Contents
Introduction
Role of platelets in atherogenesis
Tomatoes: Platelet anti-aggregation activity and
endothelial protection
Conclusion
Introduction
According to the World Health Organization (WHO),
cardiovascular disease (CVD) (i.e., acute myocardial infarction,
cerebrovascular disease and peripheral arterial thrombosis) is the
cause of approximately 30% of deaths worldwide (1), with a relative increase over time due
to the aging of the population (2). The development and progression of CVD
lies in the interactive processes of atherosclerotic lesions and
thrombus formation, an interaction established primarily by
platelet-endothelial binding (3).
The activation of vascular endothelium occurs early
in the development of atherosclerosis (4), where the inflammatory component,
present in all phases of atherosclerosis, is a vascular response to
guard against cardiovascular risk factors (i.e., hypertension,
diabetes, smoking and obesity) (5,6).
This inflammatory process generates a microenvironment
characterized by oxidative stress and cell damage (7). The process triggers a loss of
endothelial function through a decrease in the bioavailability of
nitric oxide (NO) and the physiological mechanisms of
cardiovascular protection that are derived from it (8).
The role of platelets in arterial thrombosis is well
known (9). When there is a damaged
atheromatous plaque, platelets adhere, secrete their contents, and
then attach to it (10). This
activation causes a redistribution of anionic phospholipids,
creating a negatively charged surface (11) which, in addition to the synthesis
and expression of tissue factor (12), favors the consecutive formation of
protein complexes in coagulation and fibrin and the consolidation
of the thrombus (13).
Epidemiological studies have demonstrated the
cardiovascular protective role of a healthy diet (14). In this context, the beneficial
effects of fruits and vegetables (F&V) may be related to the
bioactive compounds found therein (15), which explains the increasing amount
of attention in research on phytochemicals in the prevention of CVD
(16). In addition to their
nutritional value, tomatoes (Solanum lycopersicum L.), fresh
or processed, have been found to provide a cardioprotective effect
at both the endothelial and platelet levels (17).
This article discusses current knowledge of
platelet-endothelial interaction during the development of
atherosclerosis, and platelet anti-aggregation activity and the
endothelial protective effects from tomatoes.
Role of platelets in atherogenesis
In the last decade, it has been shown that platelets
are not only involved in the inflammatory complications of the
atheromatous lesion (18) but are
also involved in the initiation and progression of atherosclerotic
plaque (19). Accordingly,
platelets act as a bridge between the inflammatory processes
characteristic of atherosclerosis and thrombosis (20). The interaction of platelets with
endothelial cells (ECs) occurs in three forms: activated platelets
join with ECs in the normal state; resting platelets adhere to
activated ECs; or an interaction can occur between the two types of
activated cells (21).
Union of activated platelets to ECs in
the normal state
Endothelium in the normal state plays a fundamental
role in regulating the hemostatic balance (22) through various mechanisms of
antiplatelets, anticoagulants and fibrinolysis, which are regulated
by the secretion of NO and prostacyclin (23).
In vitro studies have demonstrated platelet
adhesion to ECs in the normal state (24). Platelet adhesion occurs because it
is activated in circulation. By contrast, in other in vivo
studies, the binding occurs under shear conditions (25). Once activated, platelets may adhere
to ECs and promote local vascular inflammation through inflammatory
mediators such as the secretion of chemokines (26), which disrupt normal functioning of
the endothelium (27). For
example, platelets store and express CD40L (inflammatory modulator)
on their surface, releasing the protein into the environment once
they are activated (28). CD40L
also induces the expression and release of metalloproteinases,
which degrade extracellular matrix proteins that are exposed to the
circulation when damage occurs at the endothelial level (29). In addition to the release of sCD40,
IL-1β, which promotes increased IL-6 and IL-8 and the expression of
cell adhesion molecules such as E-selectin, intercellular adhesion
molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)
in the EC (30,31), is also released. This leads to the
recruitment of leukocytes to the site from which the injury (i.e.,
damaged endothelium) originated (32). The leukocyte-platelet interaction
causes a wide range of responses in the innate and adaptive immune
systems (33), giving the platelet
a new function as regulator of the immune system, which contributes
to the pathogenesis of an inflammatory response (34,35).
Moreover, animal models have shown a high level of
P-selectin expression and endothelial growth factor (VEGF) in
atherosclerotic plaques with involvement in the progression of CVD
(36). Platelet factor 4 (PF-4),
CCL5 (RANTES) and platelet-derived growth factor (PDGF) molecules
released from activated platelets cause chemotaxis of monocytes and
other leukocytes on the EC, and promote the retention of low
density lipoprotein (LDL) and oxidized LDL in the subendothelium
(37). In patients with CVD, the
presence of oxidized LDL promotes the release of neutrophil-peptide
78 (ENA-78), with an increase in chemoattractant capacity (38). It has been shown that RANTES and
PF-4 have heterophile actions, and that the latter must undergo
structural modifications in order to amplify its effects on
monocytes (39). Moreover, the
deposit of RANTES on the endothelium is promoted by platelet
P-selectin (40). PDGF also
stimulates smooth muscle cell proliferation, causing hyperplasia of
the intima layer of the arterial wall, thus acting as an amplifier
of the inflammatory response (41).
Union of resting platelets with activated
ECs
Normally, ECs form neither a non-adherent nor a
thrombogenic surface (42). Under
physiological conditions this is able to exert important regulatory
functions, such as maintaining the balance between procoagulant and
anticoagulant factors, vascular tone regulation and control of
vascular permeability (43,44).
Under normal resting, platelets do not adhere to the endothelium
(45). However, several factors
can alter this balance, establishing what is known as endothelial
dysfunction (46). A dysfunctional
endothelium generates a proatherogenic environment characterized by
inflammation, proliferation and a prothrombotic state favoring the
development of atherosclerosis (47).
Changes in the endothelium can begin at a young age,
with slow and progressive developments of atherosclerotic lesions
occurring as an individual ages (48). The first evidence of the process is
the decrease in NO synthesis (49), causing a decrease in vasodilatory
capacity and the establishment of a proinflammatory and
prothrombotic state in the endothelium (50). Furthermore, this decrease in NO
creates an oxidative environment that eventually oxidizes LDL from
the plasma passing the site of endothelial injury, where the
presence of lysophosphatidylcholine increases oxidative stress
(51), adhesion of monocytes
(52) and induces in the EC the
expression of adhesion molecules (ICAM-1, VCAM-1, P-selectin, among
others) through two signaling pathways: i)
lysophosphatidylcholine/nuclear factor κB (NF-κB) and ii)
lysophosphatidylcholine/receivers coupled G protein 4/adenosine
monophosphate (cAMP)/protein kinase A/cAMP response element binding
protein (53,54). Endothelial P-selectin allows
platelets to roll on the activated endothelium (55), permitting adhesion, activation and
platelet aggregation in disturbed areas of the endothelium. This
process represents one of the main events in the initiation and
development of atherosclerosis (56).
