Introduction
Despite its declining incidence, non-small cell lung
cancer remains the most common type of malignancy in Northern
China. The estimated overall 5-year survival rate is only 16%
(1). The development of new
treatment modalities, diagnostic technologies and preventive
approaches requires an improved understanding of the molecular
mechanisms of lung cancer. Therefore, the study of molecular
markers to identify risk factors associated with the prognosis of
NSCLC and to develop a more aggressive treatment is required.
Survivin, the smallest member of the inhibitor of
apoptosis (IAP) gene family (2),
is a 142-amino-acid, 16.5-kDa protein encoded by a single-copy gene
located on the human chromosome 17q25. Survivin has been
demonstrated to be involved in the regulation of cell
proliferation, apoptosis and angiogenesis in cancer (3,4).
Increased survivin expression has been observed in a variety of
human neoplasms, including colorectal, breast and rectal cancer,
ovarian carcinoma and lymphoma (3,5–7).
Multiple pathways of transcription and post-transcription control
the expression of survivin, particularly in tumor cells. Survivin
is one of the relatively few genes that is actively repressed by
wild-type p53 (8,9). Certain developmental gene expression
pathways, including Notch, characterize the survivin gene for
differential expression in transformed cells, which is related to
tumorigenesis (10). In addition,
insulin-like growth factor I/mTOR signaling has been reported to
upregulate survivin through rapid changes in mRNA translation
(11). Previous studies have
reported various rates of expression, but little is known about the
prognostic value of survivin. Therefore, in the present large case
study, we performed statistical analyses to determine the
significance of survivin and vascular endothelial growth factor A
(VEGF-A) expression in stage III NSCLC, and the association between
survivin expression and clinical outcome.
Patients and methods
Patients and samples
A total of 210 patients with pathologically proven
stage III NSCLC, who underwent potentially curative tumor resection
at the Third Hospital of Harbin Medical University, Harbin, China
between 2002 and 2004, were included in this study. The study was
approved by the Harbin Medical University Ethics Committee. The
patients had received neither chemotherapy nor radiation therapy
prior to surgery. The patients included 130 males and 80 females,
of mean age 59.8 years (range, 35–76 years). All the specimens were
confirmed as NSCLC following pathological diagnosis. Routinely
processed formalin-fixed, paraffin-embedded blocks containing the
principal tumors were selected. Serial sections (4 μm) were
prepared from the cut surface of tumor blocks at the maximum
cross-section of the tumor.
Immunohistochemical (IHC) staining for
survivin and VEGF-A
IHC staining for survivin and VEGF-A antigen was
conducted using the standard streptavidin-peroxidase-biotin
technique (SP technique) using an SP kit (Zhongshan Co., Beijing,
China). Paraffin sections (4 μm) were deparaffinized in xylene and
then rehydated through graded alcohol. Hydrated autoclave
pretreatment was carried out by boiling for 5 min in citrate buffer
(10 mM, pH 6.0). After endogenous peroxidase was quenched in 3%
hydrogen peroxide and blocked for 10 min, the sections were
incubated overnight at 4°C with a primary polyclonal antibody
(Neomarkers) at a 1:200 dilution. Biotinylated immunoglobulin and
streptavidin-conjugated peroxidase were then added. Finally,
3,3′-diaminobenzidine was used for color development, and
hematoxylin was used for counterstaining. Negative control slides
processed without primary antibody were included for each staining.
The mean percentage of positive tumor cells was determined in at
least five areas at magnification ×200 for survivin and VEGF-A.
They were scored as follows: (0) <5; (1) 5–25; (2) 26–50; (3)
51–75; and (4) >75%. The intensity of immunostaining was scored
as follows: 1+, weak; 2+, moderate; and 3+, intense. The scores
indicating the percentage of positive tumor cells and the staining
intensities were multiplied together to calculate a weighted score
for each case. Cases with weighted scores 0–1 were defined as
negative; while the other cases were defined as positive.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software. Associations between survivin expression and
clinicopathological parameters were analyzed by the χ2
test. The coincident expression of survivin and VEGF-A protein in
the NSCLC tissues was analyzed using Spearman's correlation
analysis. The survival curves were plotted according to the
Kaplan-Meier method and determined by the log-rank test. Univariate
and multivariate regression analyses were performed with the Cox
proportional hazards regression model to analyze the independent
factors related to prognosis. P<0.05 was considered to indicate
a statistically significant difference.
