Introduction
Soccer is one of the sports most likely to cause not
only clinically recognized joint injuries but also unrecognized
minute ones due to the overuse, repetitive impact and torsional
loading of joints. Furthermore, these conditions may be aggravated
by several factors, including excessive mechanical loads and
repeated microtrauma. These factors are known to enhance articular
cartilage damage in soccer players. In this context, there are
supporting data demonstrating that the risk of cartilage damage is
increased in soccer (and certain other sports), where the
repetitive intense impact and torsional loading are subjected to
the joints (1), and may be
implicated in the chronic development of osteoarthritis (OA)
(2–4). Indeed, several clinical studies have
demonstrated that the risk of OA is higher in soccer players, as
well as in certain other groups of athletes, than in control
non-athlete groups (5–11).
In the course of health management of collegiate
soccer players, we noticed that most players, particularly leading
players, suffer from subtle lower extremity joint pains that are
self-recognized only when they engage in the training and/or soccer
games, although they are not diagnosed as having joint injuries.
Thus, we speculated that these conditions may result from
clinically unrecognized minute joint injuries and that such
injuries may also increase the risk of subsequent development of OA
or other degenerative joint diseases (1). The aim of the present study was to
examine at which joints the minute injury-mediated subclinical
joint pains occur and are observed in soccer players. For this
purpose, pains were measured at several different joints, mainly
lower extremity joints, using 3 pain subscales of a 100-mm visual
analog scale (VAS) in a team of collegiate soccer players.
In addition, we focused on the potential of
nutraceuticals for the prevention and treatment of such subclinical
joint pains. Glucosamine and chondroitin are two popular dietary
supplements being promoted for the maintenance of joint health
among athletes (12–15). In addition, our previous study
demonstrated that the intake of a dietary supplement containing
chicken comb extract (CCE) rich in hyaluronan (HA) (CCE supplement)
was effective in improving pain and dysfunction of afflicted joints
in patients with knee OA (16).
Based on these data, we conducted a prospective, double-blind,
controlled study, in which the effect of the CCE supplement on
joint pain was evaluated in a team of collegiate soccer players
without any previously diagnosed joint injury.
Materials and methods
Subjects
The present study was approved by the Human
Experimentation Ethics Committee of Juntendo University (Japan) and
was conducted in accordance with the principles of the amended
Declaration of Helsinki and ‘Ethical Guidelines for Epidemiological
Research’ (established by the Japanese Government in 2004). Written
informed consent was obtained from all subjects prior to their
enrollment in the study. The enrolled subjects belonged to a soccer
team of Juntendo University School of Health and Sports Science,
and included both leading and substitute players.
Exclusion criteria were: clinically recognized joint
injuries; common use of health food or cosmetics containing
constituents with analgesic and/or chondroprotective potential
(e.g., glucosamine, chondroitin and HA); known allergy to certain
constituent(s) or material(s) of the test supplement; previous or
current (within the previous 3 months) treatment or planned
treatment to be administered during the study period with
intra-articular injections of HA or corticosteroids; routine intake
of anti-resorptive drugs, including bisphosphonates or estrogen;
participation in another clinical study and the presence of any
medical condition or history judged by the medical investigator to
preclude the subject's inclusion in the study.
Among the eligible subjects, appreciable differences
in the amount of soccer-related activities were observed between
leading and substitute players. According to the estimation by the
team leader and coaches, the average amount of physical activities
in the training and soccer games for the leading players during the
study period was 1.4- to 1.7-times greater than that for the
substitute players. The amount of soccer-related activities also
varied based on the players' position within the team or on the
pitch, being the highest in midfielders and the lowest in
goalkeepers. Taking these points into consideration, enrolled
subjects were divided into 2 groups so that they were distributed
evenly in terms of their playing positions, as well as leading and
substitute players.
