Introduction
Lung cancer is the leading cause of tumor-related
mortality throughout the world, and adenocarcinoma has surpassed
squamous cell carcinoma as the most frequent type of lung cancer
(1). Lung adenocarcinoma is well
known for its ability to involve metastatic disease even at the
early stages of tumor growth, which generally results in treatment
failure. Due to the early acquisition of a metastatic phenotype and
the associated poor prognosis of lung adenocarcinoma, investigation
into the molecular mechanisms responsible for metastasis may lead
to the development of new treatments for these patients.
Regional lymph node metastasis is a major route for
tumor metastasis in non-small cell lung cancer (NSCLC), which is
also one of the most significant prognostic indicators for NSCLC
patients. Lymphangiogenesis is considered to be the initial step
and key event of lymphatic and regional lymph node metastasis, but
only peritumoral lymphangiogenesis is functional (2,3). A
number of studies have provided support for the contribution of
vascular endothelial growth factor (VEGF)-C and VEGF-D, and their
respective receptors, including vascular endothelial growth factor
receptor (VEGFR)-2 and VEGFR-3, in tumor-induced lymphangiogenesis
(4,5). Not only tumor cells, but also
inflammatory cells in tumor stroma including tumor-associated
macrophages (TAMs), express VEGF-C and/or VEGF-D, and induce
peritumoral lymphangiogenesis and lymph node metastasis (6–8).
Lymphatic microvessel density (LMVD) is a significant indicator of
tumor lymphangiogenesis. A number of studies have demonstrated that
LMVD is an independent prognostic factor in NSCLC (9–12).
However, no significant association between LMVD and lymph node
metastasis in NSCLC has been reported (13). Thus, the correlation of
lymphangiogenesis with lymph node metastasis and patient survival
is controversial and remains to be clarified. Moreover, no study
has examined whether the prognosis of patients with lung
adenocarcinoma is correlated with the peritumoral LMVD rather than
the intratumoral LMVD.
This study investigated the immunohistochemically
determined count of LMVD and found that peritumoral
lymphangiogenesis is related to poor prognosis in patients with
lung adenocarcinoma.
Materials and methods
Patients and tissue samples
A total of 65 patients with lung adenocarcinoma (38
male and 27 females; mean age, 51.5 years; age range, 32–76 years)
who underwent either lobectomy or pneumonectomy at Wuhan General
Hospital of Guangzhou Command, People’s Liberation Army, China,
were investigated. The patients underwent tumor resection between
2003 and 2006. None of the patients received any preoperative
chemotherapy or radiotherapy. The lesions of the 65 patients were
staged according to the UICC 2010 pTNM classification (7th
edition), and stage I, II, III and IV lesions were present in 14,
24, 25 and 2 patients, respectively. Histologically, according to
the new classification proposed for lung adenocarcinoma by the
IASLC/ATS/ERS 2011 (14), 16
tumors were graded as favorably differentiated (non-mucinous
lepidic), 29 as intermediately differentiated (papillary and
acinar) and 20 as poorly differentiated (solid and micropapillary)
adenocarcinoma. Lymph node metastasis occurred in 36 patients,
while the other 29 patients had no lymph node metastasis. All
patients accepted complete removal of the tumor and were treated by
standardized therapy following surgery. All patients were followed
up and their outcomes were known. The clinicopathological
parameters of those patients with lung adenocarcinoma are shown in
Table I.
 | Table I.Correlation between D2-40 positive
LMVD and clinicopathological features (mean ± SD). |
Table I.
Correlation between D2-40 positive
LMVD and clinicopathological features (mean ± SD).