Results
Association between survivin expression
and clinicopathological parameters in NSCLC
The correlation between survivin expression and
clinicopathological factors of NSCLC are shown in Table I. The expression of survivin was
significantly associated with the tumor size (P=0.015), but had no
significant correlation with age, gender, lymph node metastasis,
smoking, histological type or pathological stage.
 | Table I.Correlation between survivin
expression and clinicopathological factors. |
Table I.
Correlation between survivin
expression and clinicopathological factors.
| | Survivin expression
| |
|---|
| Variables | No. of cases
(n=210) | Positive (n=112) | Negative (n=98) | P-value |
|---|
| Age (years) | | | | |
| ≤60 | 89 | 46 | 43 | 0.681 |
| <60 | 121 | 66 | 55 | |
| Gender | | | | |
| Male | 130 | 69 | 61 | 0.924 |
| Female | 80 | 43 | 37 | |
| Tumor size (cm) | | | | |
| ≤3 | 97 | 43 | 54 | 0.015 |
| >3 | 113 | 69 | 44 | |
| Smoker | | | | |
| Yes | 105 | 61 | 44 | 0.167 |
| No | 105 | 51 | 54 | |
| Histology | | | | |
| Squamous cell
carcinoma | 101 | 54 | 47 | 0.996 |
| Adenocarcinoma | 88 | 47 | 41 | |
| Large-cell
carcinoma | 21 | 11 | 10 | |
| Lymph node
metastasis | | | | |
| Yes | 108 | 55 | 53 | 0.472 |
| No | 102 | 57 | 45 | |
| Pathological
stage | | | | |
| G1 | 43 | 22 | 21 | 0.776 |
| G2 | 91 | 47 | 44 | |
| G3 | 76 | 43 | 33 | |
Expression of survivin and VEGF-A in
NSCLC
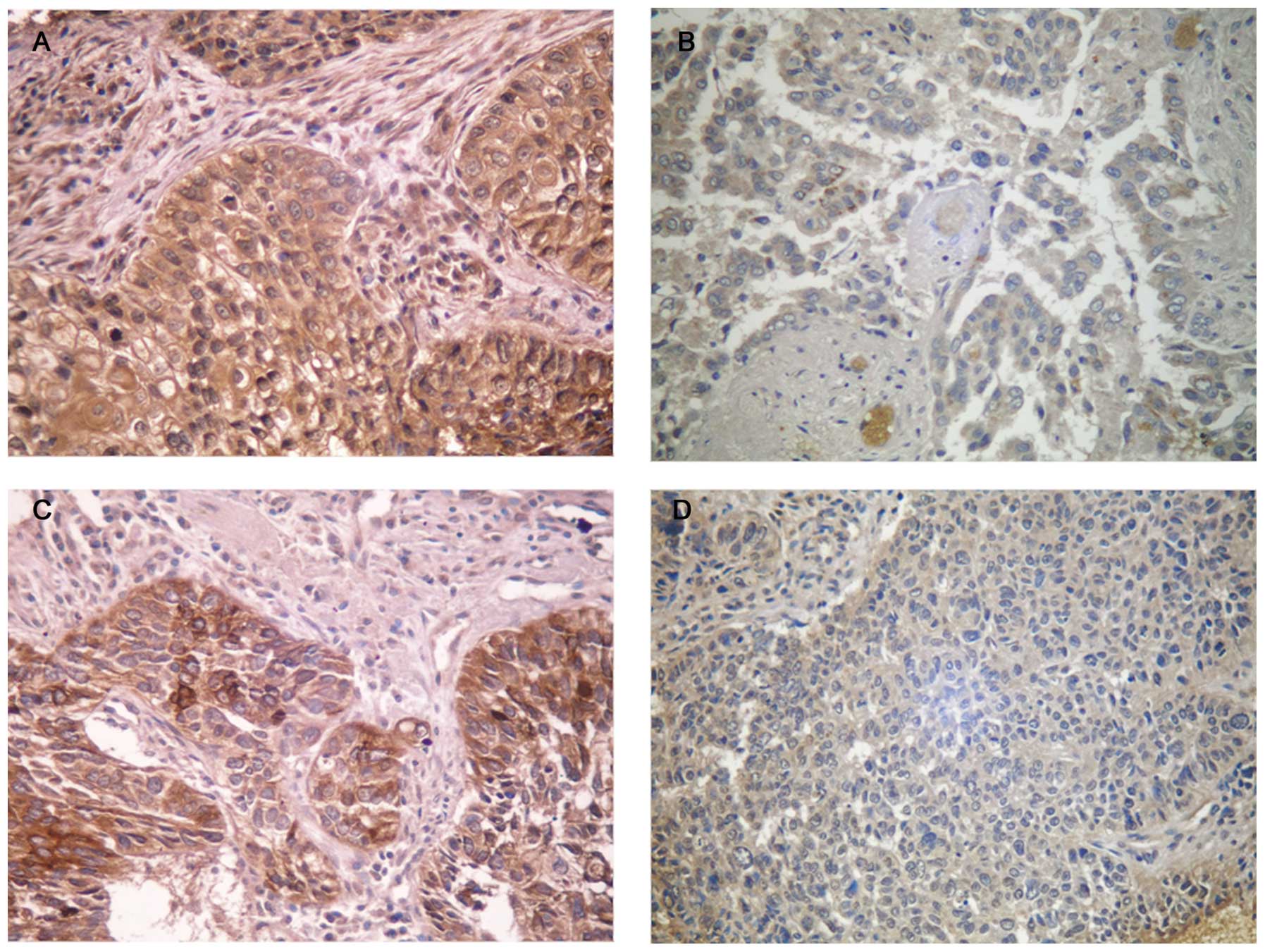
The staining of survivin and VEGF-A was mainly
localized in the cytoplasm (Fig.
1). Among the 210 stage III NSCLC cases, positive expression of
survivin was observed in 112 (53.3%), while negative expression was
observed in 98 (46.7%) cases. Survivin protein was detected in only
