Introduction
Cancer cell development and survival is a
multifactoral process, involving the genetic mutation of normal
cells as well as physiological changes within both cancer cells and
the defense mechanisms of the body (1,2).
Tumor-infiltrating lymphocytes (TILs) are regarded as a reflection
of the tumor-related immune response (3) and are recognized as principal
effectors of the local antitumor immune response. However, during
the neoplastic process, tumor cells acquire immunotolerance and
thereby evade tumor immunity through several specific immune
evasion strategies. Tumor-induced immune suppression is caused by
numerous mechanisms, many of which involve the accumulation of
immune-suppressive infiltrates in the tumor microenvironment
(4,5). One of the most potent and
well-studied suppressive phenotypes found in the tumor
microenvironment is the regulatory subpopulation among
CD4+ cells (Treg cells), constitutively expressing high
levels of CD25, CTLA-4, GITR and Foxp3, displaying anergy when
stimulated by T-cell receptor cross-linking in vitro, and
actively inhibiting CD4+CD25−T cells,
CD8+ T cells, dendritic cells, natural killer cells and
natural killer T and B cells in a cell to cell contact and
dose-dependent manner (6,7). Increasing evidence shows that Treg
cells play an important role in immune evasion mechanisms employed
by cancer (8–10). Tumors may differentiate, expand,
recruit and activate Treg (tumor Treg) cells via multiple
mechanisms and potently abrogate antitumor immunity (11). These recent advances in the
understanding of the mechanisms responsible for tumor progression
suggest the possibility to control cancer growth, not only through
chemotherapy-induced cancer cell destruction, but also by
stimulating anticancer immunity. Diverse forms of immunizations
have been suggested to have the potential to completely eradicate
cancer (12).
In addition to the existence of endogenous antitumor
molecules, several agents capable of stimulating the anticancer
immunity have also been isolated from plants. Among the great
number of natural agents derived from plants which are potentially
useful for application in the complementary therapy of cancer,
resveratrol has gained attention since it has been shown to inhibit
cellular events associated with tumor initiation, promotion and
progression in various types of solid tumors (13,14)
and to enhance immune responses in mice by promoting production of
Th1 cytokines, such as interleukin (IL)-12 and interferon (IFN)-γ,
and by enhancing lymphocyte proliferation and IL-2 production
(15). In the case of breast
cancer, resveratrol has also been reported to inhibit the in
vitro growth of a number of human and mouse breast cancer cell
lines which are both estrogen receptor (ER)-positive and
ER-negative (16). Yet, exposure
to high doses of resveratrol is required to induce chemopreventive
and chemotherapeutic properties against the tumor itself, and the
biological activity of resveratrol is limited by its
photosensitivity and metabolic instability.
Our previous study was undertaken to design and
synthesize analogues of resveratrol with potent activity (17) and we demonstrated that four
synthetic resveratrol analogues (HS-1784, -1792, -1791 and -1793)
displayed stronger antitumor effects than resveratrol in most
cancer cells tested, including the MCF-7 human breast
adenocarcinoma cell line (18). A
resveratrol analogue, 4-(6-hydroxy-2-naphthyl)-1,3-benzenediol
(HS-1793), particularly overcomes the resistance conferred by Bcl-2
by inducing apoptosis. However, considerable uncertainty remains in
regards to the effect of HS-1793 on tumor immunity. Meanwhile, it
was reported that immunomodulatory and anticancer properties can
conceivably be controlled by the suppression of the Treg cell
population, which makes the peritumoral microenvironment
unfavorable to the tumor and eventually results in growth
inhibition of tumor cells (19).
The present study was undertaken to examine whether HS-1793
exhibits a direct effect on immune responses by enhancing
lymphocyte proliferation or an immunomodulating effect by inducing
changes in the Treg cell population in tumor-bearing mice.
Materials and methods
Preparation of the resveratrol analogue
HS-1793
To obtain HS-1793, the stilbene double bond present
in resveratrol was substituted with a naphthalene ring as
previously described (17,18). A stock solution of HS-1793 was made
in absolute ethanol at 10 mM and stored at −20°C. Working dilutions
(0.3, 0.6, 1.3 and 2.5 μM), at which no toxic effect had been
observed, were directly made in the tissue culture medium. The
control vehicle used was the tissue culture medium containing
amounts of ethanol equivalent to those present in HS-1793.