Intervention and subject group
assignment
The test product was a commercially available 300-mg
capsule-form product (Kojun®), containing 157.5 mg of
CCE, of which approximately 4.5 mg was HA, together with 20 mg of
calcium lactate, 10 mg of propolis extract, 4.9 mg of chitosan
oligosaccharide, 5.0 mg each of vitamins B1 and
B6, 2.5 mg of vitamin E, 2.0 mg of ferric pyrophosphate,
0.1 mg of vitamin B12 and 192.5 mg of the vehicle
(comprising of crystalline cellulose, dextrin and fatty acid sugar
esters). Subjects were assigned to receive 16 capsules per day of
the test product (test group) or 16 capsules per day of the ‘dummy’
placebo, containing only the vehicle (placebo group). All subjects
were instructed to take allocated capsules in a dose of 8 capsules
twice daily following breakfast and dinner with the aid of a
sufficient amount of drinking water for a consecutive 12 weeks. The
study was performed during the 2009 summer-fall soccer competition
season. Adherence to the intervention was assessed on the basis of
consumption recorded in the self-administered study diary, and a
value <85% was considered to constitute a protocol
violation.
Procedures
All subjects underwent clinical and laboratory
examinations at baseline and at weeks 4, 8 and 12 following the
commencement of intervention to follow the changes in pain levels
during the 12-week intervention period. Due to the characteristics
of soccer, the lower extremity joints are expected to be at a
greater risk of suffering repetitive impact and torsional loading
than the upper extremity joints. On the basis of this, we selected
the 3 lower extremity joints (i.e., ankle, knee and hip joints) as
the main target joints, together with a shoulder joint as a
representative of upper extremity joints, for evaluating the
pain-relieving effect of the test product.
Pain levels of the individual test joints were
measured using the self-diagnosed 3 pain subscales of the 100-mm
VAS: ‘pain at rest’, ‘pain on pressing’ and ‘pain on moving’. Each
VAS pain subscale was scored from 1 to 100, where 0 indicated no
pain and 100 indicated the most intense pain ever experienced.
Tolerability and safety was assessed throughout the
study on the basis of the incidence and severity of
intervention-related adverse events (side-effects) as well as