4 (10.5%) of 38 benign tissues. Using IHC staining for VEGF-A, 103
(49.0%) cases were positive. Out of the 112 cases of NSCLC which
expressed survivin, the expression of VEGF-A was positive in 67
cases. Of the 98 cases that did not express survivin protein, 62
cases of NSCLC also did not express VEGF-A. The expression of
survivin was coincident with the expression of VEGF-A in NSCLC
(P=0.001) (Table II).
 | Table II.Correlation between survivin and VEGF
expression in NSCLC tissues. |
Table II.
Correlation between survivin and VEGF
expression in NSCLC tissues.
| VEGF expression
| |
|---|
| Survivin
expression | Negative | Positive | P-value |
|---|
| Negative | 62 | 36 | 0.001 |
| Positive | 45 | 67 | |
Association between expression of
survivin and prognosis of stage III NSCLC patients
The 5-year survival rate of the 210 patients with
clinical stage III was 23.8%. The survival rate of patients with
survivin-positive cancer was significantly lower than patients with
survivin-negative cancer (15.2 vs. 33.7%, respectively; P=0.002)
(Fig. 2A). In addition, VEGF-A
positive cancer was associated with poor prognosis (Fig. 2B). The univariate analysis
demonstrated that survivin, tumor size, lymph node metastasis and
VEGF-A expression were significantly correlated with survival time
(Table III). Multivariate analysis
with logistic regression was performed on factors related to
prognosis, and the expression of survivin, VEGF-A and lymph node
metastasis were identified as independent predictive factors of
poor prognosis (Table IV).
 | Table III.Univariate analyses of prognostic
factors for survival of stage III NSCLC patients. |
Table III.
Univariate analyses of prognostic
factors for survival of stage III NSCLC patients.