Animals and cells
All experiments were carried out on 6-week-old
female C3H/He mice obtained from Central Lab. Animal, Inc. (Seoul,
Korea). The colony was maintained under controlled conditions of
temperature (19–25°C), humidity (40–60%) and a 12-h light-dark
cycle with the light intensity of 150–300 Lux. The animals were
housed in sanitized polycarbonate cages (200 width × 260 length ×
130 height). They had free access to standard mouse food and water.
All animals were rasied under SPF condition at the Korea Institute
of Toxicology, Hospital of Dong-A University according to the Good
Laboratory Practices (GLP) OECD guidelines. All animal procedures
were performed according to approved protocols (approval no.
DIACUC-09-24) from the Institutional Animal Care and Use Committee
(IACUC) of Dong-A University, and in accordance with
recommendations for the proper use and care of laboratory animals.
FM3A murine breast cancer cells originating from the mammary gland
of the C3H/He mouse and cells were cultured in RPMI-1640 medium
(Invitrogen, Carlsbad, CA, USA) supplemented with 2 mM L-glutamine,
100 U/ml penicillin, 100 mg/ml streptomycin and 10%
heat-inactivated fetal bovine serum (FBS; Invitrogen) at 37°C in a
humidified, 5% CO2 atmosphere. Cells were used for
experiments in the log phase of growth. For the preparation of
tumor-bearing mice, FM3A cells in logarithmic growth phase
(2×106 cells/50 μl saline) were inoculated
subcutaneously in the right flank of female C3H/He mice. When the
tumor grew to a size of ∼10 mm in diameter (∼4 weeks), the spleen
was aseptically removed. A single-cell suspension was prepared by
gently teasing the cells through a sterile stainless steel screen
and the erythrocytes were lysed at room temperature using ACK lysis
buffer (NH4Cl, KHCO3 and Na2EDTA).
The isolated splenocytes were suspended in complete medium
(RPMI-1640 supplemented with 10% FBS, 50 μM 2-mercaptoethanol, 100
U/ml penicillin and 100 μg/ml streptomycin) and cultured at 37°C in
a humidified, 5% CO2 atmosphere.
Lymphocyte proliferation assay
Lymphocyte proliferation was determined by BrdU
(5-bromo-2-deoxyuridine) incorporation assay using a cell
suspension at 5×105 cells/well in flat-bottom 96-well
microculture plates. The cells were cultured for 48 h and further
incubated for 24 h in the presence of 10 μl of the BrdU solution in
RPMI medium (1:100 diluent). The BrdU incorporation was measured
using the Cell Proliferation ELISA BrdU kit (Roche
Diagnostics-Applied Science, Mannheim, German), following the
supplier’s specifications.
Lymphocyte subpopulation analysis
Lymphocyte subpopulations were determined by flow
cytometric analysis. The freshly prepared splenocytes were washed
three times with ice cold PBS-containing 0.1% FBS and then stained
with appropriately diluted labeled rat anti-mouse antibodies
[anti-CD4 FITC (BD Biosciences Pharmingen), anti-CD8 PE (BD
Biosciences Pharmingen), anti-CD25 PERCP-Cy5.5 (eBioscience) and
isotypic controls (BD Biosciences)] at 0.1-0.5 μg/ml for 40 min on
ice. For the identification of intracellular IFN-γ expression in
CD8+ T cells, the freshly prepared cells were stimulated
with PMA (50 ng/ml; Sigma) and ionomycin (500 ng/ml; Sigma) for 2 h
and further incubated with protein transport inhibitor brefeldin A
(10 μg/ml; Sigma) for 2 h to accumulate for intracellular
cytokines. At the end of the incubation, Fc Block (BD Biosciences
Pharmingen) was added for 15 min at 4°C, and standard surface
staining procedures were performed with anti-CD8 PE. The cells were
than fixed, permeabilized and stained with anti-IFN-γ FITC (BD
Biosciences Pharmingen). Following staining, cells were washed and
analyzed immediately using the FC500 flow cytometer (BD FACSAria,
USA). Two-color flow cytometric analyses were performed, and data
represent 50,000 events, unless otherwise noted. The phenotype of
Treg cells (CD4+CD25+FoxP3+) was
evaluated by three-color fluorescence flow cytometric analysis
following standard surface staining procedures combined with
intracellular FoxP3 staining method. Intracellular detection of
FoxP3 was performed using PE anti-mouse FoxP3 staining buffer set
purchased from eBioscience. Briefly, the cells stained with
anti-CD4-FITC and anti-CD25-PERCP-Cy5.5 were washed, fixed and
permeabilized according to the manufacturer’s instructions, and
then incubated with PE-conjugated rat anti-mouse FoxP3 Ab for 40
min on ice.