Furthermore, it has been demonstrated that ADAM-15
expressed in activated ECs interacts with the GPIIb/IIIa complex
and induces platelet activation (57). ADAM-15 also operates as a
metalloproteinase, degrading endothelial matrix proteins (58). The two functions of this molecule
could facilitate the instability and rupture of the fibrous cap of
atherosclerotic lesions, causing the acceleration of the formation
of a platelet thrombus (59).
Tomatoes: platelet anti-aggregation activity
and endothelial protection
The report ‘Diet, Nutrition and the Prevention of
Chronic Diseases’, published by the WHO in 2003, outlined the
scientific evidence that has been associated with a decreased risk
of CVD. According to the report, individuals who consume at least
400 g of F&V daily were found to have a reduced risk of CVD
(60). By consuming 5 servings of
F&V daily there is a 17% reduction in the risk of CVD (61). Furthermore, the effects of
consuming F&V have been replicated worldwide and are
independent of ethnicity or geographical location (62).
Presently, in addition to their recognized high
value in vitamins, minerals and dietary fiber, consuming F&V is
associated with phytochemical content (63,64),
with specific actions on target functions (65). These effects, in terms of primary
prevention, could modify cardiovascular risk without any of the
side effects normally associated with the majority of antiplatelet
drugs (66).
The antioxidant properties of F&V are well known
(67–69). However, their antithrombotic
effects on platelet-endothelial interaction are less known.
Preliminary studies have demonstrated the platelet anti-aggregation
activity of fruits (red grapes, strawberries, kiwis and pineapples)
and vegetables (garlic, onions, green onions, melons and tomatoes)
(70,71). Among those beneficial elements
mentioned above, the consumption of tomatoes, the fruit of a
dicotyledonous plant belonging to the Solanaceae family, is
emphasized (72). The possible
mechanisms of action of bioactive compounds from tomatoes that have
platelet anti-aggregation activity and endothelial protective
effects are subsequently discussed (73,74).
Platelet anti-aggregation activity of
bioactive compounds from tomatoes
It has been observed that the tomato has platelet
anti-aggregation activity in vitro and in vivo by
inhibiting platelet aggregation induced by ADP and collagen
(75–80). This finding has also been confirmed
by our research group (71). The
various platelet anti-aggregation activity levels observed in
different varieties of tomatoes can be explained by the existence
of one or more bioactive compounds or different concentrations of
the same compound (77). The
platelet anti-aggregation activity of aqueous and methanol extracts
of tomatoes (‘cluster’ type) in vitro were similar. Both
types of extract showed inhibition of platelet aggregation (30–40%)
at 1 mg/ ml induced by ADP. When collagen was used as agonist,
inhibition was lower, whereas the use of arachidonic acid and
peptide receptor activator of thrombin showed no inhibitory effect
(71).
The experimental results obtained by Fuentes et
al indicate that aqueous and methanol extracts resuspended in
0.9% saline exhibit a pH of 4.5; when resuspended in more acidic
(pH 2.0) and basic (pH 10.0) suspensions, they maintained their
inhibitory activity of maximum platelet aggregation (78). As we know that carotenoids are
unstable at pH extremes, this finding may exclude the possibility
that these antioxidant compounds exhibit platelet anti-aggregation
activity (82–84). In addition, the platelet
anti-aggregation activity is inversely related to tomato ripening
and the increase in the concentration of lycopene (77).
In the study by Fuentes et al, aqueous and
methanol extracts under various temperatures (22, 60 and 100°C)
maintained their platelet anti-aggregation activity, indicating
that the active compounds with platelet anti-aggregation activity
present in the two extracts were not affected by heat treatment
(78).
These results allow us to identify the tomato as a
functional food, with one or more bioactive compounds with
acid-base and thermal stability to exert its cardioprotective
activity. This characteristic will benefit future efforts to
protect the molecular structure and corresponding platelet
anti-aggregation activity of tomato extracts during processing,
storage, transport, management and molecular action. This will
prove useful in the search for alternative antithrombotic therapy,
a field in which most of the drugs used have a high instability in
the environment of action (85).
The mechanism of action by which the tomato inhibits
platelet aggregation has yet to be elucidated (86). It has been suggested that adenosine
and other nucleosides may be responsible for this inhibition,
possibly via a mechanism independent of cAMP generation (79) and thromboxanes (cyclooxygenase
pathway) (75).
However, there are a wide range of bioactive
compounds in tomatoes with platelet anti-aggregation activity, some
of which have known bioavailability (flavonoid derivatives), and
others whose bioavailability has yet to be identified (87). It is also necessary to determine
those anatomical sites of the tomato (skin, pulp, seeds, etc.) in
which compounds with platelet anti-aggregation activity are found.
Based on the results of our research, the bioactive compounds of
tomatoes have thermal and acid-base stability, are devoid of
lycopene and have low molecular weight (<1000 Da). Moreover, we
hypothesize that bioactive compounds exercise their antiplatelet
activity via three platelet receptors: GPVI (collagen agonist) and
P2Y1 and P2Y12 (ADP agonist) (71). Further studies of the possible
mechanisms of action found in the search strategies of alternative
pathways of platelet aggregation inhibition possessed by naturally
occurring compounds are required. These studies will allow us to
identify their therapeutic range of application (Fig. 1).