abnormal changes in blood pressure, pulse rate and laboratory
tests, including hematology, biochemical profile and
urinalysis.
Statistical analysis
Values of all variables were expressed as the means
± standard deviation (SD). Individual VAS scores for 3 pain
subscales at 4 different joints measured prior to the start of
intervention were compared by the Student's t-test. Baseline
characteristics of the total subject population and the leading
players were compared between the test and placebo groups by the
Student's t-test for continuous variables and by the Mann-Whitney U
test for category variables. Pain scores during the intervention
were compared to the baseline values by the Student's t-test.
Comparisons of the changes from the baseline in pain scores were
also performed by the Student's t-test. A p-value <0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of VAS scores for 3 pain
subscales among 4 test joints
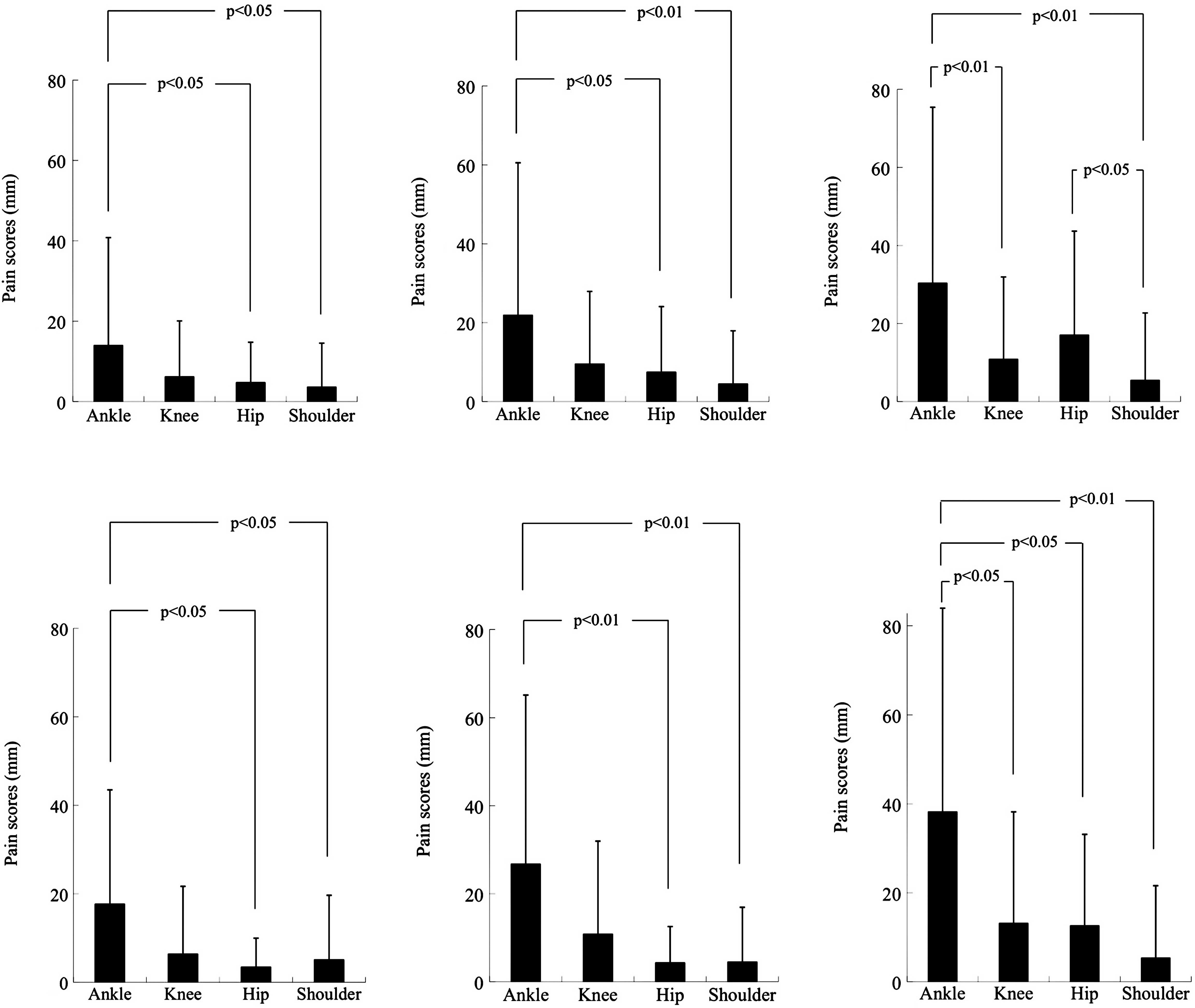
The severities of pain at 4 different joints (e.g.,
ankle, knee, hip and shoulder joints), as measured by the use of 3
pain subscales of a 100-mm VAS, were compared using 46 enrolled
collegiate soccer players, consisting of 24 leading and 22
substitute players, prior to the commencement of intervention. As
shown in Fig. 1, the total
population analysis with all the subjects indicated that the ‘pain
on moving’ subscale scores were higher than the ‘pain at rest’ and
‘pain on pressing’ subscale scores at all of the 4 test joints, and
that the values at the ankle joint were the highest and those at
the shoulder joint were the lowest among the 4 joints in the 3 pain
subscale scores (Fig. 1A–C).
Almost the same results were obtained in the subpopulation analysis
with the lead players (Fig. 1D–F).
However, no such clear results were obtained with the substitute
players, probably due to the fact that the VAS scores for every
pain subscale were too low in the substitute player group to
evaluate reliably. The ankle joint VAS scores for ‘pain at rest’
and ‘pain on moving’ subscales were 14.0±26.8 and 30.3±45.1
(p=0.037) in the total subject population, 17.7±25.8 and 38.2±45.8
(p=0.062) in the leading player subpopulation, and 9.9±27.9 and
21.8±43.7 (p=0.290) in the substitute player subpopulation,
respectively.