| Clinicopathological
features | n | Intratumoral
LMVD | Peritumoral LMVD |
|---|
| Gender | | | |
| Male | 38 | 4.02±2.11 | 11.02±10.29 |
| Female | 27 | 3.95±1.73 | 12.81±11.23 |
| Age | | | |
| ≥55 years | 39 | 3.98±2.10 | 12.98±11.04 |
| <55 years | 26 | 4.15±1.26 | 10.99±9.61 |
| Differentiation | | | |
| Favorable and
intermediate | 45 | 4.13±1.96 | 11.95±10.43 |
| Poor | 20 | 3.98±2.16 | 10.90±10.17 |
| Lymphatic
metastasis | | | |
| Positive | 36 | 3.12±2.56 | 14.98±5.60a |
| Negative | 29 | 4.86±2.27 | 9.91±2.16 |
| pTNM stage | | | |
| I and II | 38 | 4.31±2.72 | 13.54±10.32a |
| III and IV | 27 | 3.45±2.33 | 10.09±6.11 |
LMVD by immunohistochemistry
D2-40 was used as the immunohistochemical marker for
lymphatic endothelial cells (LECs) for the evaluation of LMVD
(15). Resected tissue specimens
were fixed in formalin, embedded in paraffin, and cut into 5-μm
serial sections. These sections were then deparaffinized in xylene,
and rehydrated through graded alcohol and deionized water. The
slides were immunostained with a mouse monoclonal antibody D2-40
(1:200; Signet Laboratories, Dedham, MA, USA) at 4°C overnight, and
subsequently exposed to a biotinylated secondary antibody for 20
min, followed by treatment with streptavidin peroxidase. For color
development, the slides were stained with 3,3′diaminobenzidine
(DAB), and counterstained with hematoxylin and eosin (H&E). A
reddish-brown precipitate in the cytoplasm of LECs indicated a
positive reaction.
Following scanning of the immunostained sections at
low magnification (x100), the regions with the greatest number of
distinctly highlighted lymphatic foci (hot spots) were selected by
two observers at the same time. The two observers then
independently evaluated the slides for the LMVD count using x400
magnification (field, 0.03 mm2) in three regions without
knowledge of the tumor status and the stains used. The intratumoral
compartment was the area encompassing cancer glands in the H&E
section. The peritumoral compartment was defined as the area around
the intratumoral compartment (a 1-mm band including the edge of the
tumor and just outside the tumor). Single immunoreactive
endothelial cells or endothelial cell clusters separate from other
microvessels were counted as a vessel according to previous
procedures (6).
Statistical analysis
The intratumoral or peritumoral LMVD was expressed
as the mean ± SD. Statistical differences between the means were
analyzed by the independent-samples t-test. On the basis of LMVD,
patients were classified into the high or low peritumoral LMVD
group, and the overall survival rate was compared between the two
groups. Overall survival time was calculated from the date of
surgery until mortality or, if the patient was still alive, until
the last follow-up visit. Mortality from any cause was considered
for overall survival. Two overall survival rates were calculated by
the Kaplan-Meier method and compared by the log-rank test. Each
prognostic factor was evaluated with regard to survival in a
multivariate analysis by the Cox proportional hazards regression
model. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
with SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
D2-40-positive LMVD in lung
adenocarcinoma
D2-40-positive lymph vessels were observed in all 65
lung adenocarcinoma samples examined. Staining of lymphatic vessels
with characteristic irregular morphology, empty lumina without red
blood cells and thin endothelium were strong and distinct when such
lymphatic vessels were present. Vessels containing red blood cells
were not stained. In lung adenocarcinoma, D2-40-positive vessels
were detected more in the peritumoral stroma (Fig. 1A and B) than in the intratumoral
compartment (Fig. 1C and D). The
count of D2-40-positive peritumoral LMVD was 11.56±10.73, which was
higher than that of the intratumoral LMVD (3.96±1.15)
(P<0.001).
Correlation of D2-40-positive LMVD with
clinicopathological features
Table I shows the
correlation between peritumoral or intratumoral LMVD and
clinicopathological features. Peritumoral LMVD was significantly
associated with lymph node metastasis (P=0.003) and pTNM staging
(P=0.046), but not with gender, age and differentiation.
Intratumoral LMVD did not correlate with any of the
clinicopathological features.