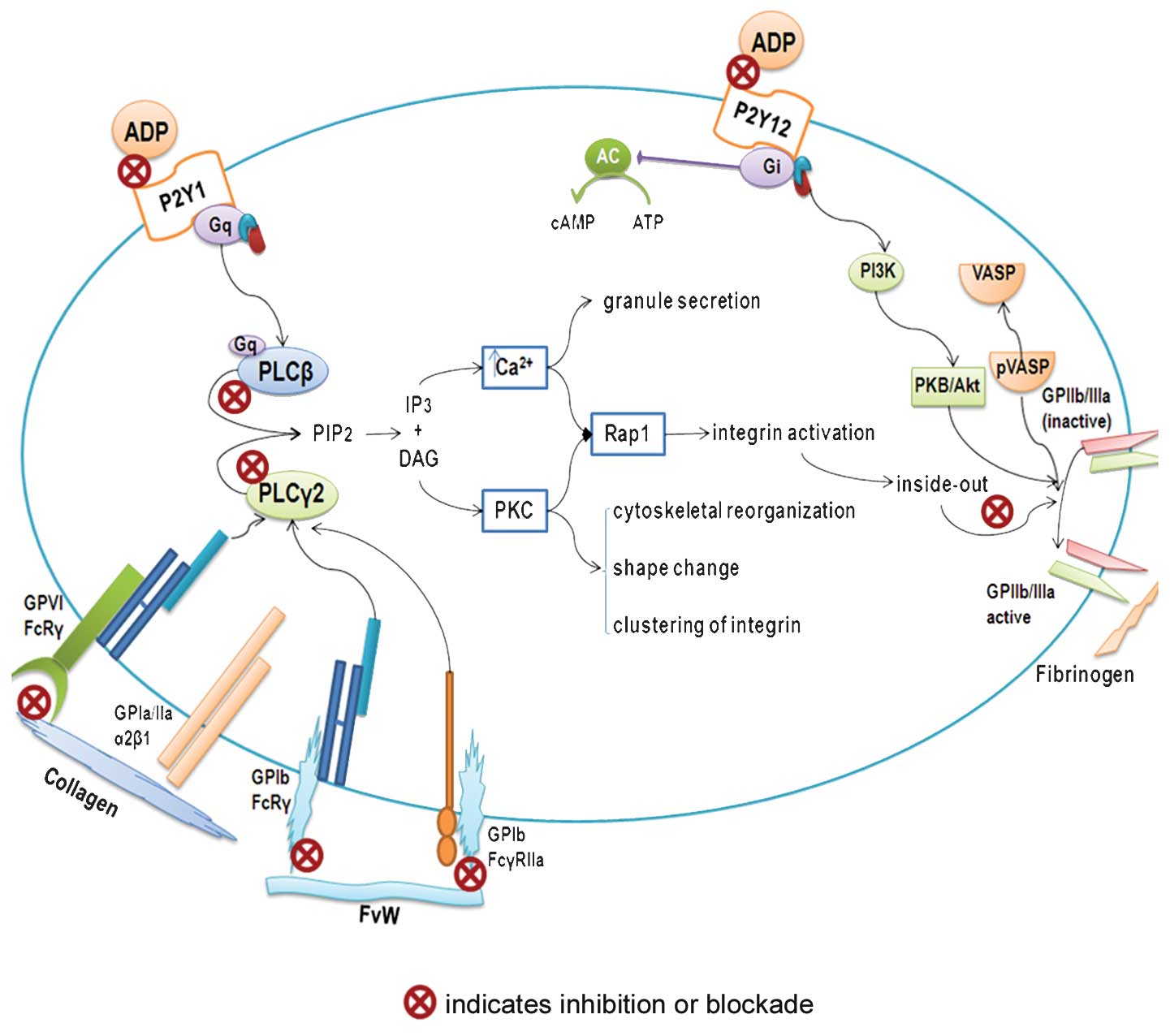 | Figure 1.Platelet anti-aggregation activity.
Schematic diagram showing possible mechanisms of action of
bioactive compounds from the tomato in inhibiting platelet
aggregation. ADP, adenosine 5′-diphosphate; AC, adenilate cyclase;
ATP, adenosine 5′-triphosphate; cAMP, adenosine 3′5′cyclic
monophosphate; DAG, dyacil glycerole; FvW, von Willebrand Factor;
GP, glycoprotein; Gq and Gi, G protein-coupled receptors; IP3,
inositol 1,4,5-trisphosphate; PLCβ, phospholipase Cβ; PLCy2,
phospholipase Cy2; PIP2, phosphatidylinositol 4, 5-bisphosphate;
PKC, protein kinase C; PI3K, phosphoinositide 3-kinase; PKB/ Akt,
protein kinase B; P2Y1 and P2Y12, ADP receptors; Rap1, ras-related
protein 1; VASP, vasodilator-stimulated phosphoprotein; pVASP,
phosphorylatedvasodilator-stimulated phosphoprotein. |
Study of intraplatelet signaling
pathways
Studies of platelet aggregation with bioactive
compounds (88) using ADP or
collagen as agonist and subsequent platelet lysate for the Western
blot test should examine: i) expression of phospholipase Cβ and ii)
expression of phospholipase Cγ2. The first is the signaling pathway
involved in the possible interaction of bioactive compounds with
ADP receptor (P2Y1) (89). It is
also necessary to study the proteins that are related to this
signaling pathway. These proteins include total Akt and phospho-Akt
(serine 473/ threonine 308) (90).
The latter, expression of phospholipase Cγ2, is involved in the
interaction of bioactive compounds with the collagen receptor
(GPVI) (91). Future studies
should examine the bioactive compounds of this signaling pathway:
total Erk, phospho-Erk 1/2 (threonine 202/ tyrosine 204, threonine
185/ tyrosine 187), total- JNK, phospho-JNK (threonine 182/tyrosine
185) and total p38 MAPK and phospho-p38 MAPK (threonine
182/tyrosine 182) (92). This
approach reveals active and inactive forms of these proteins after
they have been treated with bioactive compounds, as well as a
potential target of action in the corresponding signaling
pathway.
Analysis of cytosolic calcium
If there is a decrease in cytosolic calcium levels
when platelet aggregation is inhibited, it signifies that the
bioactive compounds are exerting their action through GPVI or P2Y1
receptors (93).
Cytosolic cAMP analysis
An increase in cytosolic calcium level may indicate
that the bioactive compounds are inhibiting platelet aggregation
through the platelet receptor P2Y12 (94). This receptor modulates the
inhibition of GPIIb/IIIa through vasodilator-stimulated
phosphoprotein (VASP) (95).
Study of platelet adhesion
After incubating platelets with bioactive compounds,
they were deposited in fibrinogen or VWF matrices (96,97).
A reduced matrix adhesion of fibrinogen (fibrinogen binding
capacity) indicates that the bioactive compounds block
GPIIb/IIIa-fibrinogen interaction via the amino acid sequence RGD
(arginine-glycine-aspartic acid), or inhibit a signaling pathway
involved in the expression of GPIIb/IIIa. Thus, complete blockage
of the GPIIb/IIIa platelet receptor inhibits aggregation from
forming a platelet thrombus (98).
Bioactive compounds may affect the interaction of platelet
(GPIb/IX/V) with endothelium (vWF) (24).
Protective activity from tomatoes on the
endothelial function of bioactive compounds
It has been observed that aqueous and methanol
extracts of tomatoes exhibit antioxidant activity in vitro
(67,99). Carotenoids (β-carotene, lycopene,
zeaxanthin, lutein and canxantina) and vitamins C and E, when they
are found in tomatoes, possess endothelium-protective activity.
These molecules have three main methods of action (Fig. 2): i) they cause antioxidant
activity by protecting LDL and increasing resistance to oxidation
(100); ii) in humans, it has
been reported that supplementation with lycopene reduces oxidative
damage to DNA and other markers of oxidative stress (101); and iii) lycopene has been found
to inhibit the expression of adhesion molecules in ECs (102).
