studies have assigned diverse functions, such as phagocytosis,
adhesion, chemotaxis, proliferation and migration of cells to
TSP-1, it has also been identified as an activator of the
TGF-β-procytokine complex. It is thought that binding of TSP-1 to
the latent complex induces a conformational rearrangement of the
latency associate peptide (LAP) in such a manner as to prevent the
LAP from conferring latency on the mature domain of TGF-β (9). Several studies have previously
determined that TSP-1 expression was greatly enhanced and
exacerbated the development of glomerulosclerosis and
tubulointerstitial fibrosis in the aging kidney (10). However, further studies concerning
TSP-1 and TGF-β expression in the heart of aging mouse models are
necessary.
In the present study, we employed 30-month-old mice
to represent the aging mouse model. The aim of the present study
was to confirm changes in TSP-1 and TGF-β expression in the aging
model and to explore the pathogenesis of interstitial fibrosis in
the aging heart, in order to provide evidence for a new clinical
treatment for the vulnerable senescent myocardium.
Materials and methods
Animal and sample preparation
Twenty 1-month-old male Kunming mice with an initial
body weight of 20–22 g were purchased from the Experiment Animal
Center of Zhejiang University and divided into two groups: 10 mice
were fed for 30 months as the aging mouse model group (AM) and
another 10 were fed for 2 months as the control group. All mice
were housed under a 12-h light/dark cycle and provided with
standard diet and water. All procedures were conducted with the
approval of the Zhejiang University Ethics and Animal Care
Committee (under NIH policies) and according to institutional
guidelines for the experimental use of animals.
Both groups of mice were sacrificed by dislocation
of the cervical vertebra. Their thoracic cavities were then opened.
The heart of each mouse was extracted, immediately fixed in
precooling saline and blood was removed and rinsed clean with
saline. Five mice per group were prepared for tissue
homogenization. Quantum satis heart tissue was accurately
weighed, cut into small pieces, placed into a glass tissue
homogenizer and precooling saline was added for preparation of the
tissue homogenate, which was centrifugalized. The supernatant was
then used for determination of superoxide dismulase (SOD) and
malondialdehyde (MDA). The remaining 5 mice per group were prepared
for paraffin sections. The hearts were fixed in 4% paraformaldehyde
for 4 h, then placed in 30% phosphate-buffered sucrose until the
tissue sank. After embedding in paraffin, the hearts were sectioned
on a rotary microtome and 8-μm-thick sections were cut on a
freezing microtome through coronary planes of the heart.
Measurement of SOD activity and MDA
content in the heart
The contents of SOD and MDA in the hearts were
determined by xanthine oxidation or TBA colorimetry. They were
measured according to the methods described in the instructions in
the SOD and MDA kits using chemicals purchased from the Nanjing
Jiancheng Bioengineering Institute.
Histopathological examination and
immunohistochemical staining
Paraffin section samples of the heart from each
mouse were stained with H&E, and then histopathological changes
in the hearts were examined under an optical microscope.
Immunohistochemistry was used to localize the TSP-1 and TGF-β. The
ABC system was used with 3,3’ diaminobenzidine hydrochloride (DAB)
as the chromagen. Briefly, heart sections were deparaffinized,
washed in 0.01 M phosphate-buffered saline (PBS; pH 7.4) three
times, each for 3 min, and treated with 3%
H2O2 for 10 min at room temperature to block
endogenous peroxidases.
The sections were subsequently washed with PBS three
times, each for 3 min, and incubated overnight at 4°C in the
primary antibody (monoclonal TSP-1 antibody or monoclonal TGF-β
antibody; Santa Cruz Biotechnology); the dilution of the primary
antibody was 1:100. The next day, the sections were incubated in a
biotinylated secondary antibody (diluted to 1:200 in PBS) and
subsequently in an avidin HRP solution. Immunolabeling was
visualized with 0.05% DAB plus 0.3% H2O2 in
PBS, and positive staining was shown as a yellow color. The
sections were then counterstained with diluted hematoxylin,
dehydrated through ethanol and xylene before being placed on
coverslips with Permount. As a negative control, PBS was used
instead of the primary antibody.