Based on all these data, the VAS scores for ‘pain on
moving’ at the ankle joint (hereafter presented as ‘pain scores’)
were utilized as the parameter for the evaluation of the efficacy
of the test product, and the total subject population and leading
player subpopulation were subjected to efficacy analyses.
Characterization of study groups
Of the 46 enrolled subjects, 23 were assigned to the
test product group and placebo group. A total of 4 of the subjects
in the 2 groups withdrew from or discontinued the study during the
intervention period for a number of reasons, including
hospitalization due to bone or joint injuries, trauma and other
medical problems, and withdrawal from the soccer team. Thus, 38
subjects (19 each in both the test and placebo groups) fulfilled
the eligibility criteria, completed the study and were analyzed for
the efficacy assessment. The eligible subjects in the test and
placebo groups comprised 11 and 10 leading players and 8 and 9
substitute players, respectively.
As shown in Table
I, the demographic and physiological characteristics (age,
height, weight, body mass index, blood pressure and pulse rate) did
not differ between the test and placebo groups. The number of
leading and substitute players and the distribution of the position
of players was well balanced between the 2 groups.
 | Table I.Baseline data of eligible subjects in
the test and placebo groups who completed the study. |
Table I.
Baseline data of eligible subjects in
the test and placebo groups who completed the study.
| Variablesa | Test group
(n=19) | Placebo group
(n=19) | p-value |
|---|
| Age (years) | 19.5±1.2 | 19.7±1.3 | 0.521 |
| Height (cm) | 172.7±5.7 | 172.2±6.2 | 0.802 |
| Weight (kg) | 66.05±5.65 | 66.45±6.52 | 0.843 |
| Body mass index
(kg/m2) | 22.14±1.32 | 22.38±1.24 | 0.579 |
| Systolic blood
pressure (mmHg) | 110.9±9.0 | 116.4±8.9 | 0.068 |
| Diastolic blood
pressure (mmHg) | 61.5±8.4 | 62.7±6.3 | 0.618 |
| Pulse rate
(beats/min) | 53.9±7.0 | 53.5±6.9 | 0.871 |
| Leading
players/substitute players (Number of subjects) | 11/8 | 10/9 | 0.795 |
| Distribution of the
position of the players (Number of subjects) MF/DF/FW/GKb | 10/6/1/2 | 9/6/4/0 | 0.246 |
| Pain scores of the
ankle joints | | | |
| Dominant
foot | 22.29±23.72 | 17.01±27.58 | 0.531 |
| Non-dominant
foot | 17.96±24.17 | 15.32±26.56 | 0.750 |
Regarding the pain scores, we analyzed the dominant
foot and the non-dominant foot separately, since the extent of
joint loads and the risk of joint injury were considered somewhat
different between the dominant and non-dominant feet. The average
pain scores for the dominant foot were slightly higher than those
for the non-dominant foot (Table
I).
Efficacy of the test supplement
Table II shows the
changes in the pain scores analyzed with the total subject
population and the leading player subpopulation in the test and
placebo groups. Pain scores of the dominant and non-dominant feet
are presented separately. Significant reductions in the pain scores
were observed only for the dominant foot (but not for the
non-dominant foot) in the test group (but not in the placebo
group); at week 4 (p<0.05) in the total population and at weeks
4 (p<0.05) and 12 (p<0.01) in the leading player
subpopulation.
 | Table II.Changes in the pain scores for the
dominant and non-dominant feet during the 12-week intervention
period in the test and placebo groups. |
Table II.
Changes in the pain scores for the
dominant and non-dominant feet during the 12-week intervention
period in the test and placebo groups.
