Introduction
In the past 50 years, the molecular bases of blood
coagulation and the anticoagulant pathways have been explained, and
several genetic risk factors for venous thrombosis have been
identified. These genetic risk factors affect the natural
anticoagulant mechanisms and result in a hypercoagulable state due
to an imbalance between procoagulant and anticoagulant forces.
Thrombosis is a lifelong risk and thrombotic events tend to occur
when one or more of the circumstantial risk factors come into play.
Venous thromboembolism (VTE) is a typical multifactorial disease
whose pathogenesis involves acquired and genetic mechanisms.
Acquired factors involve prolonged bedrest, surgery,
pregnancy and malignancies. The genetic factors include mutations
in factors involved in the coagulation-fibrinolytic system, such as
mutations in Factor V Leiden (1),
prothrombin G20210A (2,3) or methylenetetrahydrofolate reductase
C677T (4), and mutations resulting
in deficiency of antithrombin, protein C (PC) or protein S.
Evidence indicates that the PC pathway is part of the natural
anticoagulation system and plays an important role in maintaining
the balance between coagulation and anticoagulation. Previous
studies showed that the endothelial protein C receptor (EPCR), a
key component of the PC pathway (5), can increase the activation efficiency
of PC 5-fold, leading to markedly elevated anticoagulation
activity. The most recent study has shown that blocking EPCR can
accelerate thrombus development in vivo (6). These findings indicate that EPCR may
play a role in the susceptibility to and the development of venous
thrombosis. To elucidate this possibility more thoroughly, we
performed a case-control study to investigate the role of EPCR in
VTE and to examine the relationship between the presence of the
6936A/G polymorphism of EPCR and the occurrence of VTE.
Materials and methods
Study subjects
Between January 2008 and June 2010, 112 patients (64
males, 48 females) who had been diagnosed by duplex ultrasonography
with lower extremity deep venous thrombosis (DVT) at Shandong
Provincial Hospital, Shandong University, China, were recruited
into the VTE group. The mean age was 46.8±9.1 years (range, 27–92
years). Of the patients, 59 (52.7%) were outpatients and 53 (47.3%)
were hospitalized patients. Patients with hematological diseases,
liver and kidney dysfunctions, infections, autoimmune diseases,
tumors, or those receiving thrombolytic treatment or anticoagulant
treatment were excluded from this study. Venous thrombosis was
localized on the left side in 67 patients (59.8%), on the right
side in 39 patients (34.8%) and on both sides in five patients
(4.5%). A total of six patients had pulmonary embolism (PE) (5.4%),
five patients presented with DVT and PE and one patient had an
idiopathic PE. The characteristics of the patients are shown in
Table I. A total of 112 healthy
unrelated subjects were recruited into the control group after
being interviewed regarding whether they had been diagnosed with
VTE or other associated diseases. The mean age of the normal donors
was 48.5±7.4 years (range, 19–81 years). In total, 71 (63.4%) of
the healthy subjects were males and 41 (36.6%) were females.
Characteristics of the study population are shown in Table II. Informed consent was obtained
from all study subjects following explanation of the nature of the
study. The study was approved by Shandong University Research
Ethics Committee, China.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | n | % |
|---|
| Left lower extremity
DVT | 67 | 59.8 |
| Right lower extremity
DVT | 39 | 34.9 |
| Thrombosis on both
sides | 5 | 4.5 |
| DVT at iliofemoral
level | 30 | 26.8 |
| DVT at
femoropopliteal level | 27 | 24.1 |
| DVT at calf
level | 54 | 48.2 |
| Idiopathic PE | 1 | 0.9 |
| PE secondary to
DVT | 5 | 4.5 |
 | Table II.Characteristics of the case-control
study population. |
Table II.
Characteristics of the case-control
study population.
| Total | Males | Females | Mean age (years) |
|---|
| Cases | 112 | 64 | 48 | 46.8±9.1 |
| Controls | 112 | 71 | 41 | 48.5±7.4 |
DNA extraction and genotyping
Venous blood was obtained from each subject and
genomic DNA was extracted using a DNA extraction kit (Tianamp
Biotech, Beijing, China) according to the manufacturer's
instructions, then stored at −70°C until use. ELISA was applied to
detect levels of plasma sEPCR in patient plasma samples (USCNLIFE,
Beijing, China). Genomic DNA was analyzed by polymerase chain
reaction (PCR). The primers for EPCR were designed as previously
reported with the following sequences for sense,
5′-GCTTCAGTCAGTTGGTAAAC-3′ and antisense,
5′-TCTGGCTTCACAGTGAGCTG-3′, which were used in a 25-μl mixture for
amplification. The cycling conditions for PCR were 30 cycles of
denaturation (94°C for 45 sec), annealing (57°C for 45 sec) and
extension (72°C for 45 sec). A preheating step at 94°C for 5 min
and a final extension step for 7 min at 72°C were also performed.