Image analysis and statistics
The heart sections were examined at x400 with
UTHSCSA Image Tools 3.0 (University of Texas Medical School at San
Antonio, TX, USA). The number of TSP-1- and TGF-β-positive cells
was determined. Statistical analysis was performed using the SPSS
statistical software program. Values were expressed as the mean ±
SD. The significance of the difference was calculated by the
two-tailed Student’s t test. P-values <0.05 are considered to
denote statistical significance differences.
Results
General appearance
The two groups of mice showed no significant
difference in weight and behaviour, and exhibited normal reactions
and smooth hairs before modeling. However, after feeding for 6
weeks, compared to the control group, the AM group showed symptoms
of senescence, such as decreased weight, yellow, loose and dim
hairs, obtuse behaviour and reaction, and polyuria. The results
corroborate previous studies (6–8).
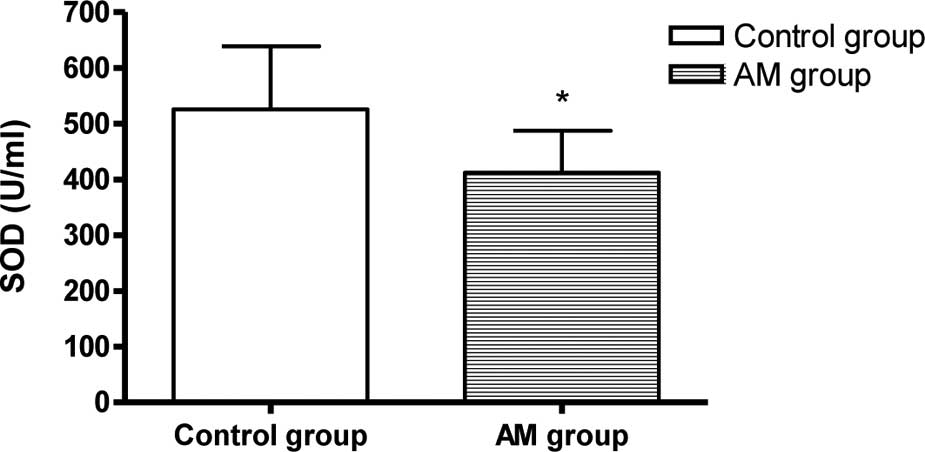
SOD activity and MDA content in the
heart
The SOD activity decreased and the MDA content
increased markedly in the hearts of the AM group mice compared to
the control group (P<0.05, Fig.
1).
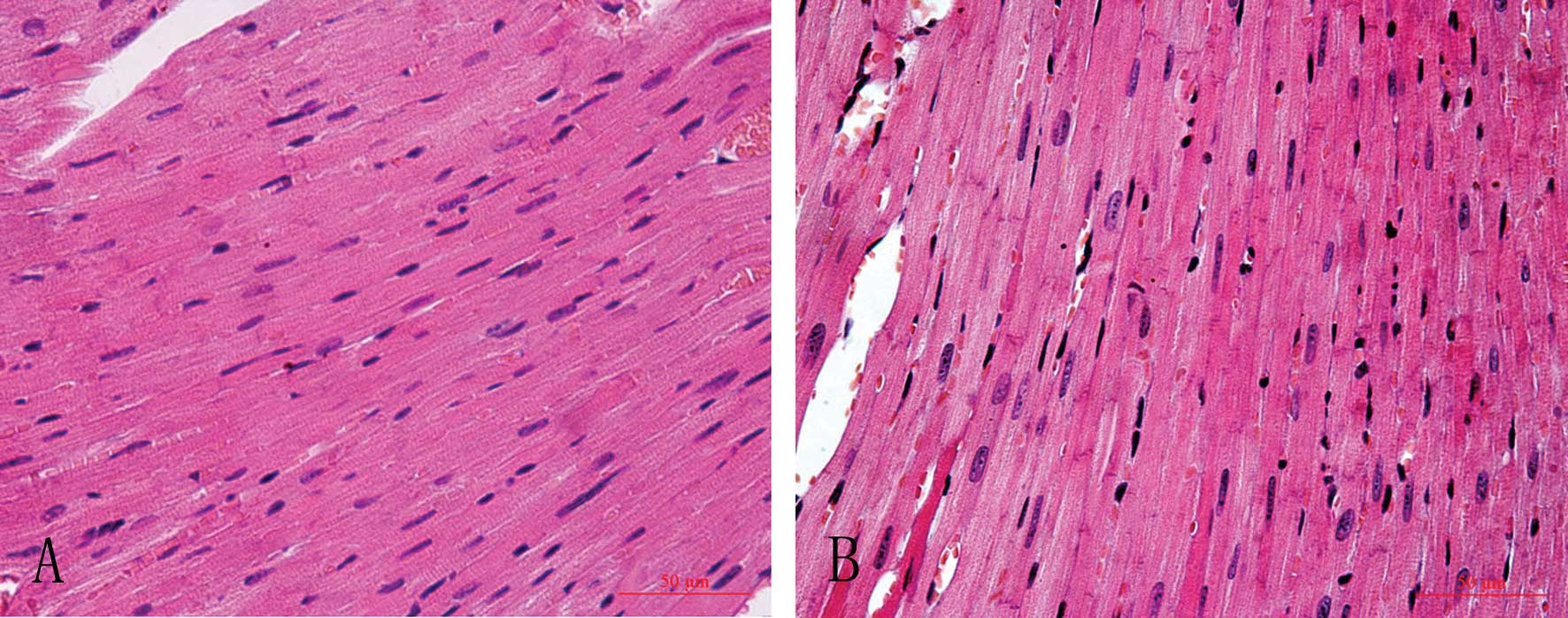
Histopathological changes in the