Cytokine production assay
The splenocytes from the mice were cultured for 24 h
at 107 cells/ml in serum-free RPMI medium containing 200
μg/ml BSA. IL-2, IL-4 and IFN-γ concentrations were determined in
the supernatant using enzyme-linked immunosorbent assay kits
(ELISA; BD Biosciences Pharmingen) according to the manufacturer’s
instructions.
Statistical analysis
All statistical analyses were performed using
commercially available statistical software (SPSS Inc., Chicago,
IL, USA). Results are expressed as the means ± standard deviation
(SD), and numerical data were analyzed by the Student’s t-test or
one-way analysis of variance (ANOVA). For all analyses, a
difference was considered to be significant at a P-value of
<0.05.
Results
HS-1793 induces immunomodulation in
tumor-bearing mice
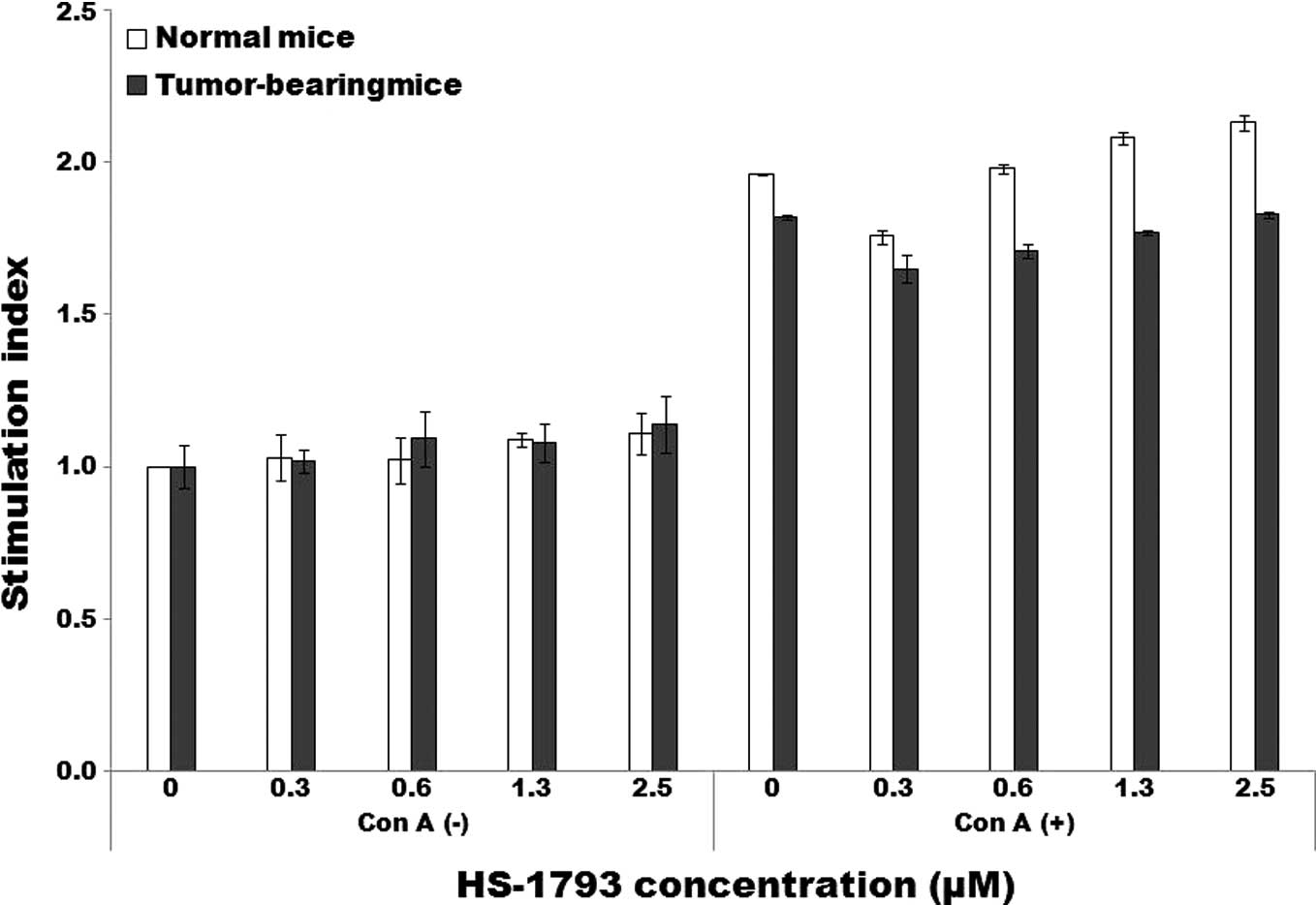
To examine whether HS-1793 gives rise to direct
immunostimulating activity, the lymphocyte proliferative response
was tested by BrdU incorporation assay. HS-1793 at doses of 0.3–2.5
μM did not induce lymphocyte proliferation in normal as well as
tumor-bearing mice. The concanavalin A (ConA)-stimulated lymphocyte
proliferative response of splenocytes from tumor-bearing mice,
which was lower than that of normal mice, was also unaltered by the
treatment of HS-1793 (Fig.