| Variables | No. of cases
(n=210) | 5-year survival rate
(%) | P-value |
|---|
| Age (years) | | | |
| ≤60 | 89 | 25 (28.1) | 0.212 |
| >60 | 121 | 22 (20.7) | |
| Gender | | | |
| Male | 130 | 28 (21.5) | 0.325 |
| Female | 80 | 22 (27.5) | |
| Tumor size
(cm) | | | |
| ≤3 | 97 | 33 (34.0) | 0.001 |
| >3 | 113 | 17 (15.0) | |
| Smoker | | | |
| Yes | 105 | 24 (22.9) | 0.746 |
| No | 105 | 26 (24.8) | |
| Lymph node
metastasis | | | |
| Yes | 108 | 35 (32.4) | 0.003 |
| No | 102 | 15 (14.7) | |
| Pathological
stage | | | |
| G1 | 43 | 16 (37.2) | 0.059 |
| G2 | 91 | 17 (18.7) | |
| G3 | 76 | 17 (22.4) | |
| Histology | | | |
|
Adenocarcinoma | 101 | 25 (24.8) | 0.759 |
| Squamous cell
carcinoma | 88 | 19 (21.6) | |
| Large-cell
carcinoma | 21 | 6 (28.6) | |
| Survivin
expression | | | |
| Negative | 98 | 33 (31.8) | 0.002 |
| Positive | 112 | 17 (15.2) | |
| VEGF
expression | | | |
| Negative | 107 | 34 (31.8) | 0.006 |
| Positive | 103 | 16 (15.5) | |
 | Table IV.Clinicopathological independent
prognostic factors for survival of stage III NSCLC patients
(multivariate Cox regression analyses). |
Table IV.
Clinicopathological independent
prognostic factors for survival of stage III NSCLC patients
(multivariate Cox regression analyses).
| Variables | Hazard ratio (95%
CI) | P-value |
|---|
| Lymph node
metastasis | | |
| No vs. yes | 1.906
(1.334–2.723) | <0.001 |
| Survivin
expression | | |
| Negative vs.
positive | 1.954
(1.345–2.839) | <0.001 |
| VEGF
expression | | |
| Negative vs.
positive | 1.461
(1.015–2.104) | 0.041 |
Discussion
Along with the development of more aggressive
surgery and new antineoplastic agents, efforts have been made to
identify patients who have a high risk of poor prognosis, as
patients with the same stage NSCLC can have different risks of
recurrence and survival.
Survivin is a recently discovered IAP, which is
undetectable in the majority of normal adult tissues, but is
overexpressed in a variety of human neoplasms, including
colorectal, uterine, esophageal, bladder and liver cancer. This
suggests that reactivation of the survivin gene frequently occurs
in cancer (3). Survivin is a
protein that is cell cycle-regulated with a robust increase in the
G2/M phase in cancer cells (12,13).
Survivin is known to be overexpressed and related with more
aggressive behavior, increased tumor recurrence, chemotherapy
resistance and increased survival in various tumors (6,14–21).
As a prognostic factor, survivin expression is significantly
associated with a poor clinical outcome in certain types of cancer,
including liver (2), colorectal
and breast (3,6). In this large case study, it was
identified that expression of the survivin protein in NSCLC tissues
was positively correlated with tumor size, suggesting that survivin
is significant in NSCLC evolution. Our results demonstrated that in
stage III NSCLC patients, the 5-year survival rate of the NSCLC
patients with survivin overexpression was significantly lower than
that of NSCLC patients with survivin low-expression; this was
significantly associated with poor survival. In addition, survivin
expression was the independent prognostic factor for stage III
NSCLC cancer. Our results demonstrated that detection of survivin
protein expression in the tumor tissue could predict the prognosis
of stage III NSCLC patients.
Tumor growth and development is a complex multistep
process, while angiogenesis is an essential step for tumor growth,
playing a critical role in tumor invasion and metastasis (22). Vascular endothelial growth factor
(VEGF) is considered to be the main growth-stimulating factor in
tumor-related angiogenesis. In esophageal, pancreatic and
colorectal cancer, VEGF overexpression was significantly correlated
with poor patient prognosis, in accordance with other malignancies
(23,24). In malignant pleural mesothelioma,
the serum level of VEGF has been reported to be significantly
correlated with poor survival of patients (25). Our study revealed that in patients
with stage III NSCLC, overexpression of VEGF-A in NSCLC tumor
tissue was significantly correlated with a decreased 5-year
survival rate. The 5-year survival rate in patients with VEGF-A
overexpression was significantly worse than that of patients with
VEGF-A low-expression. These results indicate that VEGF-A may be
signficant in local recurrence and metastasis through the induction
of angiogenesis in NSCLC. VEGF-A expression could be a valuable
marker for prognosis prediction in stage III NSCLC.
