Introduction
Malignant pleural mesothelioma (MPM) is a thoracic
tumor that arises from surface serosal cells of the pleura. It has
been reported to be associated with inhalation exposure to asbestos
(1). MPM is characterized by
rapidly progressive and diffusely local growth, and a poor
prognosis. Unfortunately, the incidence of MPM has been predicted
to steadily increase and peak over the next two decades (2). However, there is no known curative
modality for MPM (3), and long
term survival is rare even with aggressive multimodal therapy
including extrapleural pneumonectomy (4). Therefore, a new treatment strategy is
required for MPM patients.
Among various molecules, the Wnt gene family encodes
multi-functional signaling glycoproteins that are involved in the
regulation of a wide variety of normal and pathological processes,
including embryogenesis and tumorigenesis (5,6).
Recently, we found that three members of the Wnt family, including
Wnt1, Wnt2B and Wnt5A, are associated with tumorigenesis (7). In fact, many clinical studies have
reported that overexpression of Wnt members is associated with
various tumorigenic processes, such as proliferation, angiogenesis
and patient survival (8–11). However, there are only a few
clinical studies on the molecular biology of human MPM (12).
Therefore, to clarify the tumor biology of MPM, we
performed a comprehensive clinical study on the intratumoral
expression of Wnt1, Wnt2B and Wnt5A, in relation to the
tumor-associated Wnt targets, survivin (13,14)
and c-Myc (15).
Materials and methods
Clinical characteristics of patients
One hundred and seven consecutive MPM patients who
were diagnosed at Kyoto University Hospital, Hyogo Prefectural
Amagasaki Hospital, or Hyogo Prefectural Tsukaguchi Hospital, from
January 1998 to December 2010 were studied. This study was approved
by the Ethics Committee of Kyoto University, and informed consent
was obtained from each patient. All tumors were clinically staged
according to the IMIG staging system (16), and histological classification was
based on the WHO classification (17). The clinical records and
histopathological diagnosis of all patients were fully documented.
This report includes the follow-up data up to December 28,
2010.
Immunohistochemistry
The following antibodies were used: a rabbit
polyclonal antibody for Wnt1 (H89; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) diluted at 1:200, a rabbit polyclonal antibody
for Wnt2B (LS-C31588; LifeSpan Biosciences, Seattle, WA, USA)
diluted at 1.5 μg/ml, a goat polyclonal antibody for Wnt5B (C-16)
diluted at 1:100, a mouse monoclonal antibody for survivin
(sc17779) diluted at 1:50, a mouse monoclonal antibody for c-Myc
(9E10) (all from Santa Cruz Biotechnology, Inc.) diluted at 1:100,
and a mouse monoclonal antibody for the Ki-67 antigen (MIB-1; Dako,
Glostrup, Denmark) diluted at 1:40. Formalin-fixed
paraffin-embedded tissue was cut into 4-μm sections and mounted on
poly-lysine-coated slides. After deparaffinization and rehydration,
the slides were heated in a microwave for 10 min in 10 μmol/l
citrate buffer solution at pH 6.0. After quenching the endogenous
peroxidase activity with 0.3% H2O2 (in
absolute methanol) for 30 min, the sections were treated with 5%
bovine serum albumin. Duplicate sections were incubated overnight
with the primary antibodies, respectively. Slides were then
incubated for 1 h with biotinylated secondary antibodies (Vector
Laboratories, Burlingame, CA, USA). The sections were incubated
with the avidin-biotinperoxidase complex (Vector) for 1 h, and
antibody binding was visualized with 3,3′-diaminobenzidine
tetrahydrochloride. Lastly, the sections were lightly
counterstained with Mayer’s hematoxylin.
The immunostained sections were examined by two
authors (M.K. and C.H.) without knowledge of the patient
characteristics. Cases with discrepancies were jointly reevaluated
until a consensus was reached. At least 200 cells were scored per
×40 field about tumour cells. The percentage of carcinoma cells
with positive staining for Ki-67 in a given specimen was scored as
the Ki-67 proliferation index (9).