| Subjects | Group | Foot, dominant or
non-dominant | Pain scores
(mm)a
|
|---|
| Baseline | Week 4 | Week 8 | Week 12 |
|---|
| Total population
(n=38) | Test (n=19) | Dominant | 22.29±23.72 | 8.92±15.39b | 14.09±18.57 | 12.05±17.11 |
| Non-dominant | 17.96±24.17 | 18.55±25.11 | 17.55±19.86 | 15.55±17.70 |
| Placebo (n=19) | Dominant | 17.01±27.58 | 15.93±22.14 | 16.11±26.40 | 13.32±17.50 |
| Non-dominant | 15.32±26.56 | 23.61±30.84 | 12.57±23.01 | 13.69±17.48 |
| First-line players
(n=21) | Test (n=11) | Dominant | 29.91±23.68 | 13.72±18.90b | 18.51±21.92 | 7.86±12.26c |
| Non-dominant | 19.70±22.69 | 19.20±26.27 | 21.01±20.95 | 12.88±11.89 |
| Placebo (n=10) | Dominant | 19.93±27.63 | 20.26±22.76 | 24.29±32.48 | 18.79±21.04 |
| Non-dominant | 16.85±26.05 | 28.35±36.99 | 14.36±27.41 | 14.50±18.73 |
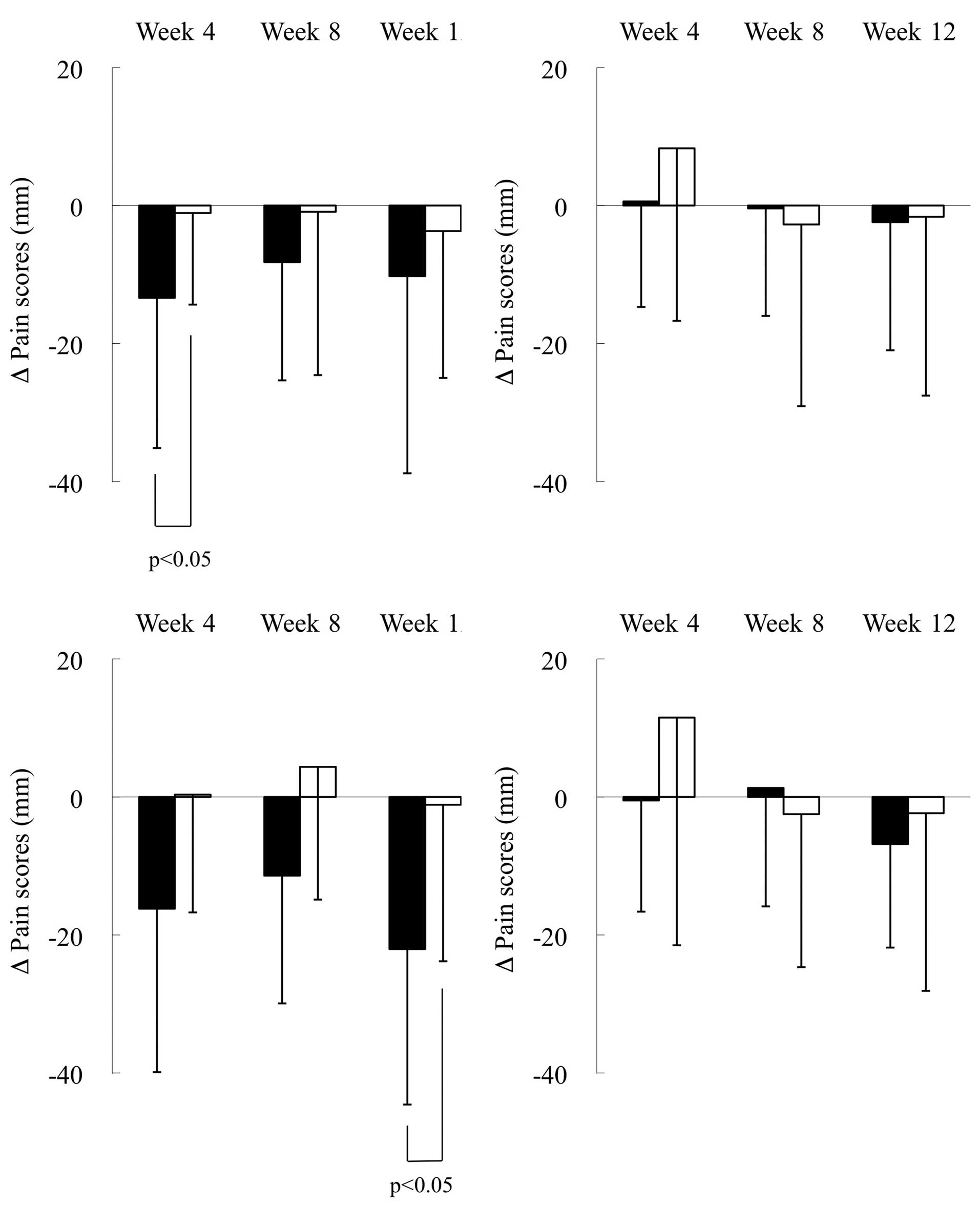
Fig. 2 shows the
changes from baseline in the pain scores for the dominant and
non-dominant feet of the total population and leading players in
the 2 groups. Of note, the pain scores were significantly reduced
in the dominant foot of the test group compared to the placebo
group at week 4 (p<0.05) in the total population (Fig. 2A) and at week 12 (p<0.05) in the
leading player subpopulation (Fig.