1A).
Having confirmed the presence of cytokines for the
development of effector T cells and Treg cells, the cultured
supernatants of ConA-stimulated lymphocytes were analyzed for the
production of IL-2 and IL-4. Tumor-bearing mice showed a decrease
in IL-2 production after HS-1793 treatment in a dose-dependent
manner and an increase in IL-4 production (Fig. 1B and C), which indicates that
HS-1793 may induce changes in the subpopulations of tumor-derived T
lymphocytes. Thus, immunofluorescent analysis for cell surface
markers using flow cytometric analysis was performed to investigate
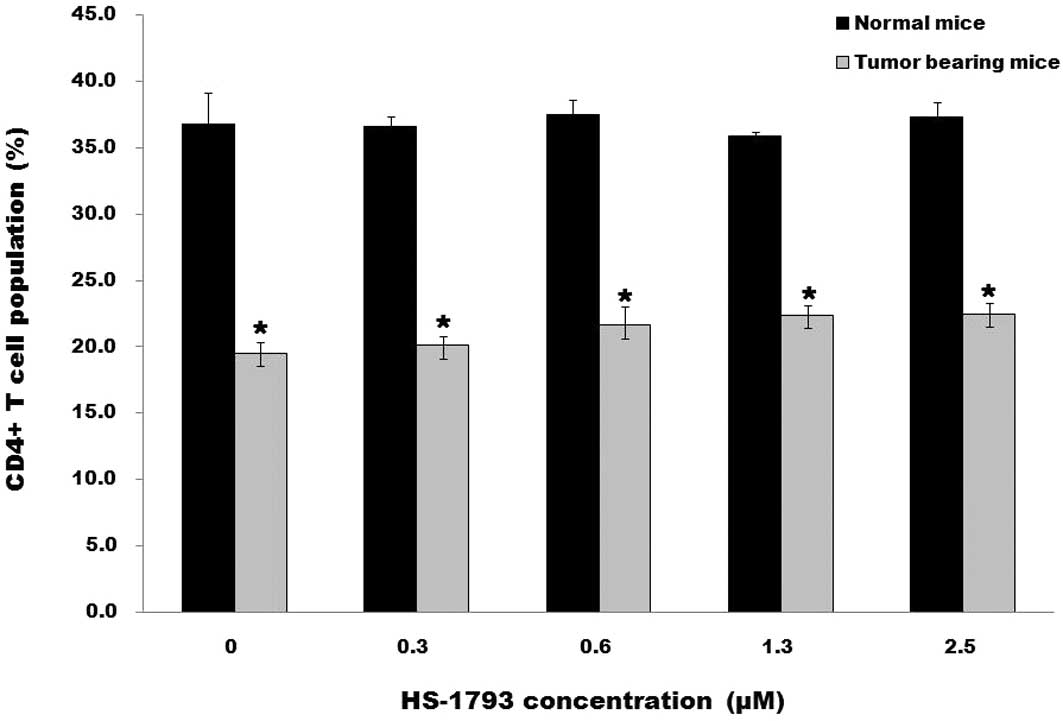
T lymphocyte subpopulations. The average frequency of
CD4+ T cells among total splenocytes from the
tumor-bearing mice was generally lower compared to that in the
normal mice and was not significantly changed by HS-1793 treatment
(Fig. 2A). CD8+ T cells
exhibited a similar profile of frequency when comparing the
tumor-bearing mice to normal mice; however, HS-1793 treatment
prominently recovered the frequency of CD8+ T cells in
tumor-bearing mice (Fig. 3A).
These results suggest that HS-1793 does not induce direct
immunostimulation, but has immunomodulating activity on
tumor-derived T lymphocytes.