Detection of apoptosis
The TUNEL method was performed using the In
Situ Apoptosis Detection kit (Takara Biomedicals, Otsu, Japan).
After deparaffinization and rehydration, the sections were treated
with 20 μg/ml proteinase K for 15 min. After quenching the
endogenous peroxidase activity with 3% H2O2
for 5 min, the sections were incubated for 90 min at 37°C with the
TUNEL reaction mixture, including terminal deoxynucleotidyl
transferase (TdT). Next, the sections were incubated for 30 min at
37°C with anti-FITC horseradish peroxidase conjugate. Staining was
detected by 3,3′-diaminobenzidine tetrahydrochloride incubation for
15 min. Lastly, the sections were lightly counterstained with
Mayer’s hematoxylin. In each case, a total of 10,000 tumor cells
were evaluated by two authors (M.K. and C.H.) independently,
without knowledge of the patient characteristics. The apoptotic
index was defined as the number of apoptotic cells per 1,000 tumor
cells (18).
Statistical analysis
The statistical significance of Wnt1, Wnt2B, Wnt5A,
survivin, and c-Myc expression was assessed by t-test, ANOVA with
Bonferroni/Dunn test or Pearson’s correlation coefficient. The
Kaplan-Meier method was used to estimate the probability of overall
survival as a function of time, and differences in the survival of
subgroups of patients were compared by using Mantel’s log-rank
test. A multivariate analysis was performed using the Cox
regression model to study the effects of different variables on
survival. The sample was classified as a Wnt1-high tumor when the
percentage of Wnt1-positive tumor cells was >50%, and as a
Wnt5A-high tumor when the percentage of Wnt5A-positive tumor cells
was >30%, as reported previously (8,9). The
sample was classified as a Wnt2B-high tumor when the percentage of
Wnt2B-positive tumor cells was >50%, a nuclear survivin-high
tumor when the percentage of nuclear survivin-positive tumor cells
was >20%, and a c-Myc-high tumor when the percentage of
c-Myc-positive tumor cells was >30%, as this had the highest
significance value in relation to the Ki-67 proliferation index.
The sample was classified as a Ki-67-high tumor when the Ki-67
proliferation index was >30% as this had the highest
significance value in relation to patient survival. All p-values
were based on two-tailed statistical analysis, and a p-value of
<0.05 was considered to indicate statistical significance.
Results
Wnt1 expression in MPMs
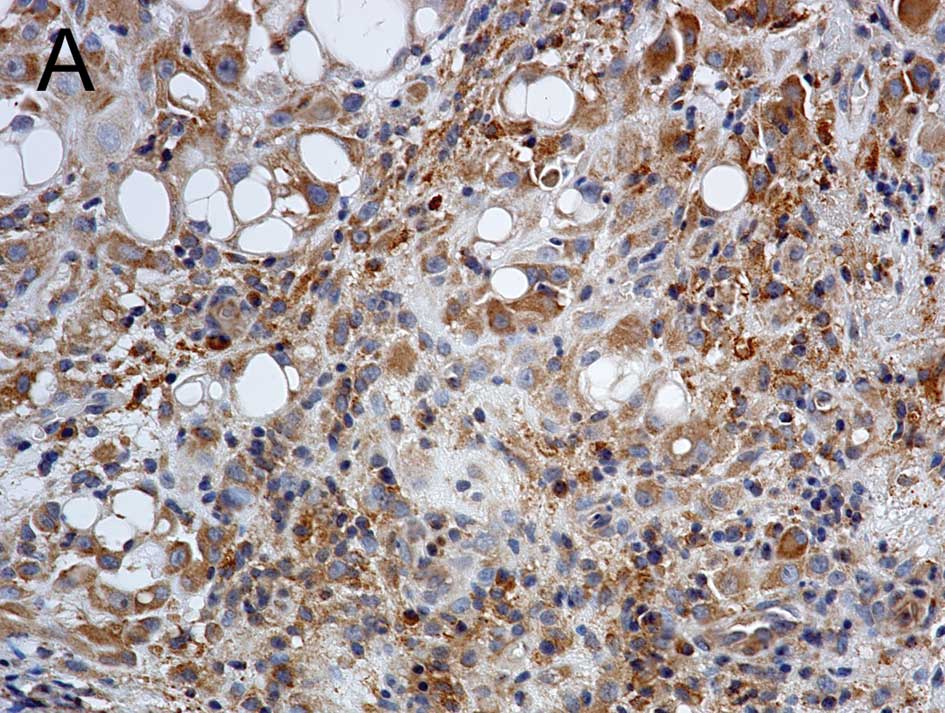
The Wnt1 expression appeared in a cytoplasmic
staining pattern (Fig. 1A). The
percentage of Wnt1-positive tumor cells varied greatly among the
MPM cases (median, 15.0%; mean ± SD, 25.9±26.4%) (Fig. 2). Regarding clinical and
pathological characteristics, the percentage of Wnt1-positive
tumors was significantly higher in stage I patients (p=0.0107)
(Table I). However, no significant
difference was observed in the Wnt1 status according to tumor
histology.