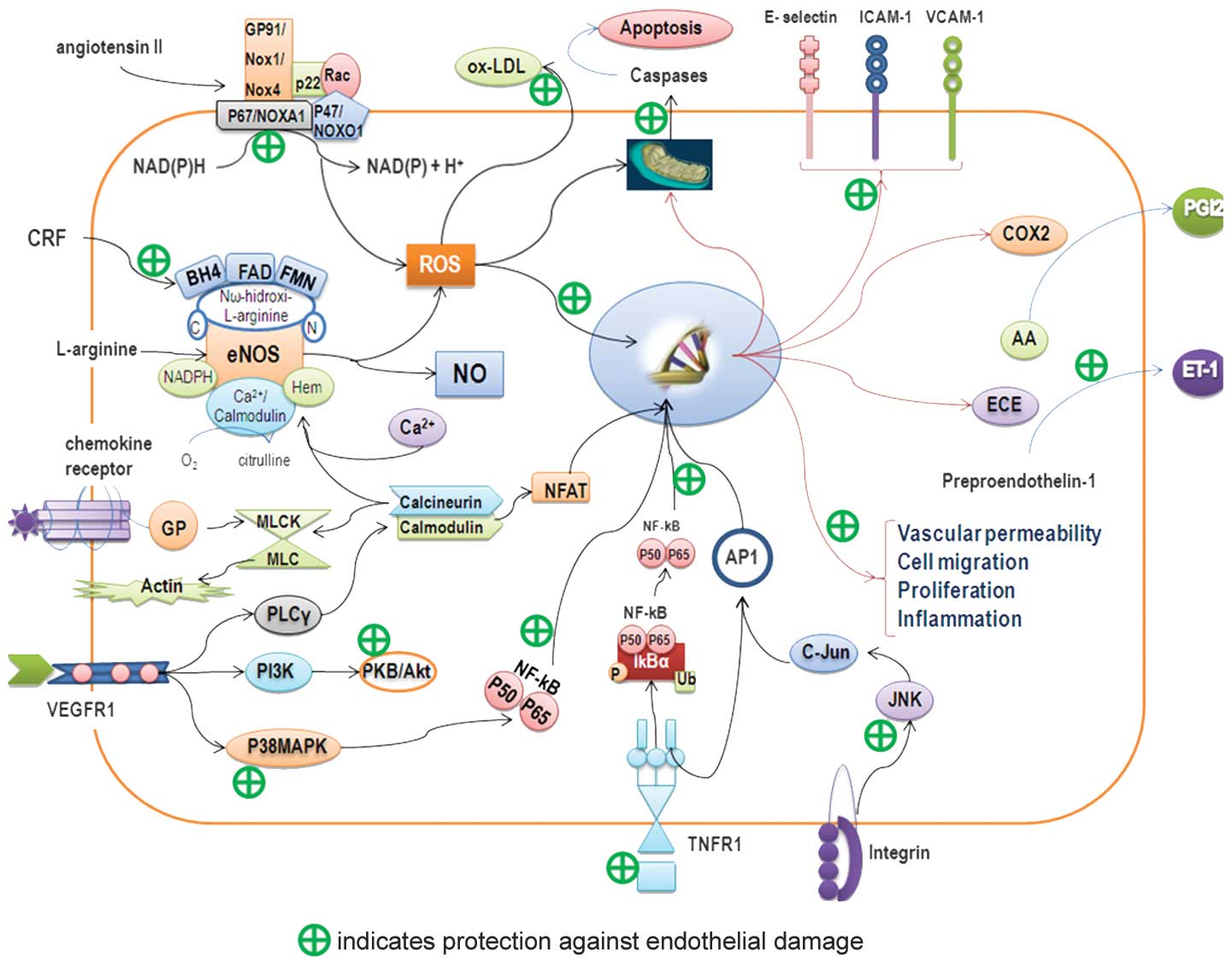 | Figure 2.Endothelial protective mechanism.
Schematic diagram showing possible mechanisms of action of
bioactive compounds from the tomato in protecting endothelium. AA,
arachidonic acid; AP-1, activator protein-1; BH4,
tetrahydrobiopterin; COX2, cyclooxygenase 2; CRF, cardiovascular
risk factor; ECE, endothelin-converting enzyme; eNOS, endothelial
nitric oxide synthase; ET-1, endothelin-1; FAD, flavin adenine
dinucleotide; FMN, flavin mononucleotide; ICAM-1, intercellular
adhesion molecule 1; IkBα, inhibitor of I-κ-B-α; JNK, c-Jun
N-terminal kinase; ox-LDL, oxidized low-density lipoprotein; MLCK/
MLC, myosin light chain kinase-myosin light chain; NADPH,
nicotinamide adenine dinucleotide phosphate; NFAT, nuclear factor
of activated T-cells; NFkB, nuclear factor κB; NO, nitric oxide;
GP, G protein; PGI2, prostacyclin I2; PI3K, phosphoinositide
3-kinase; PLCy, phospholipase Cy; PKB/Akt, protein kinase B;
p38MAPK, p38 mitogen-activated protein kinase; ROS, reactive oxygen
species; TNFR1, tumor necrosis factor receptor-1; VCAM-1, vascular
cell adhesion molecule-1; VEGFR1, vascular endothelial growth
factor receptor-1. |
Cystine-knot miniproteins from tomato with low
concentrations and low toxicity have active anti-angiogenic effects
through their inhibition of Erk phosphorylation and do not affect
the normal viability and proliferation of ECs (103). They have a similar function to
carotenoids, which prevent the phosphorylation of Akt, p38 MAPK and
JNK and are sensitive to reactive oxygen species (ROS) (104,105).
Lycopene is considered to be a chemopreventive agent
(106), as it maintains the
integrity of the vascular barrier, inhibits the expression of cell
adhesion molecules and leukocytes, and inhibits EC migration by
blocking the expression of NF-κB, CD14 and TLR4, and TNF-α
production (107). In addition to
decreased levels of malondialdehyde, programmed cell death prevents
apoptosis by attenuating the expression of p53 and caspase-3 in ECs
treated with H2O2 (108).
Newly discovered mechanisms include: the reduction
of endothelial injury, control of lipid metabolism during the
synthesis of cholesterol and oxysterol toxic activity and reduction
of the inflammatory response through changes in cytokine production
(109,110). While it is known that the
consumption of tomato products is associated with a significant
increase in plasma levels of lycopene, this has no substantial
effect on endothelial function (111). Studies have also found that there
are other potent bioactive compounds in tomatoes, such as
flavonoids, whose nanomolar concentrations protect the cofactor
tetrahydrobiopterin from peroxynitrite radicals and maintain the
action of endothelial nitric oxide synthase (eNOS) (112). The inhibition of arginase enzyme
and of NADPH oxidase combined with O2, which causes a
positive NO balance in the EC (113) and prevents apoptosis through p53,
has also been shown to inhibit the synthesis of endothelian-1
(ET-1) (114). Its immediate
anti-apoptotic function is to block the JNK and p38 MAPK signaling
pathways, and its resistance to LDL oxidation through ROS takes
place through action on the JAK2/ STAT3 pathways (115).