The products were stored at 4°C. Amplified products were later
mixed and buffered with restriction endonuclease PstI and
sustained in a water bath overnight for digestion. Finally, all
products were observed following the polyacrylamide gel
electrophoresis.
Statistical approach
All of the statistical analyses were carried out
using SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA)
and data were presented as the mean ± standard deviation.
Comparisons between two groups were performed by the independent
t-test; Chi-square analysis was applied to determine the difference
in the genotype and gene frequency. Genotype and the risk for VTE
were expressed by odds ratio (OR) at a 95% confidence interval
(CI). A value of P<0.05 was considered to indicate statistical
significance.
Results
Three genotypes, AA, AG and GG, in the EPCR gene at
position 6936 were noted in the VTE group as well as in the control
group. Fig. 1 shows the EPCR gene
6936A/G enzyme cut electrophoresis of restriction endonuclease
PstI. The frequencies of EPCR 6936A/G genotypes and alleles
in the VTE and healthy subjects are shown in Table III. In the VTE group, the frequency
of mutational genotypes (AG+GG) was 36.6% (OR=1.912; 95% CI,
1.064–2.818), which was significantly higher than that in the
control group (20.5%, P<0.05). Furthermore, the frequency of the
G allele (19.6%; OR=1.784; 95% CI, 1.113–2.891; P<0.05) in the
VTE group was significantly higher than that in the control group
(10.8%).
 | Table III.Frequencies of genotypes and
alleles. |
Table III.
Frequencies of genotypes and
alleles.
| Genotype frequency n
(%)
| Allele frequency
|
|---|
| AA n (%) | AG n (%) | GG n (%) | A n (%) | G n (%) |
|---|
| VTE group | 69 (61.6) | 38 (33.9) | 3 (2.7) | 180 (80.4) | 44 (19.6) |
| Control group | 89 (79.5) | 22 (19.6) | 1 (0.9) | 200 (89.2) | 24 (10.8) |
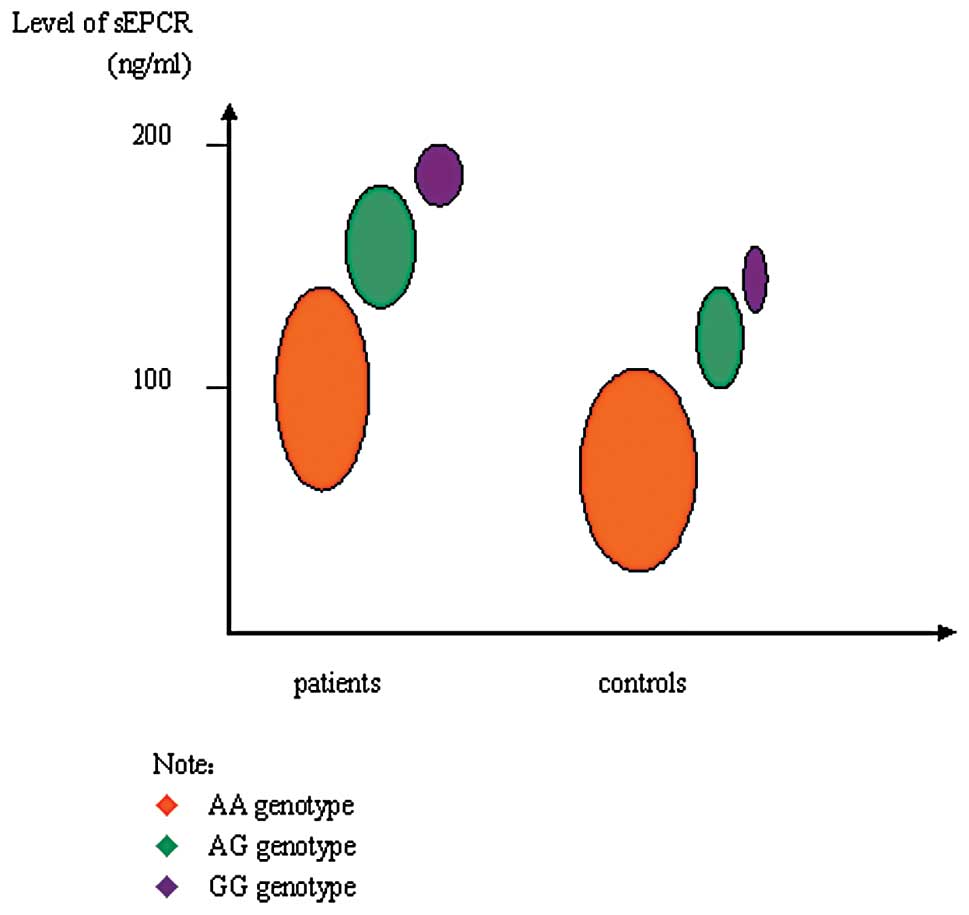
The plasma level of sEPCR in the VTE group
(132.6±61.3 ng/ ml) was significantly higher than that in the
control group (94.1±30.6 ng/ml; P<0.05). Levels of sEPCR among
different genotypes are shown in Fig.