 | Table I.Expression of Wnt family members in
107 patients with malignant pleural mesothelioma according to
clinicopathological characteristics. |
Table I.
Expression of Wnt family members in
107 patients with malignant pleural mesothelioma according to
clinicopathological characteristics.
| Wnt1
| Wnt2B
| Wnt5A
|
|---|
| Positive tumors
(%) | P-value | Positive tumors
(%) | P-value | Positive tumors
(%) | P-value |
|---|
| Age (years) | | | | | | |
| <65 | 25.5±28.5 | 0.8504 | 55.7±33.8 | 0.6150 | 34.6±29.8 | 0.3764 |
| ≥65 | 26.4±24.4 | | 52.6±29.7 | | 29.8±25.8 | |
| Gender | | | | | | |
| Male | 24.3±25.2 | 0.2947 | 54.3±31.4 | 0.9470 | 32.4±28.1 | 0.8772 |
| Female | 30.5±29.9 | | 53.8±33.2 | | 31.5±27.6 | |
| Asbestos
exposure | | | | | | |
| Yes | 19.7±22.6 | 0.0588 | 52.5±30.2 | 0.2384 | 28.3±27.4 | 0.1411 |
| No | 30.3±27.0 | | 60.5±29.6 | | 37.3±26.0 | |
| Smoking | | | | | | |
| Non-smoker | 25.1±26.3 | 0.7582 | 55.7±30.6 | 0.6461 | 33.6±26.3 | 0.6194 |
| Smoker | 26.7±26.7 | | 52.8±32.8 | | 30.9±29.3 | |
| Pathological
stage | | | | | | |
| I | 44.6±32.3 | 0.0107 | 67.4±29.2 | 0.2180 | 46.5±27.1 | 0.0466 |
| II | 29.8±30.0 | | 56.7±27.6 | | 38.3±29.7 | |
| III–IV | 21.6±22.9 | | 51.1±32.9 | | 28.0±26.7 | |
| Histology | | | | | | |
| Epithelioid | 29.2±28.5 | 0.2649 | 58.6±31.0 | 0.1654 | 34.9±29.0 | 0.4713 |
| Sarcomatoid | 19.1±19.8 | | 47.0±30.1 | | 32.9±25.5 | |
| Biphasic | 27.5±26.3 | | 54.9±32.8 | | 23.4±23.4 | |
| Desmoplastic | 13.1±21.5 | | 33.9±34.9 | | 32.1±36.3 | |
| Total number of
patients | 25.9±26.4 | | 54.1±31.7 | | 32.1±27.9 | |
Wnt2B expression in MPMs
The Wnt2B expression was also detected in a
cytoplasmic staining pattern (Fig.
1B). The percentage of Wnt2B-positive tumor cells also varied
greatly among the MPM cases (median, 60.0%; mean ± SD, 54.1±31.7%)
(Fig. 2). No significant
difference was observed in the Wnt2B status according to
pathological stage and tumor histology (Table I).