hearts
H&E staining showed that myocardial cells of the
control group lined up in order with clear structure and stained
equably, while myocardial cells of the AM group lined up in
disorder with an augmented cell body, uneven pigmentation, and the
appearance of many granules and interstitial fibrosis (Fig. 2).
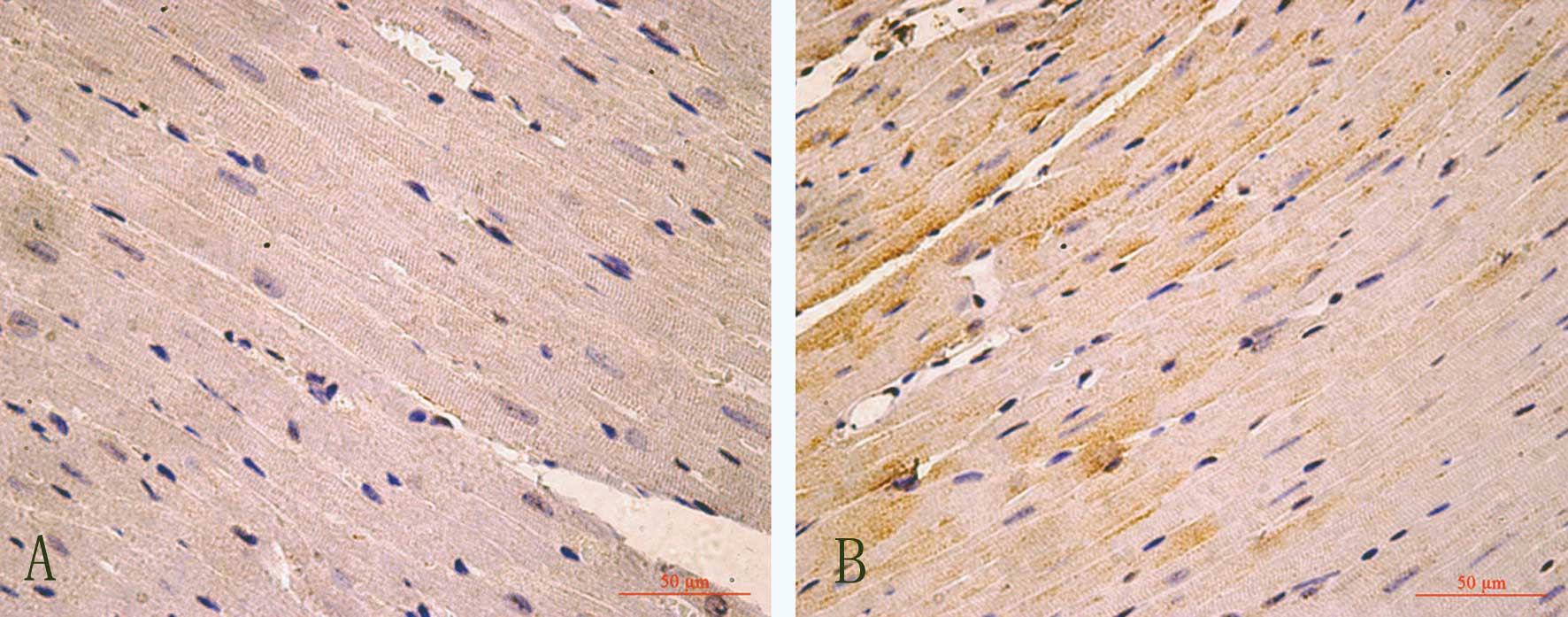
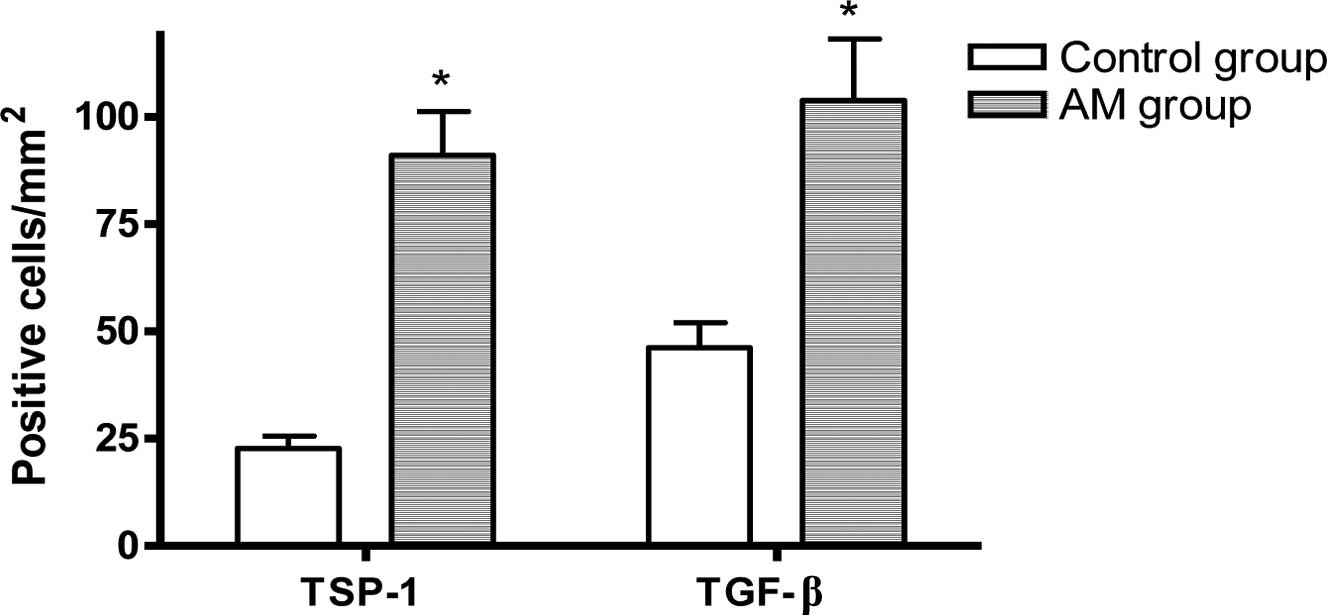
TSP-1 and TGF-β expression and
localization
Compared to the control group, in the hearts of the
AM group, the numbers of TSP-1 or TGF-β-positive-immunostained
myocardial cells were significantly increased (P<0.05, Figs. 3–5).
