Introduction
Lung cancer is the leading cause of cancer-related
death in the world (1). In 2010,
an estimated 222,520 new cases and 157,300 deaths were anticipated
in the US (2). Non-small cell lung
cancer (NSCLC) accounts for more than 85% of all cases of lung
cancer (3). Approximately 40% of
patients with NSCLC present with advanced-stage disease at the time
of diagnosis (3). The standard
treatment for these patients is systemic chemotherapy, which
improves both quality of life and survival (4). Until recently, platinum- or
non-platinum-based, two-drug regimens were considered the standard
of care for advanced NSCLC patients (4,5).
However, the vast majority of patients with advanced NSCLC failed
to benefit from combined chemotherapy (6). The WHO and Response Evaluation
Criteria in Solid Tumors (RECIST) criterias, based on radiologic
detections, were used to assess objective response after combined
chemotherapy (7). Usually, the
objective response (OR) of unmeasurable lesions, such as for
atelectasis, pericardial effusion, pleural effusion, lymphatic
vessel invasion and pleural-type tumors are difficult to evaluate
using radiologic results (8).
Moreover, a decrease in tumor volume as determined by radiologic
images cannot accurately predict the survival of patients with
advanced NSCLC (9). Therefore,
more effective and feasible markers are required for the prediction
of chemotherapy response and prognosis in patients with advanced
NSCLC. Serum tumor markers, as a potential and more effective
method to determine chemotherapy response and predict prognosis,
have been studied extensively in the past. It has been proven that
cytokeratin 19 fragment (CYFRA21-1) and carcinoembryonic antigen
(CEA) may be useful predictive factors of chemotherapy response and
prognosis in advanced NSCLC patients. The aim of this study was to
investigate the clinical value of serum CYFRA21-1 and CEA in the
prediction of chemotherapy response and prognosis in patients with
advanced NSCLC.
Materials and methods
Patient inclusion criteria
To be eligible for inclusion in this study, the
following criteria were established: i) patients had a histological
or cytological confirmation of clinical stage IIIB or IV NSCLC; ii)
at least one measurable lesion; iii) patients were able to
withstand at least 2 cycles of first-line platinum-based combined
chemotherapy; iv) Eastern Cooperative Oncology Group (ECOG)
performance status (PS) 0–2; v) no main organ dysfunction and
hematopoietic function, normal liver and renal function, without
any serious complications.
Assessment criteria of objective
response
Assessments of the objective response (OR) were
based on WHO and RECIST criteria (7), including complete response (CR),
partial response (PR), stable disease (SD) or progressive disease
(PD). OR was defined as CR plus PR, and no response (NR) was
defined as SD plus PD. OR was evaluated and a confirmative chest
computed tomography (CT) scan was performed after every 2 cycles of
chemotherapy.
Assessment criteria of serum markers
Two serum samples from untreated lung cancer
patients were prospectively collected: the first prior to the first
cycle of chemotherapy, and the second after the second cycle of
chemotherapy. All samples were frozen and stored at −80°C. All
assays were performed using commercial kits: CYFRA21-1 and CEA
(ELSA; CIS Biointernational, France) (10), with investigators blinded to
clinical information. The cut-off value of CYFRA21-1 and CEA was
3.2 and 3.4 ng/ ml, respectively. Patients were defined as
assessable when at least one serum level of either CYFRA21-1 or CEA
was above the normal cut-off values.
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 software (SPSS Inc., Chicago, IL, USA). Comparisons of
serum CYFRA21-1 or CEA prior to and after 2 cycles of chemotherapy
were analyzed by Wilcoxon's signed rank test. Associations between
categorical variables were evaluated using the χ2-test.
The ROC curve was used to assess the optimal cut-off levels of the
declines of serum CYFRA21-1 and CEA in the diagnosis of radiologic
OR. The Youden index was used to identify the optimal cut-off
levels.
All survival data were updated in May 1, 2011.
Overall survival (OS) was calculated from the initiation of
chemotherapy until death. Time to progression (TTP) was calculated
from the date of registration to progression or last contact.
Survival curves were generated with the Kaplan-Meier method and
compared by the log-rank test and generalized Wilcoxon's test.
Meanwhile, the multivariate survival analysis was performed to
investigate the independent prognostic factors using Cox
proportional hazards regression model. All tests were two-sided,
and a value of P<0.05 was considered statistically
significant.
Results
Baseline characteristics of patients
Between May 2006 and May 2010, a total of 98
patients with advanced NSCLC who were admitted to The First
Affiliated Hospital of China Medical University, were enrolled in
this study. The median age was 58 years (range 27–84), including 65
males and 33 females. There were 61 patients with adenocarcinomas,
28 with squamous carcinomas and 9 with adenosquamous carcinomas.