2C). By contrast, the pain scores were not significantly
different between the test and placebo group for the non-dominant
foot in the total population (Fig.
2B) and the leading player subpopulation (Fig. 2D).
Safety of the test supplement
A total of 11 subjects of the test group (n=23) and
12 subjects of the placebo group (n=23) reported at least 1 adverse
event, but not for injuries and trauma during the intervention
period. The total number of reported adverse events was 26 in the
test group and 21 in the placebo group, and there was no
significant difference in the frequency between the 2 groups.
Individual adverse events and their frequencies in the test group
compared to the placebo group were: upper respiratory infections
(20 vs. 17); rhinitis (0 vs. 2); diarrhea (2 vs. 0); dehydration (1
vs. 0); retinal detachment (0 vs. 1); urticaria (0 vs. 1); herpes
(1 vs. 0); anemia (1 vs. 0) and pneumonia (1 vs. 0). All adverse
events were judged by the medical investigator to be unrelated to
the intervention. In the 2 groups, measurements of physiological
parameters (weight, body mass index, blood pressure and pulse rate)
and routine laboratory tests (hematology, blood chemistry and
urinalysis) did not reveal any significant abnormality throughout
the intervention.
Discussion
Soccer injuries have been reported to occur for the
most part in the lower extremity in youth players (17,18),
as is the case for professional (19) and senior players (20,21).
The possible explanations for this are as follows: i) in soccer
players, the lower extremity joints are subjected to severe
mechanical stresses and are easily injured due to excessive and
repetitive use of the joints; and ii) soccer players expose their
lower extremity joints to repetitive intense impacts and torsional
and/or axial loads. Such joint injuries may cause pain and other
symptoms located in the afflicted joints.
With regard to the comparison of subtle joint pain
presumably associated with minute injuries among 4 various joints
(ankle, knee, hip and shoulder joints), the highest VAS scores were
observed in the ankle joint, followed by the knee, hip or shoulder
joint for all of the 3 pain subscales. This could be explained by
the finding that relatively more sprains are localized in the ankle
joint of soccer players (22).
Furthermore, a substantial number of preceding studies have
demonstrated that among all joint injuries occurring in soccer
players the ankle sprain is the most common (17–19,23–29).
The results from the present study showed that the VAS scores for
the ‘pain on moving’ subscale were substantially higher than the
scores for the ‘pain at rest’ subscale and ‘pain on pressing’ at
the ankle joint, as well as other lower extremity joints in youth
soccer players. This suggests that VAS scores for the ‘pain on
moving’ subscale may be utilized as the most sensitive parameter
for detecting subtle joint pain and evaluating the effect of
joint-protecting treatments.