2. In the VTE group, the level of sEPCR in subjects with the AG
genotype and GG genotype as a whole (189.2±53.7 ng/ml) was
significantly higher than in subjects with the AA genotype
(92.1±25.0 ng/ml, P<0.05). In the control group, the level of
sEPCR in subjects with the AG genotype and GG genotype as a whole
(143.5±54.3 ng/ml) was significantly higher than that in subjects
with the AA genotype (76.3±26.8 ng/ml, P<0.05).
Discussion
VTE is increasingly regarded as a polygenic disease.
It was reported that approximately 60% of VTE patients have genetic
risk factors and more than one third of VTE patients have a family
history (7). Genetic risk factor
assessment has become an integral component of the diagnostic
evaluation of patients who present with the signs and symptoms of
venous thrombosis (8).
Thrombophilia is caused by a set of acquired and inherited
conditions that confer a tendency for thrombus formation. The PC
pathway is a part of the natural anticoagulation system and plays
an important role in maintaining the balance between coagulation
and anticoagulation. PC, a vitamin K-dependent zymogen, is
activated at the endothelial surface when thrombin binds to
thrombomodulin, a protein that transforms the procoagulant enzyme
into a potent activator of PC. In the presence of its cofactor,
protein S, activated protein C (aPC) inactivates factors Va and
VIIIa, thereby reducing thrombin generation. EPCR, a type 1
transmembrane protein (9) that is
homologous to the major histocompatibility complex class 1/ CDI
family of proteins, was identified more recently at the surface of
endothelial cells. EPCR demonstrates a relatively endothelial
cell-specific expression pattern, with the expression levels being
notably higher on large vessel endothelium, particularly large
arteries, and low or absent on capillaries. This receptor, which
binds PC or aPC with the same affinity, is mainly expressed on
endothelial cells of large vessels. The EPCR concentration plays a
major role in determining protein C activation. Up-regulation of
EPCR by thrombin (10) or
down-regulation of EPCR expression by cytokines or proteolytic
attack (11) would, based on the
present study, contribute directly to PC activation and serve to
modulate the critical control of the blood clotting process.
The mechanism relating elevated sEPCR levels to
venous thrombosis remains to be determined. Increased sEPCR
concentrations result in decreased aPC generation and inhibit
generated aPC, with possible implications for the regulation of
coagulation, since a low circulating aPC level has been shown to be
a risk factor for venous thromboembolism (11). Extensive analyses of the EPCR gene
identified several polymorphisms, which involved 3 haplotypes
(13,14). The A3 haplotype, one of the three,
was markedly associated with high sEPCR levels. The high
constitutive sEPCR levels observed in A3 haplotype carriers may
reduce the efficiency of the PC system and predispose these
subjects to venous thrombosis. The molecular mechanism by which the
A3 haplotype increases the plasma sEPCR level remains to be
identified. Several polymorphisms defining the A3 haplotype are
located within intronic regions. One of the possible explanations
for the increased sEPCR levels associated with the A3 haplotype is
a putative conformational change in the protein due to the Ser 219
to Gly substitution (resulting from the 6936 A to G mutation). This
residue is located in the trans-membrane (15) domain, near another glycine residue,
and these two adjacent Gly residues may destabilize the helical
transmembrane domain and thus may change the exposure of the
cleavage site, resulting in a protein that is more sensitive to
metalloproteinase cleavage.
In our study, the frequencies of the 6936AG and
6936GG genotypes in the VTE group were significantly higher than
that in the control group, which suggest an increased risk for
thrombosis in patients with the 6936AG and 6936GG genotypes. In our
study, the G allele frequency in the VTE patients was increased
when compared with the healthy control subjects, which was
consistent with other studies (16,17).
