Introduction
Prostate cancer (PC) is the most common malignancy
and the second leading cause of cancer-related death in men in
industrialized countries (1,2). Its
incidence is at a relatively low rate in the Asian population
(3), but is increasing rapidly
(4). It is supposed that complex
elements, such as hormones, age, family history of PC, cultural and
enviromental factors and genetic background (3), contribute to the cancerization and
progression of PC. However, the specific mechanism remains
undetermined.
Folate is indispensably required for DNA synthesis
and methylation of DNA and histones. Epidemiological studies have
shown an effective association between low folate intake and an
increased cancer risk (5,6). MTHFR plays a vital role in the
metabolism of folates by irreversibly converting
5,10-methylenetetrahydrofolate to 5-methylenetetrahydrofolate
(7), which donates a methyl group
for the remethylation of homocysteine to methionine used for DNA
synthesis and repair (8).
Therefore, MTHFR deficiency may lead to DNA hypomethylation to
initialize cancerization and affect the progression of malignant
tumors (9,10).
The human MTHFR gene, composed of 11 exons, is
located at chromosome 1p36.3, codes cDNA of 2.2-kb in length and
produces a protein of 656 amino acids (11). The 1298A>C polymorphism, marked
as rs1801131 in the NCBI database, is located at exon 7 and results
in a glutamate-to-valine substitution at codon 429 (8). Alterations in genomic bases result in
single-nucleotide polymorphisms (SNPs), which may subsequently
affect the genetic instability, amino acid sequence and function of
protein. Recently, SNPs have been used as a tool for predicting
diseases (12) in addition to
carcinogenesis (13,14). The MTHFR gene A1298C polymorphism
has been implicated in several diseases (15,16),
including various types of cancer (17,18),
and has been investigated in relation to the risk of PC but with
inconclusive results (19–27). Among the nine eligible case-control
studies, three considered the MTHFR gene A1298C polymorphism as a
genetic marker for PC (19,24,26),
while six reported negative associations between the two (20–23,25,27).
Hence, we carried out a meta-analysis concerning the association
between the MTHFR gene A1298C polymorphism and PC susceptibility by
pooling data from the identified studies to obtain a more
conclusive estimation.
Materials and methods
Identification of relevant studies
Publications were identified by a systematic
electronic search in the PubMed database with the following
keywords: ‘methylenetetrahydrofolate reductase’, ‘MTHFR’,
‘polymorphism’, ‘variation’, ‘mutation’, ‘prostate’ and
‘prostatic’, as well as their combinations. The last search was
updated on Aug 21, 2011. We did not set any restriction on the
language of the published literature. Additional studies were
searched by manually screening references in review articles and
original papers.
Inclusion and exclusion criteria
The inclusion criteria used for the article
selection in this meta-analysis were as follows: i) case-control
study with PC and control groups; ii) study focusing on the
association between the MTHFR gene A1298C polymorphism and the
susceptibility of PC; iii) frequencies of the various genotypes in
the publications were available.
The major exclusion criteria were studies that were
duplication of a previous publication, studies without detailed
information, or not case-control studies, such as review articles,
case reports, editorials, conference abstracts and letters.
Data extraction
Two investigators (D.L. and C.G.) reviewed and
extracted the information from all included publications
independently by a standardized protocol, according to the
inclusion and exclusion criterias. Characteristics, such as year of
publication, name of first author, country of origin, ethnicity,
source of control group, methods for detecting the MTHFR gene
A1298C polymorphism, C allele percentage in controls and
frequencies of AA, AC and CC genotypes in the case and control
groups, were respectively extracted from the included studies. In
the case of disagreement, discrepancies of included studies were
resolved by discussion.
Statistical analysis
We predicted the contribution of the MTHFR gene
A1298C polymorphism to the risk of PC by adopting the Review
Manager software version 4.2 developed by the Cochrane
Collaboration. The strength of association was estimated by
calculating summary crude odds ratios (ORs) and the corresponding
95% confidence intervals (CIs). We evaluated the risk of the
dominant (CC + AC vs. AA), recessive (CC vs. AA + AC), allele (C
vs. A) and codominant models (AC vs. AA; CC vs. AA), respectively.
Hardy-Weinberg equilibrium (HWE) for the control groups in each
study was checked by the goodness-of-fit test. Heterogeneity
assumption was assesed by the Chi-square-based Q test and was
regarded to be statistically significant at P<0.10. The
random-effects model was used when the test of heterogeneity was
significant, otherwise the fixed-effects model was applied in the
analysis. Potential publication bias was primarily appraised by the
funnel plot. An asymmetric plot suggested a possible publication
bias. Funnel plot asymmetry was further evaluated by Egger's linear
regression and Begger's rank correlation tests with STATA software,
version 7.0. All P-values were two-tailed.