In this prospective, placebo-controlled study, in
which the ankle joint was used as the target, the test product
exhibited a pain-relieving effect on the ankle of the dominant foot
in the subjects, particularly leading players. The pain scores were
significantly reduced from the baseline in the total subpopulation
and the leading player subpopulation of the test groups at week 4
and at weeks 4 and 12, respectively (Table II). Moreover, the pain score
reduced significantly for the dominant feet of the leading players
in the test group, compared with those in the placebo group at week
12 (Fig. 2C). By contrast, the
pain scores were not significantly changed in the non-dominant feet
of both the total and leading player subpopulations in the test
group, as well as the dominant and non-dominant feet of the total
and leading player subpopulations in the placebo group (Table II and Fig. 2). Although there is no explanation
for these results, it may be speculated that the ankle joint of the
dominant foot in the leading players may be subjected to increased
opportunities of suffering injuries compared to the ankle joint of
the opposite (non-dominant) foot. Thus, the test product may
exhibit a pain-relieving effect on the ankle joint of the dominant
foot in the leading player subpopulation.
Glucosamine is sometimes used by soccer players for
preventing OA or relieving joint pain. However, there is currently
no evidence indicating such efficacy in healthy athletes. Regarding
the CCE supplement, we previously demonstrated in a clinical study
that the CCE supplement has a pain-relieving effect on middle-aged
and older patients with knee OA (16). Thus, it is likely that the
pain-relieving effect could be extrapolated to healthy young
athletes experiencing subclinical pains at the afflicted
joints.
There are as yet no data available for explaining
the mechanism by which the CCE supplement can alleviate joint pain
in collegiate soccer players, as observed in the present study. HA,
a presumable bioactive constituent of the CCE supplement, has been
reported to display anti-inflammatory activities through an
inhibition of the production of several pro-inflammatory cytokines
(e.g., interleukin-1β) or inflammatory mediators (e.g.,
Prostaglandin E2) (30,31).
These observations indicate a that HA may be implicated in the
favorable effect of the CCE supplement by suppressing inflammation
of the affected joints, thereby relieving joint pain.
References
|
1.
|
M YoshimuraK SakamotoA TsurutaEvaluation
of the effect of glucosamine administration on biomarkers for
cartilage and bone metabolism in soccer playersInt J Mol
Med24487494200919724889
|
|
2.
|
AS LevyJ LohnesS SculleyM LeCroyW
GarrettChondral delamination of the knee in soccer playersAm J
Sports Med24634639199610.1177/0363546596024005128883684
|
|
3.
|
JA BuckwalterNE LaneAthletics and
osteoarthritisAm J Sports
Med25873881199710.1177/0363546597025006249397280
|
|
4.
|
AC GelberMC HochbergLA MeadNY WangFM
WigleyMJ KlagJoint injury in young adults and risk for subsequent
knee and hip osteoarthritisAnn Intern
Med133321328200010.7326/0003-4819-133-5-200009050-0000710979876
|
|
5.
|
T BoyerM DelaireL BeranekPP LasserreM
TakeyaMF KahnIncidence of previous engagement in sports among
patients with symptomatic arthrosisControlled study Rev Rhum Mal
Osteoartic4879379719816461056
|
|
6.
|
UM KujalaJ KaprioS SarnaOsteoarthritis of
weight bearing joints of lower limbs in former élite male
athletesBMJ2223123419948111258
|
|
7.
|
H RoosH LindbergP GärdsellLS LohmanderH
WingstrandThe prevalence of gonarthrosis and its relation to
meniscectomy in former soccer playersAm J Sports
Med22219222199410.1177/0363546594022002118198190
|
|
8.
|
UM KujalaJ KettunenH PaananenKnee
osteoarthritis in former runners, soccer players, weight lifters,
and shootersArthritis
Rheum38539546199510.1002/art.17803804137718008
|
|
9.
|
TD SpectorPA HarrisDJ HartRisk of
osteoarthritis associated with long-term weight-bearing sports: a
radiologic survey of the hips and knees in female ex-athletes and
population controlsArthritis
Rheum39988995199610.1002/art.1780390616
|
|
10.
|
F RannouS PoiraudeauM RevelCartilage: from
biomechanics to physical therapyAnn Readapt Med
Phys44259267200111587668
|
|
11.