Wnt5A expression in MPMs
The Wnt5A expression appeared in a cytoplasmic
staining pattern (Fig. 1H). The
percentage of Wnt5A-positive tumor cells varied greatly among the
MPM cases (median, 30.0%; mean ± SD, 32.1±27.9%) (Fig. 2). The percentage of Wnt5A-positive
tumors was significantly higher in stage I patients (p=0.0466)
(Table I). However, no significant
difference was observed in the Wnt5A status according to tumor
histology (Table I).
Predominant expression of different Wnt
proteins in MPMs
Among the 107 MPMs, 23 MPMs (21.5%) were Wnt1-high
tumors, 72 MPMs (67.3%) were Wnt2B-high tumors, and 54 MPMs (50.5%)
were Wnt5A-high tumors. There was no correlation between the
expression levels of the different Wnt proteins. Furthermore, the
percentage of Wnt2B-positive tumors was significantly higher than
that of the other Wnt members (p<0.0001 vs. Wnt1 and p<0.0001
vs. Wnt5A) (Fig. 2).
Survivin expression in MPMs
Immunostaining of the antibody against survivin
showed various patterns of nuclear staining and cytoplasmic
staining (Fig. 1C). The percentage
of nuclear survivin staining was significantly higher than that of
cytoplasmic survivin staining (27.2±30.2 vs. 15.6±25.6%,
p=0.0025).
Regarding tumor biology, the Ki-67 proliferation
index was significantly higher in nuclear survivin-high tumors than
in nuclear survivin-low tumors (60.6±33.8 vs. 37.8±27.8%, p=0.0002)
(Fig. 3A). In contrast, there was
no difference in the apoptotic index according to the cytoplasmic
survivin expression in MPMs (7.0±3.7 in cytoplasmic survivin-high
tumors and 5.7±2.3 in cytoplasmic survivin-low tumors).
Regarding the Wnt status, the percentage of
survivin-positive tumor cells significantly correlated with the
percentage of Wnt2B-positive tumor cells (r=0.335, p<0.001).
However, the percentage of survivin-positive tumor cells did not
correlated with the percentage of Wnt1-positive tumor cells
(p=0.1101) or the percentage of Wnt5A-positive tumor cells
(p=0.4631).
c-Myc expression in MPMs
The percentage of c-Myc-positive tumor cells varied
greatly among the MPM cases (median, 35.0%; mean ± SD, 37.3±30.3%)
(Fig. 1D). Regarding tumor
proliferation, the Ki-67 proliferation index was significantly
higher in c-Myc-high tumors than in c-Myc-low tumors (53.8±31.4 vs.
39.7±31.7%; p=0.0231) (Fig. 3B).
In contrast, there was no difference in the apoptotic index in
relation to the c-Myc status in MPMs (5.2±2.5 in c-Myc-high tumors
and 7.1±3.0 in c-Myc-low tumors).
In regards to the Wnt status, the percentage of
c-Myc-positive tumor cells significantly correlated with the
percentage of Wnt2B-positive tumor cells (r=0.364, p<0.001).
However, the percentage of c-Myc-positive tumor cells did not
correlated with the percentage of Wnt1-positive tumor cells
(p=0.3937) or the percentage of Wnt5A-positive tumor cells
(p=0.3498).
Tumor biology and intratumoral Wnt1
status in MPMs
There was no difference in the Ki-67 proliferation
index in relation to the Wnt1 status in MPMs (35.4±28.2% in
Wnt1-high tumors and 50.1±32.7% in Wnt1-low tumors) (Fig. 3C). Furthermore, there was no
difference in the apoptotic index in relation to the Wnt1 status in
MPMs (11.2±6.0 in Wnt1-high tumors and 4.7±1.8 in Wnt1-low
tumors).
Tumor biology and intratumoral Wnt2B
status in MPMs
Regarding tumor proliferation, the Ki-67
proliferation index was 51.3±31.4% in Wnt2B-high tumors, and
37.9±32.4% in Wnt2B-low tumors (Fig.
3D). The Ki-67 proliferation index was significantly higher in
Wnt2B-high tumors than in Wnt2B-low tumors (p=0.0438). In contrast,
there was no difference in the apoptotic index in relation to the
Wnt2B status in MPMs (7.7±2.8 in Wnt2B-high tumors and 2.9±1.3 in
Wnt2B-low tumors).