Results
Study characteristics
Nine published studies were eligible for this
meta-analysis on the association between MTHFR gene A1298C
polymorphism and PC susceptibility in the electronic PubMed
database (19–27). All of the qualified articles were
case-control designed studies, consisting of a total of 2,723 PC
cases and 3,442 controls. The detailed characteristics of the
studies, such as year of publication, name of first author, country
of origin, ethnicity, source of control groups, genotyping methods,
C allele percentage in controls, HWE and the genotype distribution
of the MTHFR gene A1298C polymorphism, are documented in Table I. Among the nine studies, two
involved Asian populations (25,27),
four involved Caucasian populations (21,22,24,26),
and the remaining three were of mixed ethnicities (19,20,23).
All studies, but one (24), were
consistent with HWE. As to the source of control groups, four were
population-based studies (21,23,26,27),
four were hospital-based studies (20,22,24,25)
and the other one (19) was a
family-based study. Only four studies provided data on disease
stage, including advanced and localized PC (19,20,22,26).
PCR-RFLP was used to distinguish genotype in six studies (19,20,24–27),
and TaqMan SNP genotyping assay was chosen for the other three
(21–23). All of the research studies made use
of DNA samples extracted from peripheral blood cells for
genotyping, except one, which employed fixed tissue samples
(20). Thus, subgroup analysis was
conducted in this meta-analysis.
 | Table I.Main characteristics of all studies
included in the meta-analysis. |
Table I.
Main characteristics of all studies
included in the meta-analysis.
| Ref. | Year | First author | Method | Country | Ethnicity | Study design | Cases
| Controls
| Allele C %
incontrols | HWE
|
|---|
| AA | AC | CC | AA | AC | CC |
χ2-test | P-value |
|---|
| 19 | 2004 | Cicek | PCR-RFLP | USA | Mixed | F | 195 | 205 | 39 | 233 | 201 | 44 | 30.23 | 0.00 | 0.95 |
| 20 | 2004 | Singal | PCR-RFLP | USA | Mixed | H | 29 | 43 | 9 | 18 | 17 | 7 | 36.90 | 0.72 | 0.40 |
| 21 | 2006 | Van Guelpen | TaqMan | Sweden | Caucasian | P | 87 | 108 | 27 | 176 | 203 | 55 | 36.06 | 0.09 | 0.77 |
| 22 | 2008 | Marchal | TaqMan | Spain | Caucasian | H | 98 | 62 | 17 | 108 | 79 | 22 | 29.43 | 1.69 | 0.19 |
| 23 | 2008 | Stevens | TaqMan | USA | Mixed | P | 481 | 518 | 105 | 491 | 493 | 125 | 33.50 | 0.01 | 0.94 |
| 24 | 2009 | Muslumanoglu | PCR-RFLP | Turkey | Caucasian | H | 31 | 16 | 44 | 77 | 45 | 44 | 40.06 | 31.49 | 0.00 |
| 25 | 2010 | Cai | PCR-RFLP | China | Asian | H | 150 | 63 | 4 | 144 | 71 | 5 | 18.41 | 1.21 | 0.27 |
| 27 | 2010 | Wu | PCR-RFLP | China | Asian | P | 138 | 70 | 10 | 287 | 135 | 14 | 18.69 | 0.15 | 0.70 |
| 26 | 2010 | Safarinejad | PCR-RFLP | Iran | Caucasian | P | 90 | 70 | 14 | 158 | 150 | 40 | 33.05 | 0.23 | 0.63 |
Test of heterogeneity and main results of
the meta-analysis
Significant heterogeneity between studies
(P<0.10) was observed in several comparisons, and the data are
listed in Table II. The
random-effects model (R) was chosen in the analysis when the
P-value for the heterogeneity test was <0.10, otherwise the
fixed-effects model (F) was applied.
 | Table II.Main results of the meta-analysis in
codominant, dominant, recessive and alleles models. |
Table II.