Moreover, we found that overexpression of survivin
was significantly positively correlated with overexpression of
VEGF-A in stage III NSCLC. To date, survivin expression appears to
be regulated by growth factors, cytokines, hormones, anticancer
agents and kinase inhibitors. The anti-apoptotic properties of
VEGF-A and IL-11 appear to mediate the induction of survivin in
endothelial cells (13). It has
been revealed that VEGF-A is markedly associated with the
expression of survivin in hepatocellular cancer (26), thyroid carcinoma (27) and breast cancer (28). Recently, it has been considered
that the induction of survivin expression by VEGF-A employs a
PI3K/Akt pathway in neuroblastoma cells (29). Consistent with these findings, we
found a significantly positive correlation between survivin and
VEGF-A expression in NSCLC. Results from our multivariate analysis
indicated that survivin and VEGF-A expression in NSCLC tissues were
strong independent factors of poor prognosis (Table IV). These results indicated that
co-analysis of VEGF-A and survivin protein expression were valuable
for prognosis evaluation of an NSCLC patient.
Since the role and prognostic significance of
survivin in various types of cancer were discovered, different
types of survivin molecular antagonists have been studied (30). Further investigation, however,
concerning this is required. In conclusion, our study demonstrates
that survivin expression is associated with tumor size in stage III
NSCLC. The expression of survivin is consistent with expression of
VEGF-A in NSCLC; it was correlated with a poor prognosis of stage
III NSCLC. Increased systemic treatments or targeted antagonists of
survivin and VEGF-A are required for cases overexpressing survivin
and VEGF-A.
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
2.
|
Srinivasula SM and Ashwell JD: IAPs:
what's in a name? Mol Cell. 30:123–135. 2008.
|
|
3.
|
Hernandez JM, Farma JM, Coppola D, Hakam
A, Fulp WJ, Chen DT, Siegel EM, Yeatman TJ and Shibata D:
Expression of the antiapoptotic protein survivin in colon cancer.
Clin Colorectal Cancer. 10:188–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pavlyukov MS, Antipova NV, Balashova MV,
Vinogradova TV, Kopantzev EP and Shakhparonov MI: Survivin monomer
plays an essential role in apoptosis regulation. J Biol Chem.
286:23296–23307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nowak-Markwitz E, Puła B, Szajnik M,
Dziegiel P, Piotrowska A, Zabel M and Spaczyński M: Expression of
survivin, SDF-1 and CXCR on tumor cells in ovarian cancer. Ginekol
Pol. 81:674–677. 2010.PubMed/NCBI
|
|
6.
|
Kim K, Chie EK, Wu HG, Kim SG, Lee SH,
Kang GH, Hyun CL and Ha SW: High survivin expression as a predictor
of poor response to preoperative chemoradiotherapy in locally
advanced rectal cancer. Int J Colorectal Dis. 26:1019–1023. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Adamkov M, Halasova E, Kajo K, Machalekova
K, Vybohova D, Varga I and Rajcany J: Survivin: a promising
biomarker in breast carcinoma. Neoplasma. 57:572–577. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hoffman WH, Biade S, Zilfou JT, Chen J and
Murphy M: Transcriptional repression of the anti-apoptotic survivin
gene by wild type p53. J Biol Chem. 277:3247–3257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Mirza A, McGuirk M, Hockenberry TN, Wu Q,
Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, Nielsen
LL, Pickett CB and Liu S: Human survivin is negatively regulated by
wild-type p53 and participates in p53-dependent apoptotic pathway.
Oncogene. 21:2613–2622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lee CW, Raskett CM, Prudovsky I and
Altieri DC: Molecular dependence of estrogen receptor-negative
breast cancer on a notch-survivin signaling axis. Cancer Res.