Discussion
The aging heart is associated with suppressed
inflammation, delayed phagocytosis of dead cardiomyocytes, and
markedly diminished collagen deposition following myocardial
infarction, due to a blunted response of fibroblasts to fibrogenic
growth factors (2). The present
study revealed that the SOD activity decreased and the MDA content
increased markedly in the hearts of the AM group, demonstrating
that the classical features of an aging mouse model were induced in
the 30-month-old mice.
TGF-β is a group of potent regulatory cytokines
involved in many biological processes. Cell culture studies have
indicated that TGF-β inhibits mitotic growth of cardiomyocytes, and
stimulates hypertrophic growth, fibrosis and re-expression of the
fetal isoforms of myofibrillar protein genes (11). Experimental studies have
demonstrated that age-related cardiac fibrosis and atherosclerosis
are strongly associated with myocardial extracellular matrix (ECM)
(12), and TGF-β is known to play
a significant role in fibrotic cardiac remodeling and multiple
factors altered in the aging heart. Fibrogenic pathways of TGF-β
are involved in the regulation of the balance between
matrix-degrading matrix metalloproteinases (MMPs) and their
inhibitors (TIMPs) (13),
mediating a sequential increase in connective tissue growth factor
synthesis (14), which is a
downstream target of the TGF-β/Smad pathway (7). These regulators may induce
age-dependent changes in this proteolytic system and unusually
increased deposition of ECM. In the present study, we found that
TGF-β expression in myocardial cells of aging mice was
significantly increased; it stimulated the proliferation of
interstitial fibroblasts, aggravated fibrosis and produced
decreased compliance in the aging heart.
TSP-1 is a multifunctional ECM glycoprotein and acts
as a potent inhibitor of angiogenesis in vitro and in
vivo. Studies have demonstrated that overexpression of TSP-1 in
the aging kidney is correlated with glomerulosclerosis and
tubulointerstitial fibrosis. TGF-β is secreted as a latent complex
of the LAP and the mature domain, which must be activated for TGF-β
to signal. Previous studies have identified TSP-1 as a
physiological activator of TGF-β in vitro and in
vivo. TSP-1 utilizes a two-step mechanism to activate latent
TGF-β (15,16). Recent research has implied that
there is a mutual activation between TGF-β and TSP-1. Studies of
other pathological models proved that TGF-β upregulates TSP-1
synthesis (16), and TGF-β is
involved in the fibrogenic process in hepatic stem cells through
upregulation of fibronectin expression (17,18).
The results of our present study showed that the aging heart model
(30-month-old mice) led to the upregulation of TSP-1 and TGF-β
protein expression in the myocardium, which suggest that TSP-1 and
TGF-β play a role in the development of age-related cardiac
fibrosis.
Our findings revealed quantitative changes in TSP-1
and TGF-β in the myocardium in 30-month-old mice. This indicates
that the TSP-1–TGF-β axis may positively participate in the process
of age-related myocardial fibrosis. Further study is required to
identify the exact underlying mechanism.
Acknowledgements
This study was supported by Projects
of the Zhejiang Education Bureau.