Main results of the meta-analysis in
codominant, dominant, recessive and alleles models.
| Genetic models | No. of studies | PC (n) | OR | 95% CI | I2
(%) | Ph | Statistical
model | P-value |
|---|
| Codominant
models | | | | | | | | |
| AC vs. AA | | | | | | | | |
| Total | 9 | 2,454 | 1.04 | 0.93–1.16 | 0 | 0.67 | F | 0.46 |
| PBC DNA | 8 | 2,382 | 1.03 | 0.93–1.15 | 0 | 0.68 | F | 0.55 |
| Caucasian | 5 | 612 | 0.94 | 0.77–1.16 | 0 | 0.68 | F | 0.59 |
| Asian | 2 | 421 | 0.97 | 0.75–1.27 | 0 | 0.39 | F | 0.85 |
| H-based | 4 | 492 | 0.92 | 0.71–1.19 | 0 | 0.60 | F | 0.51 |
| P-based | 4 | 1,562 | 1.04 | 0.91–1.19 | 0 | 0.61 | F | 0.58 |
| HWE | 8 | 2,407 | 1.05 | 0.94–1.17 | 0 | 0.59 | F | 0.42 |
| Advanced
PC | 4 | 395 | 1.12 | 0.87–1.44 | 39.9 | 0.17 | F | 0.40 |
| Localized
PC | 4 | 355 | 0.95 | 0.73–1.24 | 0 | 0.67 | F | 0.69 |
| CC vs. AA | | | | | | | | |
| Total | 9 | 1,568 | 1.03 | 0.79–1.34 | 41.7 | 0.09 | R | 0.84 |
| PBC DNA | 8 | 1,530 | 1.04 | 0.78–1.39 | 48.4 | 0.06 | R | 0.78 |
| Caucasian | 5 | 432 | 1.01 | 0.59–1.73 | 66.4 | 0.02 | R | 0.98 |
| Asian | 2 | 302 | 1.22 | 0.60–2.49 | 0 | 0.41 | F | 0.58 |
| H-based | 4 | 382 | 1.18 | 0.60–2.33 | 58.9 | 0.06 | R | 0.63 |
| P-based | 4 | 952 | 0.88 | 0.70–1.10 | 0 | 0.41 | F | 0.26 |
| HWE | 8 | 1,493 | 0.90 | 0.74–1.09 | 0 | 0.84 | F | 0.27 |
| Advanced
PC | 4 | 233 | 0.78 | 0.50–1.20 | 44.5 | 0.14 | R | 0.26 |
| Localized
PC | 4 | 242 | 1.03 | 0.68–1.54 | 0 | 0.89 | F | 0.90 |
| Dominant model | | | | | | | | |
| CC + AC vs.
AA | | | | | | | | |
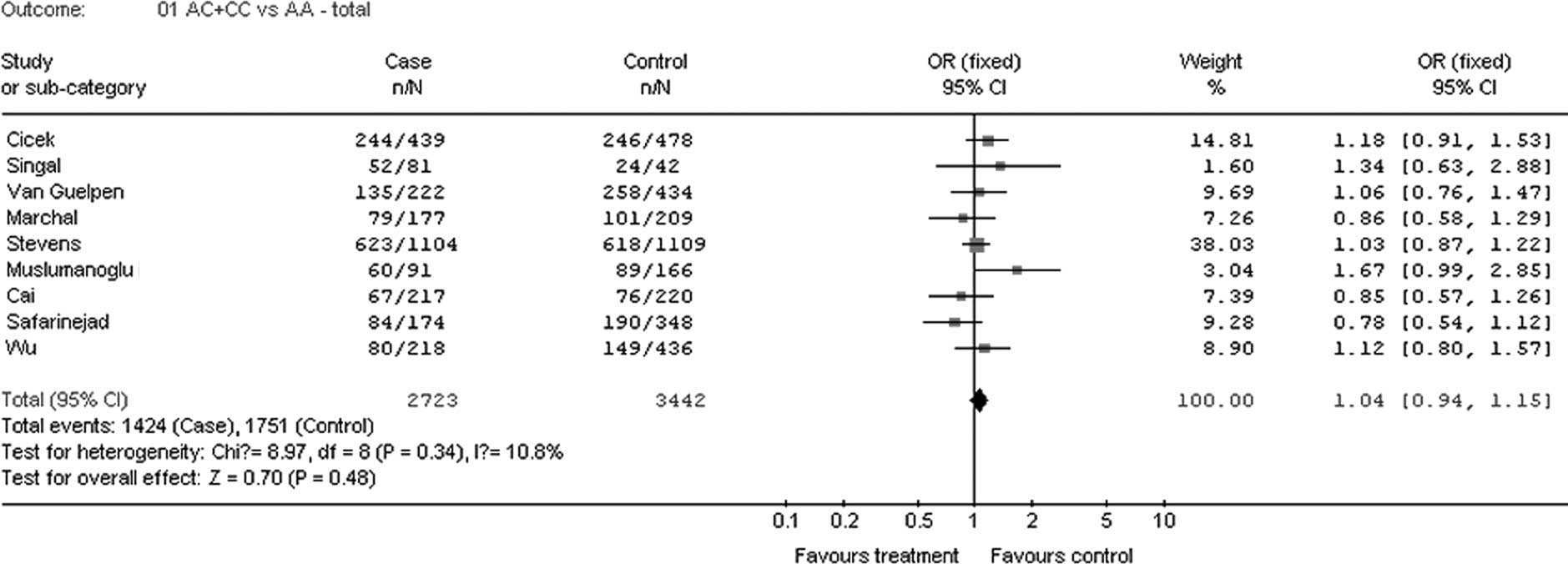
| Total | 9 | 2,723 | 1.04 | 0.94–1.15 | 10.8 | 0.34 | F | 0.48 |
| PBC DNA | 8 | 2,642 | 1.03 | 0.93–1.15 | 19.0 | 0.28 | F | 0.53 |
| Caucasian | 5 | 720 | 0.99 | 0.82–1.20 | 36.7 | 0.18 | F | 0.94 |
| Asian | 2 | 435 | 0.99 | 0.77–1.29 | 6.8 | 0.30 | F | 0.96 |
| H-based | 4 | 566 | 1.02 | 0.81–1.30 | 44.2 | 0.15 | F | 0.84 |
| P-based | 4 | 1,718 | 1.01 | 0.89–1.15 | 0 | 0.48 | F | 0.88 |
| HWE | 8 | 2,632 | 1.02 | 0.92–1.13 | 0 | 0.56 | F | 0.72 |
| Advanced