To further understand the endothelial protective
effects of bioactive compounds, such as those mentioned above, or
those of other bioactive compounds that may be present in the
tomato, future studies should focus on the three most important
properties of the endothelium: i) markers of vascular tone control,
i.e., concentrations of asymmetric dimethylarginine (ADMA), NO,
eNOS and ET-1; ii) markers of the regulation of hemostasis, i.e.,
concentrations of prostacyclin, plasminogen activator inhibitor-1
(PAI-1) and tissue plasminogen activator (tPA); and iii) markers of
the immune system, i.e., the presence of sICAM-1, sE-selectin,
sVCAM-1, IL-6, TNF-α and CRP high-sensitivity (hsCRP).
Conclusion
The initiation and development of CVD is marked by
platelet-endothelial interaction. This interaction promotes the
expression of adhesion molecules on the endothelium and the
recruitment of inflammatory cells, and stimulates the activation of
circulating platelets. In the prevention of CVD, the consumption of
F&V is crucial. At the level of primary prevention, tomato
consumption promotes cardiovascular health through its role in
platelet anti-aggregation activity and its endothelium-protective
effects. Platelet anti-aggregation activity is regulated by one or
more bioactive compounds that act on ADP and collagen receptors.
Further research is required in the identification of mechanisms of
action of bioactive compounds. In the endothelium, carotenoids and
polyphenols act mainly on eNOS and NAPDH-oxidase in order to
control the levels of NO and to ensure a reduction in the
inflammatory response.
References
|
1.
|
World Health Organization: Informe sobre
la salud en el mundo. Technical Report.WHO; Geneva, Switzerland:
2002
|
|
2.
|
Jackson CF and Wenger NK: Cardiovascular
disease in the elderly. Rev Esp Cardiol. 64:697–712.
2011.PubMed/NCBI
|
|
3.
|
Palomo I, Toro C and Alarcón M: The role
of platelets in the pathophysiology of atherosclerosis (Review).
Mol Med Rep. 1:179–184. 2008.PubMed/NCBI
|
|
4.
|
Palomo I, Alarcón M, Moore-Carrasco R and
Argilés J: Hemostasis alterations in metabolic syndrome (Review).
Int J Mol Med. 18:969–974. 2006.PubMed/NCBI
|
|
5.
|
Palomo I, Torres G, Alarcon M, Maragaño P,
Leiva E and Mujica V: Alta prevalencia de factores de riesgo
cardiovascular clásicos en una población de estudiantes
universitarios de la región centro-sur de Chile. Rev Esp Cardiol.
59:1099–1105. 2006.PubMed/NCBI
|
|
6.
|
Palomo I, Icaza G, Mujica V, et al:
Prevalencia de factores de riesgo cardiovascular clásicos en
población adulta de Talca, Chile, 2005. Rev Med Chile. 135:904–912.
2007.PubMed/NCBI
|
|
7.
|
Lubos E, Handy D and Loscalzo J: Role of
oxidative stress and nitric oxide in atherothrombosis. Front
Biosci. 13:5323–5344. 2009.PubMed/NCBI
|
|
8.
|
Badimón L, Vilahur G and Padró T:
Lipoproteínas, plaquetas y aterotrombosis. Rev Esp Cardiol.
62:1161–1178. 2009.
|
|
9.
|
Roldán I: Nuevos antiagregantes en el
síndrome coronario agudo. El futuro es hoy Rev Esp Cardiol Suppl.
10:12D–22D. 2010.
|
|
10.
|
Santos M, Aranda E, Vallés J and Palomo I:
Hemostasia primaria. Hematología: Fisiopatología y Diagnóstico.
Palomo I, Pereira J and Palma J: Editorial Universidad de Talca;
Talca: pp. 459–492. 2005
|
|
11.
|
Zhang J, Blackmore PF, Hargrave BY, Xiao
S, Beebe SJ and Schoenbach KH: Nanosecond pulse electric field
(nanopulse): a novel non-ligand agonist for platelet activation.
Arch Biochem Biophys. 471:240–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Panes O, Matus V, Sáez CG, Quiroga T,
Pereira J and Mezzano D: Human platelets synthesize and express
functional tissue factor. Blood. 109:5242–5250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Aukrust P, Halvorsen B, Ueland T, et al:
Activated platelets and atherosclerosis. Expert Rev Cardiovasc
Ther. 8:1297–1307. 2010. View Article : Google Scholar
|
|
14.
|
Dauchet L, Amouyel P, Hercberg S and
Dallongeville J: Fruit and vegetable consumption and risk of
coronary heart disease: a meta-analysis of cohort studies. J Nutr.
136:2588–2593. 2006.PubMed/NCBI
|
|
15.
|
Koleckar V, Brojerova E, Rehakova Z, et
al: In vitro antiplatelet activity of flavonoids from Luzea
carthamoides. Drug Chem Toxicol. 31:27–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Khan AN, Fatima I, Khaliq UA, Malik A,
Miana GA, Qureshi ZU and Rasheed H: Potent anti-platelet
constituents from Centaurea iberica. Molecules.
16:2053–2064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Palomo I, Moore-Carrasco R, Carrasco G,
Villalobos P and Guzmán L: El consumo de tomates previene el
desarrollo de enfermedades cardiovasculares y cáncer: antecedentes
epidemiológicos y mecanismos de acción. Idesia. 28:121–129.
2010.
|
|
18.
|
Silverstein R: Inflammation,
atherosclerosis, and arterial thrombosis: role of the scavenger
receptor CD36. Cleve Clin J Med. 76:S27–S30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nesbitt WS, Westein E, Tovar-Lopez FJ, et
al: A shear gradient-dependent platelet aggregation mechanism
drives thrombus formation. Nat Med. 15:665–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lindemann S, Kramer B, Seizer P and Gawaz
M: Platelets, inflammation and atherosclerosis. J Thromb Haemost.
5:203–211. 2007. View Article : Google Scholar
|
|
21.
|
Cimmino G, D’Amico C, Vaccaro V, D’Anna M
and Golino P: The missing link between atherosclerosis,
inflammation and thrombosis: is it tissue factor? Expert Rev
Cardiovasc Ther. 9:517–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Verhamme P and Hoylaerts M: The pivotal
role of the endothelium in haemostasis and thrombosis. Acta Clinica
Belgica. 61:213–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rau JC, Beaulieu LM, Huntington JA and
Church FC: Serpins in thrombosis, hemostasis and fibrinolysis. J
Thromb Haemost. 5:102–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Van Gils J, Zwaginga J and Hordijk P:
Molecular and functional interactions among monocytes, platelets,
and endothelial cells and their relevance for cardiovascular
diseases. J Leukoc Biol. 85:195–204. 2009.PubMed/NCBI
|
|
25.
|
Palomo I, Torres C, Moore-Carrasco R,
Alarcón M and Maragaño P: Platelet anti-aggregants: mechanisms of
action and use-associated risks. Vitae. 16:133–143. 2009.
|
|
26.
|
Schober A: Chemokines in vascular
dysfunction and remodeling. Arterioscler Thromb Vasc Biol.