Prognostic significance of D2-40-positive
LMVD
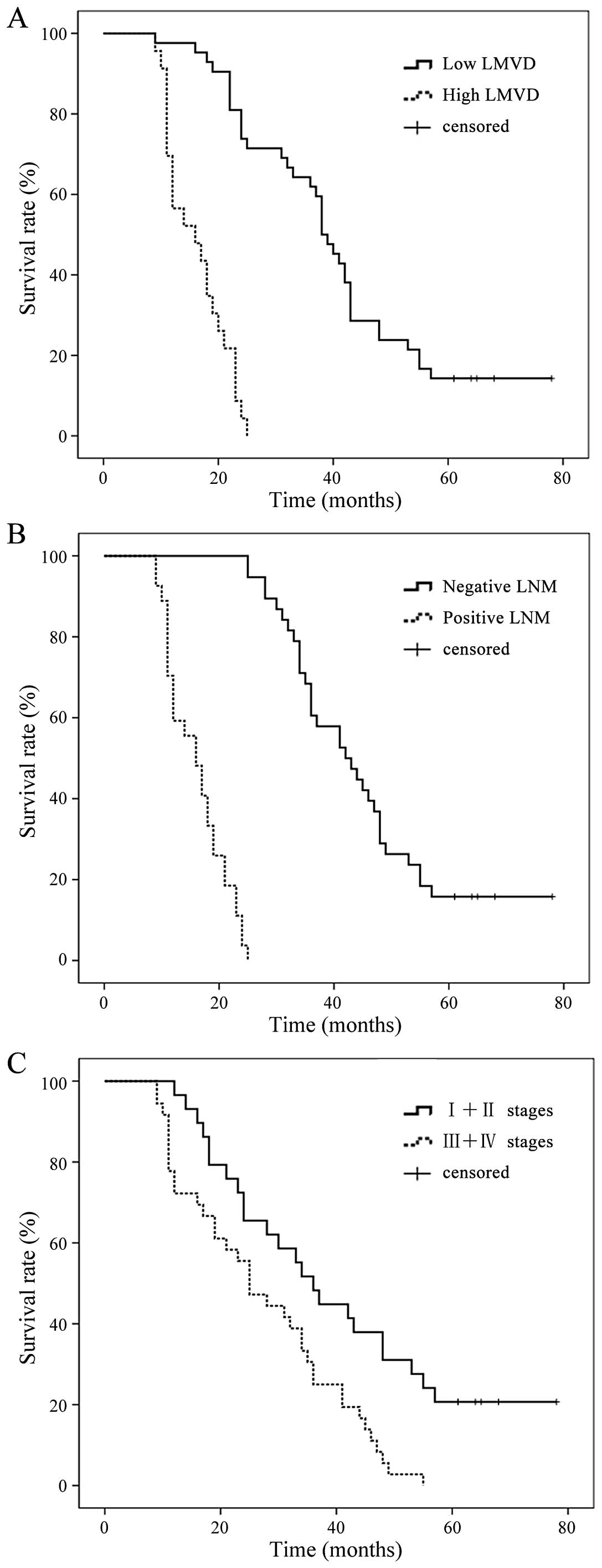
To assess the prognostic significance of
D2-40-positive peritumoral LMVD, patients were classified into two
groups on the basis of peritumoral LMVD. A median value of 11 was
used as the cut-off in peritumoral LMVD. The median overall
survival times for the patients with peritumoral LMVD ≤11 or >11
were 31 and 13 months, respectively, demonstrating a significant
difference in overall survival (P=0.005) (Fig. 2A). Meanwhile, there was a
significant difference between the survival rate curve in the
positive and negative lymphatic metastasis groups (Fig. 2B) (P=0.002). Fig. 2C shows the significant difference
between survival rate in stages I and II and stages III and IV
(P=0.000).
Multivariate analysis
Intratumoral LMVD, peritumoral LMVD and other
factors, including gender, age, differentiation, lymph node
metastasis and pTNM staging, were analyzed by Cox proportional
hazards regression models in all 65 patients with lung
adenocarcinoma. Peritumoral LMVD, as well as lymph node metastasis
and pTNM staging, were independent prognostic factors for overall
survival (Table II).
 | Table II.Multivariate analysis of various
prognostic factors in patients. |
Table II.
Multivariate analysis of various
prognostic factors in patients.