HS-1793 suppresses Treg cells in
tumor-bearing mice
To investigate the effect of HS-1793 on the Treg
cell population, CD4+CD25+ cell proportions
were determined by flow cytometric analysis after HS-1793 treatment
in splenocytes from normal and tumor-bearing mice. Based on the
changes in the average frequency of CD4+ T cells,
comparison of the CD4+CD25+ to
CD4+ cell ratio was better for discriminating the
differences between groups. The ratio of
CD4+CD25+ to CD4+ cells was not
changed in the normal mice; however, a significant decrease was
noted in the tumor-bearing mice in a dose-dependent manner
(Fig. 2B). The expression of
FoxP3, a specific Treg cell marker, was further investigated to
confirm that the CD4+CD25+ cells reduced by
HS-1793 are true Treg cells. The proportion of the
FoxP3+ cells among CD4+CD25+ cells
in the FM3A tumor-bearing mice, which was higher than that from the
normal mice, showed a similar, but more prominent pattern of
decrease (Table I). In contrast,
the FoxP3+ cells among the
CD4+CD25− cells were almost unchanged at a
minimal level. These results showed that HS-1793 effectively
suppressed the Treg cell population without affecting
CD4+ cells in the tumor-bearing mice.
 | Table I.Expression of FoxP3 on gated
CD4+CD25+ or CD4+CD25−
cells in tumor-bearing mice after HS-1793 treatment. |
Table I.
Expression of FoxP3 on gated
CD4+CD25+ or CD4+CD25−
cells in tumor-bearing mice after HS-1793 treatment.
| Test group | Concentration of
HS-1793 (μM) | FoxP3-expressing
cells/CD4+CD25+ cells
| FoxP3-expressing
cells/CD4+CD25− cells
|
|---|
| | Events no. | % Gated | Events no. | % Gated |
|---|
| Normal mice | - | 125/684 | 18.2±4.8 | 569/10,214 | 5.5±0.2 |
| Tumor bearing
mice | 0.0 | 502/745 | 67.6±9.4a | 502/5,808 | 8.6±0.4 |
| 0.3 | 328/584 | 56.3±5.0 | 561/8,721 | 6.4±0.1 |
| 0.6 | 213/450 | 48.2±15.3 | 421/9,680 | 4.3±0.4 |
| 1.3 | 125/346 | 36.7±10.2b | 509/11,115 | 4.5±0.1 |
| 2.5 | 56/195 | 28.7±6.8b | 687/13,352 | 5.1±0.1 |
HS-1793 enhances IFN-γ-expressing CD8 T
cells in tumor-bearing mice
We finally investigated whether HS-1793 enhances
effector T cells involved in antitumor immunity in tumor-bearing
mice. The number and percentage of IFN-γ-expressing CD8+
T cells in tumor-bearing mice were significantly increased in a
dose-dependent manner after HS-1793 treatment, compared to the
values in normal mice (Fig. 3B).
In addition, IFN-γ secreted in the cultured supernatants of
splenocytes from tumor-bearing mice also showed an increase after
HS-1793 treatment in a dose-dependent manner, which indicates an
unfavorable change in the tumor microenvironmental and the
induction of effective antitumor immunity (Fig. 3C).
Discussion
In recent years, there has been considerable
interest in resveratrol as a potential cancer chemotherapeutic and
immunomodulating agent. However, resveratrol is not a potent
cytotoxic compound when compared to other chemotherapeutic drugs,
and its stilbene double bonds are readily oxidized. Resveratrol
analogue HS-1793 containing a different position of two of three
hydroxyl groups at the aromatic ring without the unstable double
bond, was, on the other hand, described to display stronger
antitumor effect than resveratrol in most cancer cells, to overcome
the resistance conferred by Bcl-2 in U937 cells via 14-3-3, and to
exert its antitumor activity via Bad (18). The results presented in this study
clarified the modulating effect of HS-1793 on tumor-derived T
lymphocytes, which implies that HS-1793, having both
immunomodulatory and antitumor effects, may be an appropriate
candidate agent for production of an ideal conditioning strategy
against pre-established cancer.
We investigated the direct immunostimulating effect
of HS-1793, which had no effect on lymphocyte proliferation at
doses of 0.3–2.5 μM. However, it is noteworthy that IL-2 and IL-4
secretion of ConA-stimulated lymphocytes from tumor-bearing mice
was modulated in a dose-dependent manner by HS-1793 at these doses
(Fig. 1). The result suggests that
HS-1793 induces changes in the subpopulations of tumor-derived T
lymphocytes. The average frequency of the CD4+ T cell
population among total splenocytes from tumor-bearing mice, which
was lower than that from normal mice, was not significantly changed
by HS-1793 treatment. However, the CD4+CD25+
cell population from the tumor-bearing mice, which was higher than
that in the normal mice, exhibited a HS-1793-dose-dependent
decrease, and this decreasing trend in the
CD4+CD25+ cell population became more
definite when it was illustrated in terms of the
CD4+CD25+ to CD4+ cell ratio
(Fig. 2). The regulatory
subpopulation among CD4+ cells constitutively expressing
high levels of CD25 (Treg cells) is one of the most potent and
well-studied suppressive phenotypes found in the tumor
microenvironment.