28:1950–1959. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Badimón JJ, Santos-Gallego C, Torres F,
Castillo J and Kaski J: Nuevas herramientas en la estratificación
del riesgo cardiovascular. Rev Esp Cardiol Suppl. 11(B): 21–28.
2011.
|
|
28.
|
Lievens D, Zernecke A, Seijkens T, et al:
Platelet CD40L mediates thrombotic and inflammatory processes in
atherosclerosis. Blood. 116:4317–4327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Martín M, Rodríguez I, Palacín M and Coto
E: Polimorfismos de metaloproteasas y válvula aórtica bicúspide.
Rev Esp Cardiol. 63:1382–1389. 2010.
|
|
30.
|
Lievens D, Eijgelaar WJ, Biessen EA,
Daemen MJ and Lutgens E: The multi-functionality of CD40L and its
receptor CD40 in atherosclerosis. Thromb Haemost. 102:206–214.
2009.PubMed/NCBI
|
|
31.
|
Giannini S, Falcinelli E, Bury L,
Guglielmini G, Rossi R, Momi S and Gresele P: Interaction with
damaged vessel wall in vivo in humans induces platelets to
express CD40L resulting in endothelial activation. No effect of
aspirin intake. Am J Physiol Heart Circ Physiol. 300:2072–2079.
2011.
|
|
32.
|
Gleissner C, Von Hundelshausen P and Ley
K: Platelet chemokines in vascular disease. Arterioscler Thromb
Vasc Biol. 28:1920–1927. 2008. View Article : Google Scholar
|
|
33.
|
Totani L and Evangelista V:
Platelet-leukocyte interactions in cardiovascular disease and
beyond. Arterioscler Thromb Vasc Biol. 30:2357–2361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Semple J, Italiano J and Freedman J:
Platelets and the immune continuum. Nat Rev Immunol. 11:264–274.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Siegel-Axel D and Gawaz M: Platelets and
endothelial cells. Semin Thromb Hemost. 33:128–135. 2007.
View Article : Google Scholar
|
|
36.
|
Von Hundelshausen P and Weber C: Platelets
as immune cells: bridging inflammation and cardiovascular disease.
Circ Res. 100:27–40. 2007.PubMed/NCBI
|
|
37.
|
Badimon L, Storey R and Vilahur G: Update
on lipids, inflammation and atherothrombosis. Thromb Haemost.
105:S34–S42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Zineh I, Beitelshees AL, Welder GJ, et al:
Epithelial neutrophilactivating peptide (ENA-78), acute coronary
syndrome prognosis, and modulatory effect of statins. PLoS One.
3:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Zernecke A, Shagdarsuren E and Weber C:
Chemokines in atherosclerosis. Arterioscler Thromb Vasc Biol.
28:1897–1908. 2008. View Article : Google Scholar
|
|
40.
|
An G, Wang H, Tang R, et al: P-Selectin
glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and
a major determinant for Ly-6Chi monocyte recruitment to sites of
atherosclerosis in mice. Circulation. 117:3227–3237. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Cha BY, Shi WL, Yonezawa T, Teruya T,
Nagai K and Woo JT: An inhibitory effect of chrysoeriol on
platelet-derived growth factor (PDGF)-induced proliferation and
PDGF receptor signaling in human aortic smooth muscle cells. J
Pharmacol Sci. 110:105–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Vestweber D, Broermann A and Schulte D:
Control of endothelial barrier function by regulating vascular
endothelial-cadherin. Curr Opin Hematol. 17:230–236. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Komarova Y and Malik A: Regulation of
endothelial permeability via paracellular and transcellular
transport pathways. Annu Rev Physiol. 72:463–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Rojas A and Morales-Segura M: Nitric
oxide, an iceberg in cardiovascular physiology: far beyond vessel
tone control. Arch Med Res. 35:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Jennings L: Mechanisms of platelet
activation: need for new strategies to protect against
platelet-mediated atherothrombosis. Thromb Haemost. 102:248–257.
2009.PubMed/NCBI
|
|
46.
|
Badimon L and Martínez-González J:
Disfunción endotelial. Rev Esp Cardiol Suppl. 6:21A–30A.
2006.PubMed/NCBI
|
|
47.
|
Badimon L, Martinez-Gonzalez J,
Llorente-Cortes V, Rodriguez C and Padro T: Cell biology and
lipoproteins in atherosclerosis. Curr Mol Med. 6:439–456. 2006.
View Article : Google Scholar
|
|
48.
|
Deanfield J, Halcox J and Rabelink T:
Endothelial function and dysfunction: testing and clinical
relevance. Circulation. 115:1285–1295. 2007.PubMed/NCBI
|
|
49.
|
Martinez-Gonzalez J and Badimon L:
Influence of statin use on endothelial function: from bench to
clinics. Curr Pharm Des. 13:1771–1786. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Palomo I, Moore-Carrasco R, Alarcon M,
Rojas A, Espana F, Andres V and Gonzalez-Navarro H: Pathophysiology
of the proatherothrombotic state in the metabolic syndrome. Front
Biosci. 2:194–208. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Matsumoto T, Kobayashi T and Kamata K:
Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med
Chem. 14:3209–3220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Erdogan A, Schaefer MB, Kuhlmann CR, et
al: Activation of Ca2+ activated potassium channels is
involved in lysophosphatidylcholine-induced monocyte adhesion to
endothelial cells. Atherosclerosis. 190:100–105. 2007.
|
|
53.
|
Zou Y, Kim CH, Chung JH, et al:
Upregulation of endothelial adhesion molecules by
lysophosphatidylcholine. Involvement of G protein-coupled receptor
GPR4. FEBS J. 274:2573–2584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Kim EA, Kim JA, Park MH, Jung SC, Suh SH,
Pang MG and Kim YJ: Lysophosphatidylcholine induces endothelial
cell injury by nitric oxide production through oxidative stress. J
Matern Fetal Neonatal Med. 22:325–331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Barbaux S, Poirier O, Pincet F, Hermand P,
Tiret L and Deterre P: The adhesion mediated by the P-selectin
P-selectin glycoprotein ligand-1 (PSGL-1) couple is stronger for
shorter PSGL-1 variants. J Leukoc Biol. 87:727–734. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Ruggeri Z and Mendolicchio L: Adhesion
mechanisms in platelet function. Circ Res. 100:1673–1685. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Lu D, Scully M, Kakkar V and Lu X: ADAM-15
disintegrin-like domain structure and function. Toxins.