| Factor | P-value | HR | 95% CI |
|---|
| Gender | 0.169 | 0.620 | 0.312–1.226 |
| Age | 0.558 | 1.008 | 0.982–1.033 |
| Differentiation | 0.655 | 0.852 | 0.422–1.720 |
| Lymphatic
metastasis | 0.040a | 0.470 | 0.229–0.966 |
| pTNM stages | 0.000a | 24.199 | 4.552–128.6 |
| Intratumoral
LMVD | 0.761 | 0.955 | 0.710–1.285 |
| Peritumoral LMVD | 0.002a | 1.123 | 1.042–1.210 |
Discussion
Our data revealed that D2-40 was the most specific
lymphatic endothelial marker for the detection of
lymphangiogenesis. In recent years, the discovery of LEC markers
has facilitated detailed analyses of the nature and structural
organization of lymphatic vessels and their growth
(lymphangiogenesis). The understanding of the molecular mechanisms
of lymphangiogenesis and the elucidation of the development of
normal and pathological tissues are expected to lead to the
development of therapy for intractable diseases, including
malignant tumors and lymphadema. Common LEC markers include
VEGFR-3, lymphatic vessel hyaluronan receptor-1 (LYVE-1), Prox-1
and podoplanin (16). Among these,
podoplanin is mainly expressed in LECs, keratinocytes, choroid
plexus epithelial cells, alveolar cells and few tumor cells. To
date no research has reported the assessment of podoplanin in blood
capillary. Furthermore, D2-40 is a sialoglycoprotein separated from
fetal testiculoma or germinoma, which can be used to detect
podoplanin (17). We used all the
abovementioned antibodies to detect lymphatic vessels in the
preliminary experiment, and the results demonstrated that no
antibody was expressed in 100% of the LECs (data not shown), and
D2-40 was the most specific antibody for the detection, while
VEGFR-3 was the least. D2-40 can also be detected in tumor cells,
stromal fibroblasts or myofibroblasts (18). In NSCLC, D2-40 immunoreactivity in
tumor cells can be used to distinguish between adenocarcinoma and
squamous cell carcinoma (19).
Therefore, H&E staining combined with a D2-40-positive signal
was considered helpful in determining lymphangiogenesis in lung
adenocarcinoma.
The data revealed that D2-40-positive peritumoral
LMVD in lung adenocarcinoma was higher than intratumoral LMVD. The
correlation between LMVD and poor prognosis of tumor patients has
been identified in breast cancer, prostate adenocarcinoma, colon
cancer, melanoma, NSCLC and oral squamous cell carcinoma (20–24).
However, the correlation between LMVD status (intratumoral or
peritumoral) and tumor prognosis has not yet been fully clarified.
Several studies have demonstrated that functional lymphatics
existing in the tumor parenchyma are sufficient for lymphatic
metastasis; therefore, high intratumoral LMVD is essential for the
metastatic spread and prognosis in squamous cell carcinomas of the
head and neck region (25,26). In renal cell carcinoma,
intratumoral lymphatics were associated with tumor aggressiveness,
and patients with intratumoral lymphatics were found to have poor
prognosis (27). However,
additional studies demonstrated that intratumoral lymphatic vessels
may not be completely functional, as these vessels collapse under
high intratumoral pressure (28).
We favor the view that local lymphatic vessels at the tumor margin
are more vital to the spread of tumor cells. This is achieved
through the process of vessel sprouting under the effect of
interstitial fluid hypertension and tumor or stroma-secreted
VEGF-C/D (29,30). In gastric cancer, increased
peritumoral LMVD, but not intratumoral LMVD, was significantly
associated with the VEGF-C/-D/VEGFR-3 system, and could be an
independent risk factor for lymph node metastasis and a prognostic
factor (31). In endometrial
carcinoma, peritumoral LMVD was also an independent prognostic
factor for progression-free survival and overall survival (32). However, the correlation between
intratumoral and peritumoral lymphangiogenesis and the survival of
lung adenocarcinoma patients remains unknown.
In the present study, we found that peritumoral, but
not intratumoral lymphangiogenesis, played a significant role in
the progression and metastasis of lung adenocarcinoma. In a
previous study, high LMVD, induced by VEGF-C or VEGF-D expression
in cancer cells, was found to be a good indicator of lymphatic
metastases and lymphatic vessel invasion in lung adenocarcinoma
(33). In the present study, the
data did not demonstrate a positive correlation between
intratumoral lymphangiogenesis in lung adenocarcinoma and any
clinicopathological parameters; therefore, intratumoral LMVD was
not a prognostic factor. It was presumed that intratumoral LMVD may
be non-functional and may play only a minor role in primary tumor
dissemination. A recent study revealed that there was no
significant association between peritumoral LMVD and
clinicopathological parameters, including lymphatic vessel
invasion, lymph node metastasis and survival in lung adenocarcinoma
(19). However, in the present
study, a positive correlation was found between peritumoral LMVD
and lymphatic metastasis or pTNM staging, and high peritumoral LMVD
reduced the overall survival of patients. Therefore, peritumoral
LMVD, lymphatic metastasis and pTNM stage may be independent risk
factors for prognosis. These results demonstrate that peritumoral,
but not intratumoral LMVD, may predict the prognosis of lung
adenocarcinoma. The contradicting results regarding the role of
peritumoral LMVD in lung adenocarcinoma may be due to differences
in patient selection, sample number, methodology and
immunohistochemical markers.