It may be assumed that HS-1793 inhibited the IL-2
secretion and promoted the IL-4 secretion of tumor-derived T
lymphocytes, which downregulated CD25+-expressing
CD4+ T cells more sensitively. Actually, IL-2 and IL-4
are essential for naturally occurring Treg cell homeostasis and
activation. Treg cells are refractory to TCR-induced proliferation
(20,21) and depend on IL-2 for survival
(22,23). The signaling via the high-affinity
IL-2R complex, in combination with TCR engagement, is essential for
Treg cell proliferation as well as the acquisition of their potent
suppressive function (23).
Stimulation of Treg cells with IL-2 leads to phosphorylation and
activation of STAT5 and consequently, binding to the forkhead box
P3 (FoxP3) promotor, resulting in enhanced FoxP3 expression
(24). By contrast, IL-4 inhibited
TGF-β-induced Foxp3 expression and thus suppressed the new
generation of Foxp3+ Treg cells (25). The transcriptional factor FoxP3 is
a crucial developmental and functional factor expressed in Treg
cells (20), and is regarded as a
useful intracellular Treg cell marker. Thus, we investigated the
expression of FoxP3 to more clearly define Treg cells. FoxP3 was
expressed mainly in the CD4+CD25+ cell
population and was extensively increased in cells from
tumor-bearing mice. Moreover, the
CD4+CD25+FoxP3+ to
CD4+CD25+ cell ratio decreased significantly
after treatment with HS-1793 in a dose-dependent manner (Table I).
It is generally recognized that IFN-γ-producing
CD8+ T cells play an important role in inhibiting and
killing tumor cells and impeding tumor growth, which requires
effector CD4+ help (26) and can be largely affected by the
presence of regulatory CD4+ cells (27). Regulatory CD4+ cells
promote CD8+ tolerization by preventing licensing of
APCs by Th1 effector cells, or educating tolerogenic APCs (28), or directly modulating
CD8+ T cells (29). As
shown in the results of our present study, the average frequency of
the CD8+ T cell population among total splenocytes from
the tumor-bearing mice, which was lower than that from normal mice,
was significantly increased by HS-1793 treatment. The
IFN-γ-producing CD8+ T cell population as well as IFN-γ
secretion of splenocytes from tumor-bearing mice also showed a
HS-1793-dose-dependent increase (Fig.
3). IFN-γ plays a key role in T helper 1 cell responses and
tumor surveillance and immunoediting, and appears to be one of the
most significant cytokines preventing and suppressing the
development of cancers. In parallel, IFN-γ blocks TGF-β-mediated
Treg cell differentiation (30).
Although the exact mechanism of enhanced IFN-γ expression in
CD8+ cells by HS-1793 is not clear, it may be related in
part to HS-1793-induced Treg cell depletion in tumor-bearing
mice.
Based on the results of our present and previous
studies, HS-1793 itself could be applied as an adequate principal
therapeutic agent, facilitating the immune system against
pre-established cancer and inducing apoptosis of cancer cells.
However, HS-1793 may be more effective clinically when it is used
in combination with other main cancer therapies, such as
chemotherapeutic agents, ionizing radiation or tumor vaccines,
since one of the major obstacles that must be overcome to achieve
successful cancer treatment is tumor-induced immune suppression
caused by Treg cells. Actually, the presence of Treg cells is
demonstrated in the tumor microenvironment; higher numbers of Treg
cells are associated with progression in a variety of malignancies,
including a variety of solid tumors as well as haematological
malignancies. In addition, increased populations of Treg appear to
correlate with poor survival in several types of cancers.
Therefore, effective Treg cell elimination is critical for both
primary treatment and secondary prevention (i.e., relapse or
recurrence) of cancer. Indeed, our previous study demonstrated that
Treg cell depletion, treated by a low dose of cyclophosphamide,
potentiated the antitumor effect of immunization with an injection
of immature dendritic cells into irradiated tumors (31). As well as in breast cancer, Treg
cells were increased in the peripheral blood and greatly increased
in the tumor microenvironment (32); the presence of Treg cells is likely
to correlate with cancer progression (33), and specific depletion of Treg cells
markedly inhibits tumor growth and maintains a strong and
persistent antitumor immune response (34).