2:2411–2427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Charrier-Hisamuddin L, Laboisse C and
Merlin D: ADAM-15: a metalloprotease that mediates inflammation.
FASEB J. 22:641–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Badimon L and Vilahur G: Platelets,
arterial thrombosis and cerebral ischemia. Cerebrovasc Dis.
24:30–39. 2007. View Article : Google Scholar
|
|
60.
|
World Health Organization: Joint WHO/FAO
Expert Consultation on Diet, Nutrition, and the Prevention of
Chronic Diseases. WHO; Geneva, Switzerland: 2003
|
|
61.
|
He FJ, Nowson CA, Lucas M and MacGregor
GA: Increased consumption of fruit and vegetables is related to a
reduced risk of coronary heart disease: meta-analysis of cohort
studies. J Hum Hypertens. 21:717–728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Iqbal R, Anand S, Ounpuu S, et al: Dietary
patterns and the risk of acute myocardial infarction in 52
countries: results of the INTERHEART study. Circulation.
118:1929–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Carlsen MH, Halvorsen BL, Holte K, et al:
The total antioxidant content of more than 3100 foods, beverages,
spices, herbs and supplements used worldwide. Nutr J. 9:1–11. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Lako J, Trenerry V, Wahlqvist M,
Wattanapenpaiboon N, Sotheeswaran S and Premier R: Phytochemical
flavonols, carotenoids and the antioxidant properties of a wide
selection of Fijian fruit, vegetables and other readily available
foods. Food Chem. 101:1727–1741. 2007. View Article : Google Scholar
|
|
65.
|
Badimon L, Vilahur G and Padro T:
Nutraceuticals and atherosclerosis: human trials. Cardiovasc Ther.
28:202–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Badimon L and Vilahur G: Enfermedad
aterotrombótica coronaria: avances en el tratamiento
antiplaquetario. Rev Esp Cardiol. 61:501–513. 2008.
|
|
67.
|
Palomo I, Gutiérrez M, Astudillo L, et al:
Efecto antioxidante de frutas y hortalizas de la zona central de
Chile. Rev Chil Nutr. 36:152–158. 2009. View Article : Google Scholar
|
|
68.
|
Dauchet L, Péneau S, Bertrais S, et al:
Relationships between different types of fruit and vegetable
consumption and serum concentrations of antioxidant vitamins. Br J
Nutr. 100:633–641. 2008. View Article : Google Scholar
|
|
69.
|
Palomo I, Yuri J, Moore-Carrasco R,
Quilodrán A and Neira A: El consumo de manzanas contribuye a
prevenir el desarrollo de enfermedades cardiovasculares y cáncer:
antecedentes epidemiológicos y mecanismos de acción. Rev Chil Nutr.
37:377–385. 2010.
|
|
70.
|
Pierre S, Crosbie L and Duttaroy A:
Inhibitory effect of aqueous extracts of some herbs on human
platelet aggregation in vitro. Platelets. 16:469–473. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Torres-Urrutia C, Guzmán L,
Schmeda-Hirschmann G, et al: Antiplatelet, anticoagulant, and
fibrinolytic activity in vitro of extracts from selected fruits and
vegetables. Blood Coagul Fibrinolysis. 22:197–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Nuez F: El cultivo del tomate. Editorial
Mundi-Prensa; Barcelona: pp. 16–20. 1995
|
|
73.
|
Mekhfi H, Gadi D, Bnouham M, Ziyyat A,
Legssyer A and Aziz M: Effect of argan oil on platelet aggregation
and bleeding time: a beneficial nutritional property. J Complement
Integr Med. 5:1–18. 2008. View Article : Google Scholar
|
|
74.
|
Palomo I, Fuentes E, Carrasco G, González
D and Moore-Carrasco R: Actividad antioxidante, hipolipemiante y
antiplaquetaria del tomate (Solanum lycopersicum L.), y el
efecto de su procesamiento y almacenaje. Rev Chil Nutr. 37:524–533.
2010. View Article : Google Scholar
|
|
75.
|
Dutta-Roy A, Crosbie L and Gordon M:
Effects of tomato extract on human platelet aggregation in vitro.
Platelets. 12:218–227. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Lazarus S, Dutta-Roy A and Garg M: Aqueous
tomato extract inhibits platelet aggregation. Asia Pac J Clin Nutr.
11(Suppl): S2402002.
|
|
77.
|
Yamamoto J, Taka T, Yamada K, et al:
Tomatoes have natural anti-thrombotic effects. Br J Nutr.
90:1031–1038. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Fuentes E, Astudillo L, Gutiérrez M, et
al: Fractions of aqueous and methanolic extracts from tomato
(Solanum lycopersicum L.) present platelet antiaggregant
activity. Blood Coagul Fibrinolysis. 23:109–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Lazarus S and Garg M: Tomato extract
inhibits human platelet aggregation in vitro without increasing
basal cAMP levels. Int J Food Sci Nutr. 55:249–256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
O’Kennedy N, Crosbie L, van Lieshout M,
Broom JI, Webb DJ and Duttaroy AK: Effects of antiplatelet
components of tomato extract on platelet function in vitro and ex
vivo: a time-course cannulation study in healthy humans. Am J Clin
Nutr. 84:570–579. 2006.PubMed/NCBI
|
|
81.
|
O’Kennedy N, Crosbie L, Whelan S, et al:
Effects of tomato extract on platelet function: a double-blinded
crossover study in healthy humans. Am J Clin Nutr. 84:561–569.
2006.PubMed/NCBI
|
|
82.
|
Provesi J, Odebrecht C and Amante E:
Changes in carotenoids during processing and storage of pumpkin
puree. Food Chem. 128:195–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Nguyen ML and Schwartz SJ: Lycopene:
chemical and biological properties. Food Technol. 58:38–44.
1999.
|
|
84.
|
Shi J and Le M: Lycopene in tomatoes:
chemical and physical properties affected by food processing. Crit
Rev Biotechnol. 20:293–334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Agrawal H, Kaul N, Paradkar AR and Mahadik
KR: Stability indicating HPTLC determination of clopidogrel
bisulphate as bulk drug and in pharmaceutical dosage form. Talanta.
61:581–589. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
Lazarus S, Bowen K and Garg M: Tomato
juice and platelet aggregation in type 2 diabetes. JAMA.
292:805–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Murphy K, Chronopoulos A, Singh I, et al:
Dietary flavanols and procyanidin oligomers from cocoa
(Theobroma cacao) inhibit platelet function. Am J Clin Nutr.