PC | 4 | 431 | 1.04 | 0.82–1.33 | 49.0 | 0.12 | F | 0.74 |
| Localized
PC | 4 | 400 | 0.97 | 0.75–1.24 | 0 | 0.86 | F | 0.79 |
| Recessive
model | | | | | | | | |
| CC vs. AA +
AC | | | | | | | | |
| Total | 9 | 2,723 | 1.02 | 0.76–1.35 | 53.4 | 0.03 | R | 0.91 |
| PBC DNA | 8 | 2,642 | 1.05 | 0.77–1.42 | 57.5 | 0.02 | R | 0.77 |
| Caucasian | 5 | 720 | 1.00 | 0.57–1.76 | 73.5 | 0.01 | R | 0.99 |
| Asian | 2 | 435 | 1.22 | 0.60–2.47 | 0 | 0.46 | F | 0.58 |
| H-based | 4 | 566 | 1.15 | 0.55–2.41 | 68.7 | 0.02 | R | 0.71 |
| P-based | 4 | 1,718 | 0.86 | 0.69–1.07 | 0 | 0.51 | F | 0.17 |
| HWE | 8 | 2,632 | 0.87 | 0.73–1.04 | 0 | 0.89 | F | 0.14 |
| Advanced
PC | 4 | 431 | 0.71 | 0.47–1.09 | 32.4 | 0.22 | F | 0.12 |
| Localized
PC | 4 | 400 | 1.05 | 0.71–1.56 | 0 | 0.74 | F | 0.80 |
| Allele model | | | | | | | | |
| C vs. A | | | | | | | | |
| Total | 9 | 5,446 | 1.04 | 0.90–1.19 | 57.7 | 0.02 | R | 0.61 |
| PBC DNA | 8 | 5,284 | 1.04 | 0.90–1.20 | 63.0 | 0.01 | R | 0.62 |
| Caucasian | 5 | 1,440 | 1.05 | 0.77–1.43 | 76.2 | 0.00 | R | 0.76 |
| Asian | 2 | 870 | 1.02 | 0.81–1.27 | 24.6 | 0.25 | F | 0.89 |
| H-based | 4 | 1,132 | 1.12 | 0.75–1.69 | 78.0 | 0.00 | R | 0.58 |
| P-based | 4 | 3,436 | 0.98 | 0.89–1.08 | 8.9 | 0.35 | F | 0.62 |
| HWE | 8 | 5,264 | 0.98 | 0.91–1.07 | 0 | 0.62 | F | 0.69 |
| Advanced
PC | 4 | 862 | 0.96 | 0.80–1.15 | 50.8 | 0.11 | F | 0.65 |
| Localized
PC | 4 | 800 | 1.01 | 0.84–1.23 | 0 | 0.97 | F | 0.88 |
The results of the association between the MTHFR
gene A1298C polymorphism and PC risk are also shown in Table II. Overall, when all the qualified
studies were pooled into the meta-analysis, no evidence of
significant association was found between PC risk and MTHFR gene
1298A>C polymorphism in any genetic model (codominant models: CC
vs. AA, OR=1.03, 95% CI 0.79–1.34, P=0.84; AC vs. AA, OR=1.04, 95%
CI 0.93–1.16, P=0.46; dominant model: AC + CC vs. AA, OR=1.04, 95%
CI 0.94–1.15, P=0.48; recessive model: CC vs. AC + AA, OR=1.02, 95%
CI 0.76–1.35, P=0.91; allele model: C vs. A, OR=1.04, 95% CI
0.90–1.19, P=0.61) (Fig. 1).