The present study revealed that HS-1793 effectively
suppressed the Treg cell population and enhanced IFN-γ production
of CD8+ T cells in FM3A breast cancer cell-bearing
C3H/He mice. Furthermore, another advantage of HS-1793 involves the
fact that the CD4+ cell pool did not decrease, which may
include tumor-specific CD4+ cells involved in antitumor
immunity. In light of this, HS-1793, free from the restriction of
metabolic instability and high-dose requirement of resveratrol, is
a promising candidate agent for adjuvant therapy in breast cancer
immunotherapy, and is able to confer a bystander effect in
eradicating chemotherapy or radiotherapy-resistant cancer cells and
synergistically act when treated with dendritic cell vaccine.
Acknowledgements
This study was supported by the
Nuclear R&D Program through the Dong Nam Institute of
Radiological and Medical Sciences funded (code 50593-2011) by the
Ministry of Education, Science and Technology, and by a grant of
the Korea Healthcare Technology R&D Project, Ministry of Health
and Welfare, Republic of Korea (A090314).
References
|
1.
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar
|
|
2.
|
Raza SA, Clifford GM and Franceschi S:
Worldwide variation in the relative importance of hepatitis B and
hepatitis C viruses in hepatocellular carcinoma: a systematic
review. Br J Cancer. 96:1127–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Odunsi K and Old LJ: Tumor infiltrating
lymphocytes: indicators of tumor-related immune responses. Cancer
Immun. 7:32007.PubMed/NCBI
|
|
4.
|
Zou W: Immunosuppressive networks in the
tumour environment and their therapeutic relevance. Nat Rev Cancer.
5:263–274. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yu P and Fu YX: Tumor-infiltrating T
lymphocytes: friends or foes? Lab Invest. 86:231–245. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Beyer M and Schultze JL: Regulatory T
cells in cancer. Blood. 108:804–811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Schabowsky RH, Madireddi S, Sharma R,
Yolcu ES and Shirwan H: Targeting
CD4+CD25+FoxP3+ regulatory T-cells
for the augmentation of cancer immunotherapy. Curr Opin Investig
Drugs. 8:1002–1008. 2007.
|
|
9.
|
Leon K, Garcia K, Carneiro J and Lage A:
How regulatory CD25+CD4+ T cells impinge on
tumor immunobiology: the differential response of tumors to
therapies. J Immunol. 179:5659–5668. 2007.PubMed/NCBI
|
|
10.
|
Curiel TJ: Regulatory T cells and
treatment of cancer. Curr Opin Immunol. 20:241–246. 2008.
View Article : Google Scholar
|
|
11.
|
Liu Z, Kim JH, Falo LD Jr and You Z: Tumor
regulatory T cells potently abrogate antitumor immunity. J Immunol.
182:6160–6167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Malmberg KJ: Effective immunotherapy
against cancer: a question of overcoming immune suppression and
immune escape? Cancer Immunol Immunother. 53:879–892. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, Moon RC and Pezzuto JM: Cancer chemopreventive activity of
resveratrol, a natural product derived from grapes. Science.
275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
15.
|
Bove K, Lincoln DW and Tsan MF: Effect of
resveratrol on growth of 4T1 breast cancer cells in vitro and in
vivo. Biochem Biophys Res Commun. 291:1001–1005. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Feng YH, Zhou WL, Wu QL, Li XY, Zhao WM
and Zou JP: Low dose of resveratrol enhanced immune response of
mice. Acta Pharmacol Sin. 23:893–897. 2002.PubMed/NCBI
|
|
17.
|
Song S, Lee H, Jin Y, Ha YM, Bae S, Chung
HY and Suh H: Syntheses of hydroxy substituted
2-phenyl-naphthalenes as inhibitors of tyrosinase. Bioorg Med Chem
Lett. 17:461–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jeong SH, Jo WS, Song S, Suh H, Seol SY,
Leem SH, Kwon TK and Yoo YH: A novel resveratrol analog, HS-1793,
overcomes the resistance conferred by Bcl-2 in human leukemic U937
cells. Biochem Pharmacol. 7:1337–1347. 2009. View Article : Google Scholar
|
|
19.
|
Yang Y, Paik JH, Cho D, Cho JA and Kim CW:
Resveratrol induces the suppression of tumor-derived
CD4+CD25+ regulatory T cells. Int
Immunopharmacol. 8:542–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Itoh M, Takahashi T, Sakaguchi N, Kuniyasu
Y, Shimizu J, Otsuka F and Sakaguchi S: Thymus and autoimmunity:
production of CD25+CD4+ naturally anergic and
suppressive T cells as a key function of the thymus in maintaining
immunologic self-tolerance. J Immunol. 162:5317–5326.