77:1466–1473. 2003.PubMed/NCBI
|
|
88.
|
Born G and Cross M: The aggregation of
blood platelets. J Physiol. 168:178–195. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
89.
|
Unsworth AJ, Smith H, Gissen P, Watson SP
and Pears CJ: Submaximal inhibition of protein kinase c restores
ADP-induced dense granule secretion in platelets in the presence of
Ca2+. J Biol Chem. 286:21073–21082. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90.
|
Kim S, Jin J and Kunapuli S: Akt
activation in platelets depends on Gi signaling pathways. J Biol
Chem. 279:4186–4195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91.
|
Guidetti GF, Bernardi B, Consonni A, Rizzo
P, Gruppi C, Balduini C and Torti M: Integrin α2β1 induces
phosphorylation-dependent and phosphorylation-independent
activation of phospholipase Cγ2 in platelets: role of Src kinase
and Rac GTPase. J Thromb Haemost. 7:1200–1206. 2009.
|
|
92.
|
Kim SD, Lee IK, Lee WM, et al: The
mechanism of anti-platelet activity of davallialactone: involvement
of intracellular calcium ions, extracellular signal-regulated
kinase 2 and p38 mitogen-activated protein kinase. Eur J Pharmacol.
584:361–367. 2008. View Article : Google Scholar
|
|
93.
|
Liu FC, Liao CH, Chang YW, Liou JT and Day
YJ: A new insight of anti-platelet effects of sirtinol in platelet
aggregation via cyclic AMP phosphodiesterase. Biochem Pharmacol.
77:1364–1373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94.
|
Ferreiro J, Gómez-Hospital J, Angiolillo D
and Cequier A: Los nuevos antagonistas del receptor P2Y12 pueden
reemplazar a los inhibidores de la glucoproteína IIb/IIIa? Rev Esp
Cardiol Suppl. 11(A): 14–19. 2011.
|
|
95.
|
Angiolillo DJ and Ferreiro JL: Platelet
adenosine diphosphate P2Y12 receptor antagonism: benefits and
limitations of current treatment strategies and future directions.
Rev Esp Cardiol. 63:60–76. 2010.
|
|
96.
|
Olas B, Wachowicz B, Tomczak A, Erler J,
Stochmal A and Oleszek W: Comparative anti-platelet and antioxidant
properties of polyphenol-rich extracts from: berries of Aronia
melanocarpa, seeds of grape and bark of Yucca schidigera
in vitro. Platelets. 19:70–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
97.
|
Spiel AO, Gilbert JC and Jilma B: Von
Willebrand factor in cardiovascular disease: focus on acute
coronary syndromes. Circulation. 117:1449–1459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98.
|
Fernández-Ortiz A, Núñez-Gil I,
Ruiz-Mateos B and Ibáñez B: Propiedades de los diferentes
inhibidores de la glucoproteína IIb/ IIIa: se puede aceptar el
efecto de clase? Rev Esp Cardiol Supl. 11:3–7. 2011.
|
|
99.
|
Paran E, Novack V, Engelhard YN and
Hazan-Halevy I: The effects of natural antioxidants from tomato
extract in treated but uncontrolled hypertensive patients.
Cardiovasc Drugs Ther. 23:145–151. 2009. View Article : Google Scholar
|
|
100.
|
Silaste ML, Alfthan G, Aro A, Kesäniemi YA
and Hörkkö S: Tomato juice decreases LDL cholesterol levels and
increases LDL resistance to oxidation. Br J Nutr. 98:1251–1258.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
101.
|
Kim JY, Paik JK, Kim OY, Park HW and Lee
JH, Jang Y and Lee JH: Effects of lycopene supplementation on
oxidative stress and markers of endothelial function in healthy
men. Atherosclerosis. 215:189–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
102.
|
Hung CF, Huang TF, Chen BH, Shieh JM, Wu
PH and Wu WB: Lycopene inhibits TNF-alpha-induced endothelial
ICAM-1 expression and monocyte-endothelial adhesion. Eur J
Pharmacol. 586:275–282. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
103.
|
Cavallini C, Trettene M, Degan M, Delva P,
Molesini B, Minuz P and Pandolfini T: Anti-angiogenic effects of
two cystine-knot miniproteins from tomato fruit. Br J Pharmacol.
162:1261–1273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104.
|
Lee D, Grantham R, Mannion J and Trachte
A: Carotenoids enhance phosphorylation of Akt and suppress tissue
factor activity in human endothelial cells. J Nutr Biochem.
17:780–786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
105.
|
Palozza P, Simone R, Catalano A, et al:
Lycopene prevents 7-ketocholesterol-induced oxidative stress, cell
cycle arrest and apoptosis in human macrophages. J Nutr Biochem.
21:34–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
106.
|
Santangelo C, Varì R, Scazzocchio B, Di
Benedetto R, Filesi C and Masella R: Polyphenols, intracellular
signalling and inflammation. Ann Ist Super Sanita. 43:394–405.
2007.PubMed/NCBI
|
|
107.
|
Wood L and Gibson P: Dietary factors lead
to innate immune activation in asthma. Pharmacol Ther. 123:37–53.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
108.
|
Tang X, Yang X, Peng Y, Peng Y and Lin J:
Protective effects of lycopene against
H2O2-induced oxidative injury and apoptosis
in human endothelial cells. Cardiovasc Drugs Ther. 23:439–448.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
109.
|
Palozza P, Parrone N, Simone R and
Catalano A: Lycopene in atherosclerosis prevention: an integrated
scheme of the potential mechanisms of action from cell culture
studies. Arch Biochem Biophys. 504:26–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110.
|
Simone R, Russo M, Catalano A, Monego G,
Froehlich K, Boehm V and Palozza P: Lycopene inhibits
Nf-κB-mediated IL-8 expression and changes Redox and PPARγ
signalling in cigarette smoke-stimulated macrophages. PLoS One.
6:1–11. 2011.
|
|
111.
|
Stangl V, Kuhn C, Hentschel S, et al: Lack
of effects of tomato products on endothelial function in human
subjects: results of a randomised, placebo-controlled cross-over
study. Br J Nutr. 105:263–267. 2011. View Article : Google Scholar
|
|
112.
|
McCarty M: Scavenging of
peroxynitrite-derived radicals by flavonoids may support
endothelial NO synthase activity, contributing to the vascular
protection associated with high fruit and vegetable intakes. Med
Hypotheses. 70:170–181. 2008. View Article : Google Scholar
|
|
113.
|
Weseler A and Bast A: Oxidative stress and
vascular function: implications for pharmacologic treatments. Curr
Hypertens Rep. 12:154–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
114.
|
Duran X, Vilahur G and Badimon L:
Exogenous in vivo NO-donor treatment preserves p53 levels and
protects vascular cells from apoptosis. Atherosclerosis.
205:101–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
115.
|
Choi J, Choi Y, Shin S, et al: Dietary
flavonoids differentially reduce oxidized LDL-induced apoptosis in
human endothelial cells: role of MAPK- and JAK/STAT-signaling. J
Nutr. 138:983–990. 2008.
|
