In conclusion, D2-40-positive peritumoral LMVD may
be an independent prognostic factor for lung adenocarcinoma.
Detecting this indicator may predict patient prognosis in lung
adenocarcinoma, while it has been suggested that reducing
peritumoral lymphangiogenesis could antagonize the metastasis of
lung adenocarcinoma. However, this assumption is based only on
retrospective analysis of a small case series, and further
experimental and clinical support with a larger number of cases is
required.
Acknowledgements
We thank Manli Qi (Department of
Pathology, Wuhan General Hospital of Guangzhou Command, People’s
Liberation Army, Wuhan, China) for her excellent technical
assistance. This study was supported by the Natural Science
Foundation of Hubei Province, China (no. 2010CDB09204) and the
Youth Dawn Plan of Science and Technology in Wuhan, China (no.
201150431137).
References
|
1.
|
Tiseo M, Bartolotti M, Gelsomino F and
Ardizzoni A: First-line treatment in advanced non-small-cell lung
cancer: the emerging role of the histologic subtype. Expert Rev
Anticancer Ther. 9:425–435. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Alitalo K, Tammela T and Petrova TV:
Lymphangiogenesis in development and human disease. Nature.
438:946–953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Stacker SA, Farnsworth RH, Karnezis T, et
al: Molecular pathways for lymphangiogenesis and their role in
human disease. Novartis Found Symp. 281:38–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mandriota SJ, Jussila L, Jeltsch M, et al:
Vascular endothelial growth factor-C-mediated lymphangiogenesis
promotes tumour metastasis. EMBO J. 20:672–682. 2001. View Article : Google Scholar
|
|
5.
|
Stacker SA, Caesar C, Baldwin ME, et al:
Vascular endothelial growth factor-D promotes the metastatic spread
of cancer via the lymphatics. Nat Med. 7:186–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Schoppmann SF, Birner P, Stöckl J, et al:
Tumor-associated macrophages express lymphatic endothelial growth
factors and are related to peritumoral lymphangiogenesis. Am J
Pathol. 161:947–956. 2002. View Article : Google Scholar
|
|
7.
|
Schoppmann SF, Fenzl A, Nagy K, et al:
VEGF-C expressing tumor-associated macrophages in lymph node
positive breast cancer: impact on lymphangiogenesis and survival.
Surgery. 139:839–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zhang B, Wang J, Gao J, et al:
Alternatively activated RAW264.7 macrophages enhance tumor
lymphangiogenesis in mouse lung adenocarcinoma. J Cell Biochem.
107:134–143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Renyi-Vamos F, Tovari J, Fillinger J, et
al: Lymphangiogenesis correlates with lymph node metastasis,
prognosis, and angiogenic phenotype in human non-small cell lung
cancer. Clin Cancer Res. 11:7344–7353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Takanami I: Lymphatic microvessel density
using D2-40 is associated with nodal metastasis in non-small cell
lung cancer. Oncol Rep. 15:437–442. 2006.PubMed/NCBI
|
|
11.
|
Kadota K, Huang CL, Liu D, et al: The
clinical significance of lymphangiogenesis and angiogenesis in
non-small cell lung cancer patients. Eur J Cancer.
44:105710–105767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Iwakiri S, Nagai S, Katakura H, et al:
D2-40-positive lymphatic vessel density is a poor prognostic factor
in squamous cell carcinoma of the lung. Ann Surg Oncol.
16:1678–1685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Faoro L, Hutto JY, Salgia R, et al:
Lymphatic vessel density is not associated with lymph node
metastasis in non-small cell lung carcinoma. Arch Pathol Lab Med.