|
M LequesneSport and osteoarthritis of the
membersSci Sports19281285200410.1016/j.scispo.2004.09.003
|
|
12.
|
TL SchwenkCD CostleyWhen food becomes a
drug: nonanabolic nutritional supplement use in athletesAm J Sports
Med30907916200212435662
|
|
13.
|
RT GorslineCC KaedingThe use of NSAIDs and
nutritional supplements in athletes with osteoarthritis:
prevalence, benefits, and consequencesClin Sports
Med247182200510.1016/j.csm.2004.08.00315636778
|
|
14.
|
P HespelRJ MaughanPL GreenhaffDietary
supplements for footballJ Sports
Sci24749761200610.1080/0264041050048297416766503
|
|
15.
|
SM OstojicM ArsicS ProdanovicJ VukovicM
ZlatanovicGlucosamine administration in athletes: effects on
recovery of acute knee injuryRes Sports
Med15113124200710.1080/1543862070140524817578751
|
|
16.
|
I NagaokaK NabeshimaS MurakamiEvaluation
of the effects of a supplementary diet containing chicken comb
extract on symptoms and cartilage metabolism in patients with knee
osteoarthritisExp Ther Med1817827201022993606
|
|
17.
|
S NilssonA RoaasSoccer injuries in
adolescentsAm J Sports Med6358361197810.1177/036354657800600608
|
|
18.
|
JA SullivanRH GrossWA GranaCA
Garcia-MoralEvaluation of injuries in youth soccerAm J Sports
Med8325327198010.1177/0363546580008005057416349
|
|
19.
|
M AlbertDescriptive three-year data study
of outdoor and indoor professional soccer injuriesJ Athl
Train182182201983
|
|
20.
|
J EkstrandJ GillguistSoccer injuries and
their mechanisms: a prospective studyMed Sci Sport
Exerc15267270198310.1249/00005768-198315030-000146621313
|
|
21.
|
J EkstrandJ GillquistThe avoidability of
soccer injuriesInt J Sports
Med4124128198310.1055/s-2008-1026025
|
|
22.
|
H InklaarE BolSL SchmikliWL
MosterdInjuries in male soccer players: team risk analysisInt J
Sports Med17229234199610.1055/s-2007-9728378739579
|
|
23.
|
WC McMasterM WalterInjuries in soccerAm J
Sports Med6354357197810.1177/036354657800600607
|
|
24.
|
B KristensenC AndersenSoccer injuries. II
The socioeconomic importance of injuries treated outside
hospitalsUgeskr Leager141270327041979531960
|
|
25.
|
S MaehlumOA DaljordFootball injuries in
Oslo: a one-year studyBr J Sports Med1818619019846487944
|
|
26.
|
S Schmidt-OlsenLK BünemannV LadeJO
BrassøeSoccer injuries of youthInt J Sports
Med1916116419854075068
|
|
27.
|
M Sadat-AliM Sankaran-KuttySoccer injuries
in Saudi ArabiaAm J Sports
Med15500502198710.1177/036354658701500513
|
|
28.
|
CS KellerFR NoyesCR BuncherThe medical
aspects of soccer injuryAm J Sports
Med15230237198710.1177/036354658701500307
|
|
29.
|
K HøyBE LindbladCJ TerkelsenHE HellelandCJ
TerkelsenEuropean soccer injuries. A prospective epidemiologic and
socioeconomic studyAm J Sports Med2031832219921636863
|
|
30.
|
T YasuiM AkatsukaK TobettoM HayaishiT
AndoThe effect of hyaluronan on interleukin-1α-induced
Prostaglandin E2 production in human osteoarthritic
synovial cellsAgents Actions371551561992
|
|
31.
|
K TakahashiRS GoomerF HarwoodT KuboY
HirasawaD AmielThe effects of hyaluronan on matrix
metalloproteinase-3 (MMP-3), interleukin-1β (IL-1 β), and tissue
inhibitor of metal-loproteinase-1 (TIMP-1) gene expression during
the development of osteoarthritisOsteoarthritis
Cartilage71821901999
|