68:5273–5281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Vaira V, Lee CW, Goel HL, Bosari S,
Languino LR and Altieri DC: Regulation of survivin expression by
IGF-1/mTOR signaling. Oncogene. 26:2678–2684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Li F: Survivin study: what is the next
wave? J Cell Physiol. 197:8–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Altieri DC, Marchisio PC and Marchisio C:
Survivin apoptosis: an interloper between cell death and cell
proliferation in cancer. Lab Invest. 79:1327–1333. 1999.PubMed/NCBI
|
|
14.
|
Shu MG, Guo XT, Zhen HN, Han Y, Chen FL,
Li LW and Guo SZ: Enhancing skin flap survival by a cell-permeable
wild-type survivin. Med Hypotheses. 69:888–891. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhu H, Wang Q, Hu C, Zhang W, Quan L, Liu
M, Xu N and Xiao Z: High expression of survivin predicts poor
prognosis in esophageal squamous cell carcinoma following
radiotherapy. Tumour Biol. 32:1147–1153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Trabulo S, Cardoso AM, Santos-Ferreira T,
Cardoso AL, Simões S and Pedroso de Lima MC: Survivin silencing as
a promising strategy to enhance the sensitivity of cancer cells to
chemotherapeutic agents. Mol Pharm. 8:1120–1131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Park E, Gang EJ, Hsieh YT, Schaefer P,
Chae S, Klemm L, Huantes S, Loh M, Conway EM, Kang ES, Hoe Koo H,
Hofmann WK, Heisterkamp N, Pelus L, Keerthivasan G, Crispino J,
Kahn M, Müschen M and Kim YM: Targeting survivin overcomes drug
resistance in acute lymphoblastic leukemia. Blood. 118:2191–2199.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Takizawa BT, Uchio EM, Cohen JJ, Wheeler
MA and Weiss RM: Downregulation of survivin is associated with
reductions in TNF receptors' mRNA and protein and alterations in
nuclear factor kappa B signaling in urothelial cancer cells. Cancer
Invest. 25:678–684. 2007.PubMed/NCBI
|
|
19.
|
Ai Z, Yin L, Zhou X, Zhu Y, Zhu D, Yu Y
and Feng Y: Inhibition of survivin reduces cell proliferation and
induces apoptosis in human endometrial cancer. Cancer. 107:746–756.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Malcles MH, Wang HW, Koumi A, Tsai YH, Yu
M, Godfrey A and Boshoff C: Characterisation of the anti-apoptotic
function of survivin-DeltaEx3 during TNF alpha-mediated cell death.
Br J Cancer. 96:1659–1666. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Nassar A, Lawson D, Cotsonis G and Cohen
C: Survivin and caspase-3 expression in breast cancer: correlation
with prognostic parameters, proliferation, angiogenesis, and
outcome. Appl Immunohistochem Mol Morphol. 16:113–120. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Giatromanolaki A, Sivridis E and
Koukourakis MI: Angiogenesis in colorectal cancer: prognostic and
therapeutic implications. Am J Clin Oncol. 29:408–417. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kleespies A: Vascular endothelial growth
factor in esophageal cancer. J Surg Oncol. 87:95–104. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yasumitsu A, Tabata C, Tabata R and
Hirayama N: Clinical significance of serum vascular endothelial
growth factor in malignant pleural mesothelioma. J Thorac Oncol.
5:479–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zhu H, Chen XP, Zhang WG, Luo SF and Zhang
BX: Expression and significance of new inhibitor of apoptosis
protein survivin in hepatocellular carcinoma. World J
Gastroenterol. 11:3855–3859. 2005.PubMed/NCBI
|
|
27.
|
Zhang HY, Meng X, Du ZX, et al:
Significance of survivin, caspase-3, and VEGF expression in thyroid
carcinoma. Clin Exp Med. 9:207–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ryan BM, Konecny GE, Kahlert S, Wang HJ,
Untch M, Meng G, Pegram MD, Podratz KC, Crown J, Slamon DJ and
Duffy MJ: Survivin expression in breast cancer predicts clinical
outcome and is associated with HER2, VEGF, urokinase plasminogen
activator and PAI-1. Ann Oncol. 17:597–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Beierle EA, Nagaram A, Dai W, Iyengar M
and Chen MK: VEGF-mediated survivin expression in neuroblastoma
cells. J Surg Res. 127:21–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Nakahara T, Yamanaka K, Hatakeyama S, Kita
A, Takeuchi M, Kinoyama I, Matsuhisa A, Nakano K, Shishido T,
Koutoku H and Sasamata M: YM155, a novel survivin suppressant,
enhances taxane-induced apoptosis and tumor regression in a human
Calu 6 lung cancer xenograft model. Anticancer Drugs. 22:454–462.
2011. View Article : Google Scholar : PubMed/NCBI
|