In the stratified analyses by ethnicity, no
significant results were found for Asian and Caucasian subjects in
the different statistical models (all P>0.05). Moreover,
meta-analyses of studies illustrating advanced and localized PC
were conducted, and these analyses again found no significant
correlations in any type of statistical model (all P>0.05).
Furthermore, insignificant statistical conclusions were found for
hospital- and population-based subjects in various statistical
models in the subgroup analyses according to source of controls
(all P>0.05).
Sensitivity analyses
In the study by Muslumanoglu et al (24), genotype frenquencies of the MTHFR
gene A1298C polymorphism in the control group deviated from HWE
(P=0.00). Sensitivity analyses were carried out by excluding the
above study and no evident changes were found for the pooled ORs.
Similarly, the pooled ORs were not qualitatively influenced after
exclusion of one heavily weighted study by Stevens et al
(23). Sensitivity analyses
suggested that the results of this meta-analysis were stable.
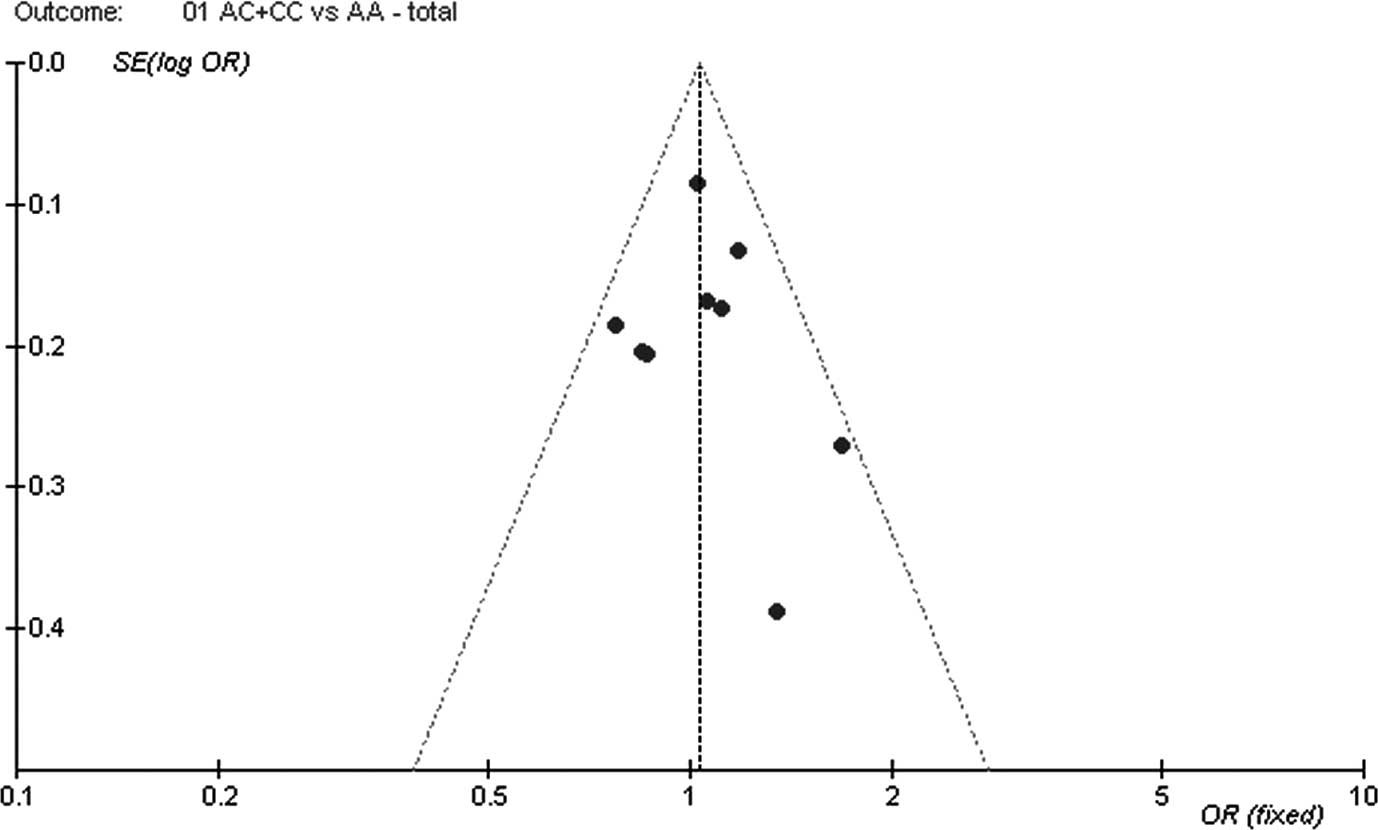
Publication bias
The shape of the funnel plots did not show any
evidence of obvious asymmetry for all genetic models in either
overall or stratified meta-analyses. Subsequently, Begger's funnel
plot, Begger's test and Egger's test were performed to assess the
publication bias of the eligible studies. Still, the results did
not reveal obvious evidence of publication bias (P=0.767 for
Egger's test in the dominant model; Fig. 2).
Discussion
MTHFR is involved in the one-carbon cycle, which is
of importance for nucleotide synthesis and methylation of DNA,
membranes, proteins and lipids. The MTHFR gene A1298C polymorphism,
one of the most popular sites, is associated with a 30% decreased
enzymatic activity without thermolability (28,29).
Thus, the MTHFR gene A1298C polymorphism is considered to produce
the potentially functional site rs1801131, which was extensively
studied.
The published literature regarding the association
between the MTHFR gene A1298C polymorphism and the risk of PC
consists of small studies mainly in Caucasian populations, which
show conflicting findings. No consensus has yet been reached. To
date, there have been two meta-analyses (30,31)
focusing on the association between the two, both of which found
that the MTHFR gene A1298C polymorphism did not contribute to the