1999.PubMed/NCBI
|
|
21.
|
Takahashi T, Kuniyasu Y, Toda M, Sakaguchi
N, Itoh M, Iwata M, Shimizu J and Sakaguchi S: Immunologic
self-tolerance maintained by CD25+CD4+
naturally anergic and suppressive T cells: induction of autoimmune
disease by breaking their anergic/suppressive state. Int Immunol.
10:1969–1980. 1998.
|
|
22.
|
De la Rosa M, Rutz S, Dorninger H and
Scheffold A: Interleukin-2 is essential for
CD25+CD4+ regulatory T cell function. Eur J
Immunol. 34:2480–2488. 2004.PubMed/NCBI
|
|
23.
|
Furtado GC, Curotto de Lafaille MA,
Kutchukhidze N and Lafaille JJ: Interleukin 2 signaling is required
for CD4+ regulatory T cell function. J Exp Med. 196:851–857.
2002.
|
|
24.
|
Yao Z, Kanno Y, Kerenyi M, Stephens G,
Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl
R, Hennighausen L, Wu C and O’Shea JJ: Nonredundant roles for
Stat5a/b in directly regulating Foxp3. Blood. 109:4368–4375. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Valérie D, Amit A, Hyoung K, George G,
Wenda G, Raymond AS, Meike M, Terry BS, Wassim E, I-Cheng H, Samia
K, Mohamed O and Vijay KK: IL-4 inhibits TGF-b-induced
Foxp3+ T cells and, together with TGF-b, generates
IL-9+ IL-10+ Foxp3-effector T cells. Nat
Immunol. 9:1347–1355. 2008.
|
|
26.
|
Dudley ME, Wunderlich JR, Robbins PF, et
al: Cancer regression and autoimmunity in patients after clonal
repopulation with antitumor lymphocytes. Science. 298:850–854.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Turk MJ, Guevara-Patino JA, Rizzuto GA,
Engelhorn ME and Houghton AN: Concomitant tumor immunity to a
poorly immunogenic melanoma is prevented by regulatory T cells. J
Exp Med. 200:771–782. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Alpan O, Bachelder E, Isil E, Arnheiter H
and Matzinger P: ‘Educated’ dendritic cells act as messengers from
memory to naive T helper cells. Nat Immunol. 5:615–622. 2004.
|
|
29.
|
Bourgeois C, Rocha B and Tanchot C: Arole
for CD40 expression on CD8+ T cells in the generation of
CD8+ T cell memory. Science. 297:2060–2063.
2002.PubMed/NCBI
|
|
30.
|
Xiaoyu H and Lionel BL: Cross-regulation
of signaling pathway by interferon-γ: implication for immune
response and auto-immune diseases. Immunity. 31:593–550. 2009.
|
|
31.
|
Park YS, Bae JH, Son CH, Lee KS, Kim W,
Jung MH, Yang K, Kim SH and Kang CD: Cyclophosphamide potentiates
the antitumor effect of immunization with injection of immature
dendritic cells into irradiated tumor. Immunol Invest. 40:383–399.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Liyanage UK, Moore TT, Joo HG, Tanaka Y,
Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ,
Goedegebuure PS and Linehan DC: Prevalence of regulatory T cells is
increased in peripheral blood and tumor microenvironment of
patients with pancreas or breast adenocarcinoma. J Immunol.
169:2756–2761. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Perez SA, Karamouzis MV, Skarlos DV,
Ardavanis A, Sotiriadou NN, Iliopoulou EG, Salagianni ML, Orphanos
G, Baxevanis CN, Rigatos G and Papamichail M:
CD4+CD25+ regulatory T-cell frequency in
HER-2/neu (HER)-positive and HER-negative advanced-stage breast
cancer patients. Clin Cancer Res. 13:2714–2721. 2007.PubMed/NCBI
|
|
34.
|
Knutson KL, Dang Y, Lu H, Lukas J, Almand
B, Gad E and Disis ML: IL-2 immunotoxin therapy modulates
tumor-associated regulatory T cells and leads to lasting
immune-mediated rejection of breast cancers in neu-transgenic mice.
J Immunol. 177:84–91. 2006. View Article : Google Scholar
|