Tumor biology and intratumoral Wnt5A
status in MPMs
There was no difference in the Ki-67 proliferation
index in relation to the Wnt5A status in MPMs (48.0±31.1% in
Wnt5A-high tumors and 45.9±33.6% in Wnt5A-low tumors) (Fig. 3E). In addition, there was no
difference in the apoptotic index in relation to the Wnt 5A status
in MPMs (5.6±2.6 in Wnt5A-high tumors and 6.6±2.9 in Wnt5A-low
tumors).
Survival of MPM patients in relation to
the Ki-67 proliferation index and intratumoral Wnt status
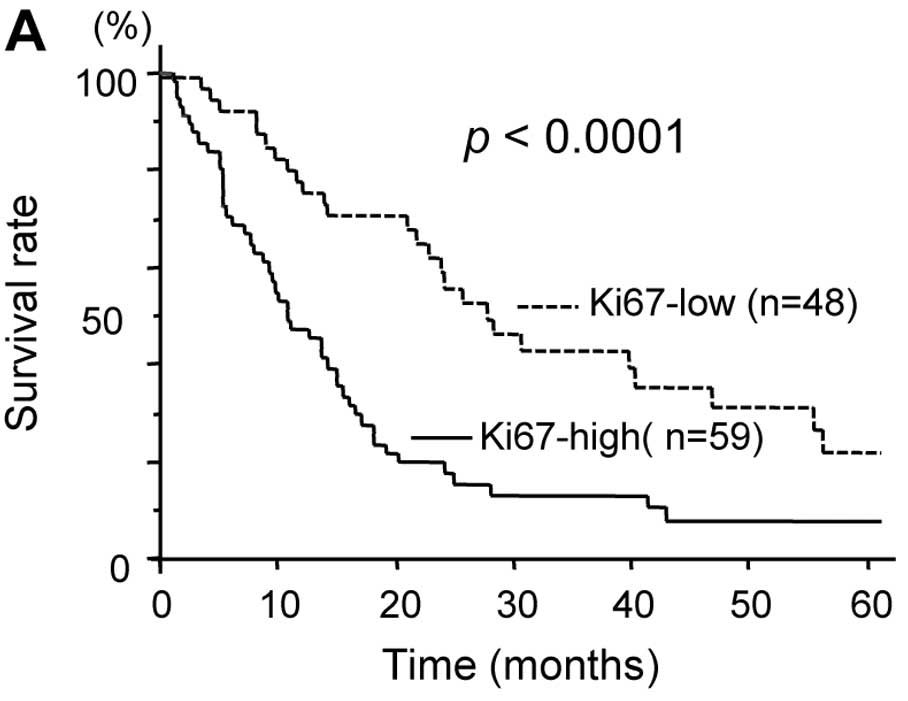
Regarding tumor proliferation, the 5-year survival
rate was significantly lower in patients with high Ki-67 tumors
than in those with low Ki-67 tumors (7.8 vs. 30.4%, p<0.0001)
(Fig. 4A). Regarding the
intratumoral Wnt status, the 5-year survival rate was 11.6% in
patients with Wnt2B-high tumors, and 32.3% in patients with
Wnt2B-low tumors (Fig. 4C). The
overall survival was significantly lower in patients with
Wnt2B-high tumors than in those with Wnt2B-low tumors (p=0.0238).
In particular, the overall survival was significantly lower in
patients with Wnt2B-high epithelioid tumors than in those with
Wnt2B-low epithelioid tumors (20.9 vs. 37.4% at 5-year survival,
p=0.0426) (Fig. 4D). In contrast,
there was no difference in patient survival according to Wnt1
status or Wnt5A status (Fig. 4B and
E). A Cox multivariate analysis demonstrated that Wnt2B status
(hazard ratio 2.396; p=0.0042), pathological stage (hazard ratio
1.455; p=0.0439), and tumor histology (hazard ratio 1.973;
p=0.0074) were significant prognostic factors for MPM patients
(Table II).
 | Table II.Multivariate regression analysis for
predicting survival of 107 patients with malignant pleural
mesothelioma. |
Table II.