References
|
1.
|
A JahangirS SagarA TerzicAging and
cardioprotectionJ Appl
Physiol10321202128200710.1152/japplphysiol.00647.200717717116
|
|
2.
|
W ChenNG FrangogiannisThe role of
inflammatory and fibrogenic pathways in heart failure associated
with agingHeart Fail
Rev15415422201010.1007/s10741-010-9161-y20213186
|
|
3.
|
A BurkauskieneZ MackiewiczI
VirtanenAge-related changes in myocardial nerve and collagen
networks of the auricle of the right atriumActa
Cardiol61513518200610.2143/AC.61.5.201776517117750
|
|
4.
|
R KhanR SheppardFibrosis in heart disease:
understanding the role of transforming growth factor-beta in
cardiomyopathy, valvular disease and
arrhythmiaImmunology1181024200610.1111/j.1365-2567.2006.02336.x16630019
|
|
5.
|
A IshihataY KatanoRole of angiotensin II
and endothelin-1 receptors in aging-related functional changes in
rat cardiovascular systemAnn NY Acad
Sci1067173181200610.1196/annals.1354.02116803983
|
|
6.
|
M WangJ ZhangS WalkerInvolvement of NADPH
oxidase in age-associated cardiac remodelingJ Mol Cell
Cardiol48765772201010.1016/j.yjmcc.2010.01.00620079746
|
|
7.
|
HJ ZhuAW BurgessRegulation of transforming
growth factor-beta signalingMol Cell Biol Res
Commun4321330200110.1006/mcbr.2001.030111703090
|
|
8.
|
ML Iruela-ArispeRegulation of
thrombospondin1 by extracellular proteasesCurr Drug
Targets9863868200810.2174/13894500878590936518855620
|
|
9.
|
S Schultz-CherryS RibeiroL
GentryThrombospondin binds and activates the small and large forms
of latent transforming growth factor-beta in a chemically defined
systemJ Biol Chem26926775267821994
|
|
10.
|
DH KangS AndersonYG KimImpaired
angiogenesis in the aging kidney: vascular endothelial growth
factor and thrombospondin-1 in renal diseaseAm J Kidney
Dis37601611200110.1053/ajkd.2001.2208711228186
|
|
11.
|
DD BonnemaCS WebbWR PenningtonEffects of
age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor
of metalloproteinases (TIMPs)J Card
Fail13530540200710.1016/j.cardfail.2007.04.01017826643
|
|
12.
|
DR EdwardsKJ LecoPP BeaudryDifferential
effects of transforming growth factor-beta 1 on the expression of
matrix metalloproteinases and tissue inhibitors of
metalloproteinases in young and old human fibroblastsExp
Gerontol131207223199610.1016/0531-5565(95)02010-18706790
|
|
13.
|
ML BeggsR NagarajanJM
Taylor-JonesAlterations in the TGF beta signaling pathway in
myogenic progenitors with ageAging
Cell3353361200410.1111/j.1474-9728.2004.00135.x15569352
|
|
14.
|
GD YoungJE Murphy-UllrichThe
tryptophan-rich motifs of the thrombospondin type 1 repeats bind
VLAL motifs in the latent transforming growth factor-beta complexJ
Biol Chem2794763347642200410.1074/jbc.M40491820015342643
|
|
15.
|
XM ZhangF ShenZY XvExpression changes of
thrombospondin-1 and neuropeptide Y in myocardium of STZ-induced
ratsInt J
Cardiol105192197200510.1016/j.ijcard.2004.12.06516243112
|
|
16.
|
XM ZhangPH ShiSH CaoExpression changes of
transforming growth factor-beta1 and thrombospondin-1 in cavernous
tissues of diabetic ratsUrol
Int84221225201010.1159/00027760220215829
|
|
17.
|
W CuiHB JinZW LiMechanism of the
transforming growth factor-beta induction of fibronectin expression
in hepatic stem-like cellsBraz J Med Biol
Res433642201010.1590/S0100-879X200900750001719936542
|
|
18.
|
M RenB WangJ ZhangSmad2 and Smad3 as
mediators of the response of adventitialfibroblasts induced by
transforming growth factor β1Mol Med Rep4561567201121468608
|