132:1882–1888. 2008.PubMed/NCBI
|
|
14.
|
Travis WD, Brambilla E, Noguchi M, et al:
International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society International
Multidisciplinary Classification of lung adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011. View Article : Google Scholar
|
|
15.
|
Kahn HJ and Marks A: A new monoclonal
antibody, D2-40, for detection of lymphatic invasion in primary
tumors. Lab Invest. 82:1255–1257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kato S, Shimoda H, Ji RC and Miura M:
Lymphangiogenesis and expression of specific molecules as lymphatic
endothelial cell markers. Anat Sci Int. 81:71–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Braun M, Wardelmann E, Debald M, et al:
Detection of lymphovascular invasion in vulvar cancer by D2-40
(podoplanin) as a predictor for inguinal lymph node metastases.
Onkologie. 32:732–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kitano H, Kageyama S, Hewitt SM, et al:
Podoplanin expression in cancerous stroma induces lymphangiogenesis
and predicts lymphatic spread and patient survival. Arch Pathol Lab
Med. 134:1520–1527. 2010.PubMed/NCBI
|
|
19.
|
Min KH, Park SJ, Lee KS, et al: Clinical
usefulness of D2-40 in non-small cell lung cancer. Lung. 189:57–63.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Raica M, Cimpean AM, Ceausu R and Ribatti
D: Lymphatic microvessel density, VEGF-C, and VEGFR-3 expression in
different molecular types of breast cancer. Anticancer Res.
31:1757–1764. 2011.PubMed/NCBI
|
|
21.
|
Kim HS, Sung W, Lee S, Chang SG and Park
YK: Lymphatic vessel densities of lymph node-negative prostate
adenocarcinoma in Korea. Pathol Res Pract. 205:249–254. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Saad RS, Kordunsky L, Liu YL, Denning KL,
Kandil HA and Silverman JF: Lymphatic microvessel density as
prognostic marker in colorectal cancer. Mod Pathol. 19:1317–1323.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Valencak J, Heere-Ress E, Kopp T,
Schoppmann SF, Kittler H and Pehamberger H: Selective
immunohistochemical staining shows significant prognostic influence
of lymphatic and blood vessels in patients with malignant melanoma.
Eur J Cancer. 40:358–364. 2004. View Article : Google Scholar
|
|
24.
|
Ali MA: Lymphatic microvessel density and
the expression of lymphangiogenic factors in oral squamous cell
carcinoma. Med Princ Pract. 17:486–492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Dadras SS, Paul T, Bertoncini J, et al:
Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous
melanoma metastasis and survival. Am J Pathol. 162:1951–1960. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Maula SM, Luukkaa M, Grénman R, Jackson D,
Jalkanen S and Ristamäki R: Intratumoral lymphatics are essential
for the metastatic spread and prognosis in squamous cell carcinomas
of the head and neck region. Cancer Res. 63:1920–1926. 2003.
|
|
27.
|
Horiguchi A, Ito K, Sumitomo M, Kimura F,
Asano T and Hayakawa M: Intratumoral lymphatics and lymphatic
invasion are associated with tumor aggressiveness and poor
prognosis in renal cell carcinoma. Urology. 71:928–932. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Padera TP, Stoll BR, Tooredman JB, Capen
D, Di Tomaso E and Jain RK: Pathology: cancer cells compress
intratumour vessels. Nature. 427:6952004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Padera TP, Kadambi A, Di Tomaso E, et al:
Lymphatic metastasis in the absence of functional intratumor
lymphatics. Science. 296:1883–1886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ji RC: Lymphatic endothelial cells, tumor
lymphangiogenesis and metastasis: new insights into intratumoral
and peritumoral lymphatics. Cancer Metastasis Rev. 25:677–694.
2006.PubMed/NCBI
|
|
31.
|
Wang XL, Fang JP, Tang RY and Chen XM:
Different significance between intratumoral and peritumoral
lymphatic vessel density in gastric cancer: a retrospective study
of 123 cases. BMC Cancer. 10:2992010. View Article : Google Scholar
|
|
32.
|
Gao Y, Liu Z, Gao F and Meng XY: High
density of peritumoral lymphatic vessels is a potential prognostic
marker of endometrial carcinoma: a clinical immunohistochemical
method study. BMC Cancer. 10:1312010. View Article : Google Scholar
|
|
33.
|
Adachi Y, Nakamura H, Kitamura Y, et al:
Lymphatic vessel density in pulmonary adenocarcinoma
immunohistochemically evaluated with anti-podoplanin or anti-D2-40
antibody is correlated with lymphatic invasion or lymph node
metastases. Pathol Int. 57:171–177. 2007. View Article : Google Scholar
|