Multivariate regression analysis for
predicting survival of 107 patients with malignant pleural
mesothelioma.
| Variables | Assigned score | Hazard ratio | 95% CI | P-value |
|---|
| Wnt2B status | | | | |
| Low | 0 | 2.396 | 1.317–4.359 | 0.0042 |
| High | 1 | | | |
| Age (years) | | | | |
| <65 | 0 | 1.114 | 0.664–1.871 | 0.6821 |
| ≥65 | 1 | | | |
| Gender | | | | |
| Male | 0 | 1.434 | 0.772–2.665 | 0.2536 |
| Female | 1 | | | |
| Asbestos
exposure | | | | |
| No | 0 | 0.745 | 0.441–1.259 | 0.2710 |
| Yes | 1 | | | |
| Smoking | | | | |
| Non-smoker | 0 | 0.989 | 0.577–1.696 | 0.9692 |
| Smoker | 1 | | | |
| Clinical stage | | | | |
| I | 0 | 1.455 | 1.010–2.095 | 0.0439 |
| II | 1 | | | |
| III–IV | 2 | | | |
| Histology | | | | |
| Epithelioid | 0 | 1.973 | 1.200–3.245 | 0.0074 |
| Others | 1 | | | |
Discussion
The clinical outcome of MPM patients is poor even in
patients with early-stage MPM (3).
In fact, 69.2% (74 of 107) of MPM patients included in the present
study had advanced-stage MPM, and the 5-year survival rate was only
22.2% even in stage I patients. The development of new treatment
strategies for MPM patients is therefore critical.
The Wnt family is involved in the regulation of a
wide variety of normal and pathological processes including
tumorigenesis (5,6). We first investigated the expression
of Wnt1, Wnt2B, and Wnt5A in tumor tissues from MPM patients. The
present study showed that the intratumoral expression of Wnt2B was
significantly higher than that of Wnt1 and Wnt5A in MPMs. In
addition, Wnt1 and Wnt5A expression levels were significantly lower
in MPMs than in non-small cell lung cancers that were analyzed
concurrently (data not shown). These results suggest that the
intratumoral Wnt2B expression has an effect on the tumor biology of
MPMs.
Wnt2B is known to stimulate the canonical
Wnt/β-catenin pathway (19). The
activation of the canonical Wnt/β-catenin pathway leads to the
transcription of Wnt-target genes including survivin (13,14),
c-Myc (15), and vascular
endothelial growth factor-A (20).
Therefore, Wnt2B overexpression may affect tumor biology during
tumor progression through the induction of these tumor-associated
Wnt targets.
Furthermore, the present study clearly demonstrated
that the tumor proliferation rate was associated with survival of
MPM patients. In fact, MPM is clinically characterized by rapid and
diffuse local growth, which results in a poor prognosis. In
contrast, the apoptotic index in MPM tissues was significantly
lower than that in non-small cell lung cancers that were analyzed
concurrently (data not shown). We, therefore, evaluated the
expression of survivin (13,14)
and c-Myc (15), Wnt-targets
associated with proliferation.
Previous studies have shown that survivin not only
inhibits the caspase-dependent apoptotic pathway (21,22),
but also accelerates cell proliferation (23). In particular, the nuclear
localization of survivin affects cell mitosis through chromosome
condensation and segregation (24,25).
Previous studies revealed that the nuclear expression of survivin
is associated with tumor proliferation and a poor prognosis in
cancer patients (26–28). The present study also demonstrated
that the nuclear expression of survivin was associated with tumor
proliferation in MPMs. Furthermore, the survival was significantly
lower in patients with nuclear survivin-high tumors than in those
with nuclear survivin-low tumors (p=0.0447) (data not shown).
c-Myc is also a target of the canonical
Wnt/β-catenin pathway (15). c-Myc
is involved in cell cycle progression through the stimulation and
repression of the expression of cell cycle regulators (29). Previous studies have revealed that
c-Myc overexpression is associated with the malignant phenotype in
various human cancers (8,30). The present clinical study also
demonstrated that c-Myc expression was associated with the tumor
proliferation of MPMs.