According to the TNM staging system for lung cancer by the 6th
edition of the International Union Against Cancer (UICC) and the
American Joint Committee on Cancer (AJCC) (11), the study included 53 patients with
stage IIIB and 45 patients with stage IV. There were 54 patients
with a ECOG PS score of 0–1. All patients received a median of 4
cycles of chemotherapy (range 2–6). Baseline characteristics of the
patients are shown in Table I.
 | Table I.Baseline characteristics of the NSCLC
patients. |
Table I.
Baseline characteristics of the NSCLC
patients.
| Characteristics | No. of patients
(%) |
|---|
| Gender | |
| Male | 65 (66.3) |
| Female | 33 (33.7) |
| Age (years) | |
| Median (range) | 58 (27–84) |
| ≤65 | 60 (61.2) |
| >65 | 38 (38.8) |
| Histology | |
| Squamous cell
carcinoma | 28 (28.6) |
| Adenocarcinoma | 61 (62.2) |
| Adenosquamous
carcinomas | 9 (9.2) |
| Clinical stage | |
| III | 53 (54.1) |
| IV | 45 (45.9) |
| ECOG PS score | |
| 0–1 | 54 (55.1) |
| 2 | 44 (44.9) |
| CYFRA21-1 baseline
(ng/ml) | |
| Median value
(range) | 6.4 (1.5–144.7) |
| Normal (≤3.2) | 22 (22.4) |
| Abnormal
(>3.2) | 76 (77.6) |
| CEA baseline
(ng/ml) | |
| Median value
(range) | 13.9 (1.1–985.1) |
| Normal (≤3.4) | 16 (16) |
| Abnormal
(>3.4) | 82 (82) |
| Chemotherapy
response | |
| CR | 1 (1.0) |
| PR | 44 (44.9) |
| SD | 30 (30.6) |
| PD | 23 (23.5) |
| Last follow-up
status | |
| Alive | 19 (19.4) |
| Dead | 79 (80.6) |
Association between chemotherapy response
and decreases in the serum markers
Among 98 patients with advanced NSCLC, 45.9% (45/98)
achieved OR after 2 cycles of chemotherapy, including 1 patient
with CR, 44 patients with PR, 30 patients with SD and 23 patients
with PD. The median values of serum CYFRA21-1 prior to and after
chemotherapy were 6.4 ng/ml (range 1.5–144.7) and 3.4 ng/ml (range
0.6–97.7), respectively. The median values of serum CEA prior to
and after chemotherapy were 13.9 ng/ml (range 1.1–985.1) and 3.9
ng/ml (range 1.0–375.5), respectively. After 2 cycles of
chemotherapy, serum CYFRA21-1 and CEA were significantly decreased
compared to baseline levels (P<0.0001).
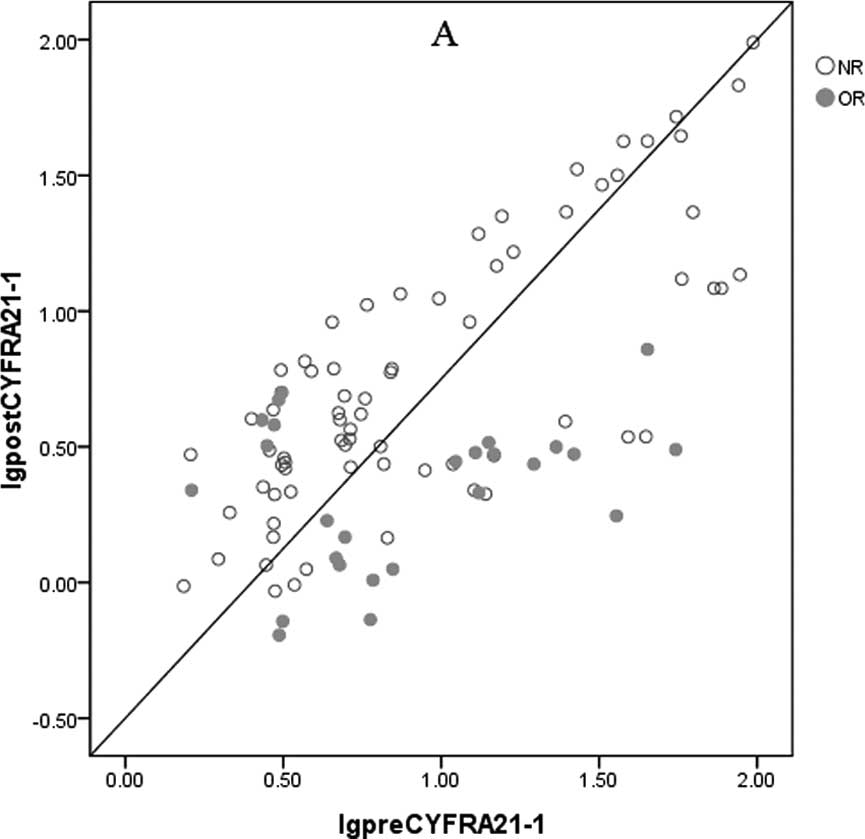
As shown in Fig. 1,
the values in the horizontal axis represent serum CYFRA21-1 and CEA
prior to chemotherapy, and those in the vertical axis represent
serum CYFRA21-1 and CEA after 2 cycles of chemotherapy. This
reflected the relationship between serum CYFRA21-1 or CEA and
radiologic OR. If there was no significant difference in serum
CYFRA21-1 or CEA prior to and after 2 cycles of chemotherapy, the
corresponding points should fall along the straight line. In fact,
most of the data points were below the line, especially for those
of serum CYFRA21-1, suggesting that chemotherapy induced declines
in serum CYFRA21-1 and/or CEA in the majority of patients.