susceptibility of PC. However, the two previous meta-analyses
included only a small number of case-control studies, with limited
Caucasian subjects. Therefore, we performed a meta-analysis of all
eligible studies, in order to derive a more conclusive estimation
of the relationship. A total of nine case-control studies were
selected in our meta-analysis, consisting of 2,723 PC cases and
3,442 controls. In the total population, we did not find an
association between the MTHFR gene A1298C polymorphism and PC risk
in the codominant, dominant, recessive and allele models (all
P>0.05).
In the eligible studies, the percentage of the C
allele was 0.1841 (25) and 0.1869
(27) in Asian population, while
it was >0.25% for other populations. Hence, the different
genetic background may have affected the results of the
meta-analysis. In the stratified analysis by ethnicity, no
significant association between the MTHFR gene A1298C polymorphism
and PC risk was found for either Caucasian or Asian populations in
the codominant, dominant, recessive and allele models (all
P>0.05). Furthermore, no statistically significant results were
found between the MTHFR gene A1298C polymorphism and PC development
in the subgroup analyses of HWE, PBC DNA, advanced PC, localized
PC, hospital-based and population-based case control studies (all
P>0.05).
The results of the present study, along with those
of other meta-analyses regarding the MTHFR gene A1298C polymorphism
(32–34), reached the same conclusions, which
found no association between the polymorphism site and the disease.
It may not be uncommon that the results of the funciional study
were not coincident with the epidemiological results. The mentioned
discrepancy may be due to complex genetic background and
multi-genetic interaction (35).
On the other hand, one recent study highlighted that the MTHFR gene
A1298C polymorphism is not associated with the modification of
MTHFR activity (36). As a result,
the specific mechanism of how the MTHFR gene A1298C polymorphism
influences the MTHFR function warrants further investigation.
In conclusion, this meta-analysis suggests that the
MTHFR gene A1298C polymorphism is not associated with prostate
cancer susceptibility in either total or stratified populations.
Furthermore, gene-gene and gene-environment interactions should
also be considered in the analysis, which may contribute to a
better understanding of the possible genetic risk of prostate
carcinoma.