Finally, we investigated the clinical significance
of Wnt expression in relation to survivin and c-Myc expression.
Consequently, the present study has revealed that the intratumoral
Wnt2B expression is associated with survivin and c-Myc expression,
which results in the acceleration of tumor proliferation.
Furthermore, overall survival was lower in patients with Wnt2B-high
tumors than in those with Wnt2B-low tumors. To our knowledge, this
is the first comprehensive clinical study clearly demonstrating the
clinical significance of intratumoral Wnt2B expression in MPMs. In
conclusion, the present study demonstrated that intratumoral Wnt2B
expression is associated with tumor proliferation and survival of
MPM patients through the induction of survivin and c-Myc. Wnt2B is
therefore a potential candidate for molecular-targeted therapy for
MPMs. In fact, it was recently demonstrated that an adenoviral
vector expressing short hairpin RNA (shRNA) against Wnt2B had a
strong antitumor effect against Wnt2B-overexpressing tumors, via
the down-regulation of survivin and c-Myc, resulting in the
inhibition of tumor proliferation and the induction of apoptosis
(31). Therefore, Wnt2B-inhibiting
gene therapy, including the intrathoracic administration of viral
vectors (32) or non-viral vectors
(33), may be effective as a
therapeutic strategy for Wnt2B-overexpressing MPMs (34).
Acknowledgements
We thank Ms. Seiko Sakai for the
excellent secretarial assistance.
References
|
1.
|
Greillier L and Astoul P: Mesothelioma and
asbestos-related pleural diseases. Respiration. 76:1–15. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bueno R: Mesothelioma clinical
presentation. Chest. 116(Suppl 6): S444–S445. 1999. View Article : Google Scholar
|
|
3.
|
Fujimoto N, Aoe K, Gamba K, Kato K,
Yamazaki K and Kishimoto T: Clinical investigation of malignant
mesothelioma in Japan. J Cancer Res Clin Oncol. 136:1755–1759.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schil PE, Bass P, Gaafar R, et al:
Trimodality therapy for malignant pleural mesothelioma: results
from an EORTC phase II multicentre trial. Eur Respir J.
36:1362–1369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Dale TC: Signal transduction by the Wnt
family of ligands. Biochem J. 329:209–223. 1998.
|
|
6.
|
You Z, Saims D, Chen S, et al: Wnt
signaling promotes oncogenic transformation by inhibiting
c-Myc-induced apoptosis. J Cell Biol. 157:429–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Huang C, Liu D, Masuya D, et al: MRP-1/CD9
gene transduction downregulates Wnt signal pathways. Oncogene.
23:7475–7483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Huang C, Liu D, Ishikawa S, et al: Wnt1
overexpression promotes tumour progression in non-small cell lung
cancer. Eur J Cancer. 44:2680–2688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Huang C, Liu D, Nakano J, et al: Wnt5a
expression is associated with the tumor proliferation and the
stromal vascular endothelial growth factor-A expression in
non-small cell lung cancer. J Clin Oncol. 23:8765–8773. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chen G, Shukeir N, Potti A, et al:
Up-regulation of Wnt-1 and β-catenin production in patients with
advanced metastatic prostate carcinoma: potential pathogenetic and
prognostic implications. Cancer. 101:1345–1356. 2004.
|
|
11.
|
Zhang WM, Lo Muzio L, Rubini C and Yan G:
Effect of WNT-1 on β-catenin expression and its relation to Ki-67
and tumor differentiation in oral squamous cell carcinoma. Oncol
Rep. 13:1095–1099. 2005.
|
|
12.
|
Lee A, Raz DJ, He B and Jablons DM: Update
on the molecular biology of malignant mesothelioma. Cancer.
109:1454–1461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kim PJ, Plescia J, Clevers H, Fearon ER
and Altieri DC: Survivin and molecular pathogenesis of colorectal
cancer. Lancet. 362:205–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ma H, Nguyen C, Lee KS and Kahn M:
Differential roles for the coactivators CBP and p300 on
TCF/β-catenin-mediated survivin gene expression. Oncogene.