This result suggests that the EPCR gene indicates susceptibility to
VTE and is associated with VTE pathogenesis. In conclusion, our
results indicate that the plasma sEPCR level is associated with the
6936A/G polymorphism of the EPCR gene. The plasma sEPCR level in
VTE patients was higher than that in the healthy control subjects
and the frequencies of the EPCR gene 6936AG and 6936GG genotypes in
the VTE patients were markedly increased when compared with the
healthy subjects. These findings suggest that the EPCR gene 6936A/G
polymorphism may be a candidate risk factor for VTE.
References
|
1.
|
Davies KA, Ireland H, Athanassiou P,
Loizou S, Lane D and Walport MJ: Factor V Leiden mutation and
venous thrombosis. Lancet. 345:132–133. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Laposata M: The prothrombin G20210A
mutation: a new high-prevalence congenital risk factor for
thrombosis. Gastroenterology. 116:213–215. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Seligsohn U and Lubetsky A: Genetic
susceptibility to venous thrombosis. N Engl J Med. 344:1222–1231.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Frosst P, Blom HJ, Milos R, et al: A
candidate genetic risk factor for vascular disease: a common
mutation in methylenetetrahydrofolate reductase. Nat Genet.
10:111–113. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Stearns-Kurosawa DJ, Kurosawa S, Mollica
JS, Ferrell GL and Esmon CT: The endothelial cell protein C
receptor augments protein C activation by the
thrombin-thrombomodulin complex. Proc Natl Acad Sci USA.
93:10212–10216. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Centelles MN, Puy C, López-Sagaseta J,
Fukudome K, Montes R and Hermida J: Blocking endothelial protein C
receptor (EPCR) accelerates thrombus development in vivo. Thromb
Haemost. 103:1239–1244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ridker PM: Inherited risk factors for
venous thromboembolism: implications for clinical practice. Clin
Cornerstone. 4:18–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Koster T, Rosendaal FR, Briët E, et al:
Protein C deficiency in a controlled series of unselected
outpatients: an infrequent but clear risk factor for venous
thrombosis (Leiden Thrombophilia Study). Blood. 85:2756–2761.
1995.
|
|
9.
|
Fukudome K and Esmon CT: Identification,
cloning and regulation of a novel endothelial cell protein
C/activated protein C receptor. J Biol Chem. 269:26486–26491.
1994.PubMed/NCBI
|
|
10.
|
Gu JM, Katsuura Y, Ferrell GL, Grammas P
and Esmon CT: Endotoxin and thrombin elevate rodent endothelial
cell protein C receptor mRNA levels and increase receptor shedding
in vivo. Blood. 95:1687–1693. 2000.PubMed/NCBI
|
|
11.
|
Kurosawa S, Stearns-Kurosawa DJ, Carson
CW, D'Angelo A, Della Valle P and Esmon CT: Plasma levels of
endothelial cell protein C receptor are elevated in patients with
sepsis and systemic lupus erythematosus: lack of correlation with
thrombomodulin suggests involvement of different pathological
processes. Blood. 91:725–727. 1998.
|
|
12.
|
Espana F, Vaya A, Mira Y, et al: Low level
of circulating activated protein C is a risk factor for venous
thromboembolism. Thromb Haemost. 86:1368–1373. 2001.PubMed/NCBI
|
|
13.
|
Navarro S, Medina P, Mira Y, et al:
Haplotypes of the EPCR gene, prothrombin levels, and the risk of
venous thrombosis in carriers of the prothrombin G20210A mutation.
Haematologica. 93:885–891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zöller B: Familial thrombophilia: clinical
and molecular analysis of Swedish families with inherited
resistance to activated protein C or protein S deficiency. Scand J
Clin Lab Invest Suppl. 226:19–46. 1996.PubMed/NCBI
|
|
15.
|
Villoutreix BO, Blom AM and Dahlbäck B:
Structural prediction and analysis of endothelial cell protein
C/activated protein C receptor. Protein Eng. 12:833–840. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Saposnik B, Reny JL, Gausem P, Emmerich J,
Aiach M and Gandrille S: A haplotype of the EPCR gene is associated
with increased plasma levels of sEPCR and is a candidate risk
factor for thrombosis. Blood. 103:1311–1318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yamagishi K, Cushman M, Heckbert SR, Tsai
MY and Folsom AR: Lack of association of soluble endothelial
protein C receptor and PROCR 6936A/G polymorphism with the risk of
venous thromboembolism in a prospective study. Br J Haematol.
145:221–226. 2009. View Article : Google Scholar : PubMed/NCBI
|