References
|
1.
|
A JemalR SiegelJ XuE WardCancer
statistics, 2010CA Cancer J Clin60277300201010.3322/caac.20073
|
|
2.
|
F BrayR SankilaJ FerlayDM ParkinEstimates
of cancer incidence and mortality in Europe in 1995Eur J
Cancer3899166200210.1016/S0959-8049(01)00350-111750846
|
|
3.
|
AW HsingAP ChokkalingamProstate cancer
epidemiologyFront Biosci1113881413200610.2741/1891
|
|
4.
|
KW JungYJ WonS ParkCancer statistics in
Korea: incidence, mortality and survival in 2005J Korean Med
Sci249951003200910.3346/jkms.2009.24.6.99519949651
|
|
5.
|
JB MasonT LevesqueFolate: effects on
carcinogenesis and the potential for cancer
chemopreventionOncology101727173619968953590
|
|
6.
|
YI KimFolate and carcinogenesis: evidence,
mechanisms, and implicationsJ Nutr
Biochem106688199910.1016/S0955-2863(98)00074-615539274
|
|
7.
|
P GoyetteJS SumnerR MilosHuman
methylenetetrahydrofolate reductase: isolation of cDNA, mapping and
mutation identificationNat Genet7195200199410.1038/ng0694-195
|
|
8.
|
P FrosstHJ BlomR MilosA candidate genetic
risk factor for vascular disease: a common mutation in
methylenetetrahydrofolate reductaseNat
Genet10111113199510.1038/ng0595-111
|
|
9.
|
JR DobosyJL RobertsVX FuDF JarrardThe
expanding role of epigenetics in the development, diagnosis and
treatment of prostate cancer and benign prostatic hyperplasiaJ
Urol177822831200710.1016/j.juro.2006.10.06317296351
|
|
10.
|
TM MurphyAS PerryM LawlerThe emergence of
DNA methylation as a key modulator of aberrant cell death in
prostate cancerEndocr Relat
Cancer151125200810.1677/ERC-07-020818310272
|
|
11.
|
P GoyetteA PaiR MilosGene structure of
human and mouse methylenetetrahydrofolate reductase (MTHFR)Mamm
Genome9652656199810.1007/s0033599008389680386
|
|
12.
|
M KurzawskiK DziewanowskiK KędzierskaA
WajdaJ LapczukM DroździkAssociation of transcription factor 7-like
2 (TCF7L2) gene polymorphism with posttrans-plant diabetes mellitus
in kidney transplant patients medicated with tacrolimusPharmacol
Rep63826833201110.1016/S1734-1140(11)70595-3
|
|
13.
|
JD LeuCY WangHY TsaiIF LinRC ChenYJ
LeeInvolvement of p53 R72P polymorphism in the association of
MDM2-SNP309 with breast cancerOncol Rep2517551763201121479369
|
|
14.
|
JW KimHM ParkYK ChoiSY ChongD OhNK
KimPolymorphisms in genes involved in folate metabolism and plasma
DNA methylation in colorectal cancer patientsOncol
Rep25167172201121109973
|
|
15.
|
MM FungRM SalemMS
LipkowitzMethylenetetrahydrofolate reductase (MTHFR) polymorphism
A1298C (Glu429Ala) predicts decline in renal function over time in
the African-American Study of Kidney Disease and Hypertension
(AASK) Trial and Veterans Affairs Hypertension Cohort (VAHC)Nephrol
Dial TransplantMay2011(E-pub ahead of print)
|
|
16.
|
S DayakarKI GoudTP ReddySP RaoSB
SesikeranM SadhnaniSequence variation of the methylene
tetrahydrofolate reductase gene (677C>T and 1298 A>C) and
traditional risk factors in a South Indian populationGenet Test Mol
BiomarkersJuly2011(E-pub ahead of print)
|
|
17.
|
K SiemianowiczJ GminskiW
GarczorzMethylenetetrahydrofolate reductase gene C677T and A1298C
poly morphisms in patients with small cell and non-small cell lung
cancerOncol Rep1013411344200312883704
|
|
18.
|
H ShenAS NewmannZ
HuMethylenetetrahydrofolate reductase polymorphisms/haplotypes and
risk of gastric cancer: a case-control analysis in ChinaOncol
Rep13355360200515643524
|
|
19.
|
MS CicekNL NockL LiDV ContiG CaseyJS
WitteRelationship between methylenetetrahydrofolate reductase C677T
and A1298C genotypes and haplotypes and prostate cancer risk and
aggressivenessCancer Epidemiol Biomarkers
Prev1313311336200415298954
|
|
20.