24:3619–3631. 2005.PubMed/NCBI
|
|
15.
|
He TC, Sparks AB, Rago C, et al:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Husain AN, Colby TV, Ordonez NG, et al:
Guidelines for pathologic diagnosis of malignant mesothelioma. Arch
Pathol Lab Med. 133:1317–1331. 2009.PubMed/NCBI
|
|
17.
|
Attanoos RL and Gibbs AR: Pathology of
malignant mesothelioma. Histopathology. 30:403–418. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nakano J, Huang C, Liu D, et al: The
clinical significance of splice variants and subcellular
localization of survivin in non-small cell lung cancer. Br J
Cancer. 98:1109–1117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Katoh M, Kirikoshi H, Terasaki H and
Shiokawa K: WNT2B2 mRNA, up-regulated in primary gastric cancer, is
a positive regulator of the WNT-beta-catenin-TCF signaling pathway.
Biochem Biophys Res Commun. 289:1093–1098. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zhang X, Gaspard JP and Chung DC:
Regulation of vascular endothelial growth factor by the Wnt and
K-ras pathways in colonic neoplasia. Cancer Res. 61:6050–6054.
2001.PubMed/NCBI
|
|
21.
|
Lu CD, Altieri DC and Tanigawa N:
Expression of a novel anti-apoptosis gene, survivin, correlated
with tumor cell apoptosis and p53 accumulation in gastric
carcinomas. Cancer Res. 58:1808–1812. 1998.PubMed/NCBI
|
|
22.
|
Kawasaki H, Altieri DC, Lu CD, Toyoda M,
Tenjo T and Tanigawa N: Inhibition of apoptosis by survivin
predicts shorter survival rates in colorectal cancer. Cancer Res.
58:5071–5074. 1998.PubMed/NCBI
|
|
23.
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Li F, Ambrosini G, Chu EY, et al: Control
of apoptosis and mitotic spindle checkpoint by survivin. Nature.
396:580–584. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Adams RR, Carmena M and Earnshaw WC:
Chromosomal passengers and the (aurora) ABCs of mitosis. Trends
Cell Biol. 11:49–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Martinez A, Bellosillo B, Bosch F, et al:
Nuclear survivin expression in mantle cell lymphoma is associated
with cell proliferation and survival. Am J Pathol. 164:501–510.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Shinohara ET, Gonzalez A, Massion PP, et
al: Nuclear survivin predicts recurrence and poor survival in
patients with resected nonsmall cell lung carcinoma. Cancer.
103:1685–1692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Brennan DJ, Rexhepaj E, O’Brien SL, et al:
Altered cytoplasmicto nuclear ratio of survivin is a prognostic
indicator in breast cancer. Clin Cancer Res. 14:2681–2689. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Dang CV, Resar LM, Emison E, et al:
Function of the c-Myc oncogenic transcription factor. Exp Cell Res.
253:63–77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yang G, Timme TL, Frolov A, Wheeler TM and
Thompson TC: Combined c-Myc and caveolin-1 expression in human
prostate carcinoma predicts prostate carcinoma progression. Cancer.
103:1186–1194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lu D, Kadota K, Ueno M, Nakashima N,
Yokomise H and Huang CL: Adenoviral vector expressing short hairpin
RNA targeting Wnt2B has an effective antitumour activity against
Wnt2B2-overexpressing tumours. Eur J Cancer. Jun 4–2011, (Epub
ahead of print).
|
|
32.
|
Rao DD, Vorhies JS, Senzer N, et al: siRNA
vs. shRNA: similarities and differences. Adv Drug Deliv Rev.
61:746–759. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Ma B, Zhang S, Jiang H, Zhao B and Lv H:
Lipoplex morphologies and their influences on transfection
efficiency in gene delivery. J Control Release. 123:184–194. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Watanabe Y, Kojima T, Kagawa S, et al: A
novel translational approach for human malignant pleural
mesothelioma: heparanase-assisted dual virotherapy. Oncogene.
29:1145–1154. 2010. View Article : Google Scholar : PubMed/NCBI
|