|
R SingalL FerdinandPM DasIM ReisJJ
SchlesselmanPolymorphisms in the methylenetetrahydrofolate
reductase gene and prostate cancer riskInt J
Oncol2514651471200415492840
|
|
21.
|
BR Van GuelpenSM WirénAR BerghG HallmansPE
StattinJ HultdinPolymorphisms of methylenetetrahydrofolate
reductase and the risk of prostate cancer: a nested case-control
studyEur J Cancer Prev154650200616374229
|
|
22.
|
C MarchalM RedondoA Reyes-EngelAssociation
between polymorphisms of folate-metabolizing enzymes and risk of
prostate cancerEur J Surg
Oncol34805810200810.1016/j.ejso.2007.09.00817967524
|
|
23.
|
VL StevensC RodriguezJ SunJT TalbotMJ
ThunEE CalleNo association of single nucleotide polymorphisms in
one-carbon metabolism genes with prostate cancer riskCancer
Epidemiol Biomarkers
Prev1736123614200810.1158/1055-9965.EPI-08-078919064578
|
|
24.
|
MH MuslumanogluE TepeliS DemirThe analysis
of the relationship between A1298C and C677T polymorphisms of the
MTHFR gene with prostate cancer in Eskisehir populationGenet Test
Mol Biomarkers13641645200910.1089/gtmb.2009.004619814618
|
|
25.
|
D CaiL NingC PanAssociation of
polymorphisms in folate metabolic genes and prostate cancer risk: a
case-control study in a Chinese populationJ
Genet89263267201010.1007/s12041-010-0037-720861582
|
|
26.
|
MR SafarinejadN ShafieiS
SafarinejadRelationship between three polymorphisms of
methylenetetrahydrofolate reductase (MTHFR C677T, A1298C, and
G1793A) gene and risk of prostate cancer: a case-control
studyProstate7016451657201010.1002/pros.21200
|
|
27.
|
HC WuCH ChangRY TsaiSignificant
association of methylenetetrahydrofolate reductase single
nucleotide polymorphisms with prostate cancer susceptibility in
TaiwanAnticancer Res30357335772010
|
|
28.
|
IS WeisbergPF JacquesJ SelhubThe
1298A-->C polymorphism in methylenetetrahydrofolate reductase
(MTHFR): in vitro expression and association with
homocysteineAtherosclerosis1564094152001
|
|
29.
|
NM van der PutF GabreëlsEM StevensA second
common mutation in the methylenetetrahydrofolate reductase gene: an
additional risk factor for neural-tube defects?Am J Hum
Genet621044105119989545395
|
|
30.
|
JL BaiMH ZhengX XiaM Ter-MinassianYP ChenF
ChenMTHFR C677T polymorphism contributes to prostate cancer risk
among Caucasians: a meta-analysis of 3511 cases and 2762
controlsEur J
Cancer4514431449200910.1016/j.ejca.2009.01.02019223177
|
|
31.
|
SM CollinC MetcalfeL ZuccoloAssociation of
folate-pathway gene polymorphisms with the risk of prostate cancer:
a population-based nested case-control study, systematic review,
and meta-analysisCancer Epidemiol Biomarkers
Prev1825282539200910.1158/1055-9965.EPI-09-022319706844
|
|
32.
|
X DongJ WuP LiangJ LiL YuanX
LiuMethylenetetrahydrofolate reductase C677T and A1298C
polymorphisms and gastric cancer: a meta-analysisArch Med
Res41125133201010.1016/j.arcmed.2010.01.00120470942
|
|
33.
|
LX QiuJ ZhangWH LiLack of association
between me thylenetetrahydrofolate reductase gene A1298C
polymorphism and breast cancer susceptibilityMol Biol
Rep3822952299201110.1007/s11033-010-0361-221052845
|
|
34.
|
B WeiZ XuJ RuanMTHFR 677C>T and
1298A>C polymorphisms and male infertility risk: a
meta-analysisMol Biol RepJune2011(E-pub ahead of print)
|
|
35.
|
JN HirschhornK LohmuellerE ByrneK
HirschhornA comprehensive review of genetic association
studiesGenet Med44561200210.1097/00125817-200203000-00002
|
|
36.
|
KJ LieversGH BoersP VerhoefA second common
variant in the methylenetetrahydrofolate reductase (MTHFR) gene and
its relationship to MTHFR enzyme activity, homocysteine, and
cardiovascular disease riskJ Mol Med
(Berl)79522528200110.1007/s001090100253
|