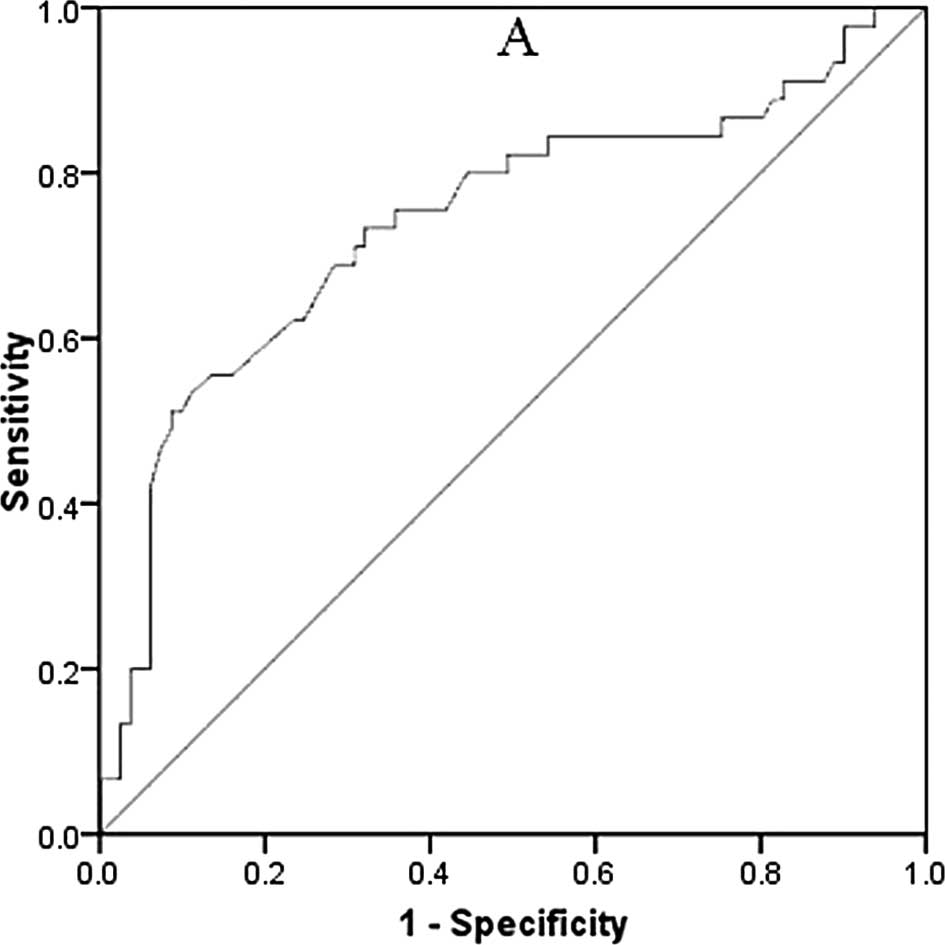
ROC curves of serum CYFRA21-1 and
CEA
Analysis of the ROC curves was carried out to assess
the correlation between declines in serum CYFRA21-1 or CEA and
radiologic OR after 2 cycles of chemotherapy (Fig. 2). The area under the ROC curve
(AUC) was 0.727 (95% CI 0.600–0.857) for CYFRA21-1 and 0.629 (95%
CI 0.48–0.771) for CEA. After 2 cycles of chemotherapy, a ≥60%
reduction in CYFRA21-1 and a ≥25% reduction in CEA were the optimal
cut-off levels with best sensitivity and specificity for the
diagnosis of radiologic OR. When there was a ≥60% reduction in
serum CYFRA21-1, the sensitivity and specificity values were 77.9
and 78.2%, respectively. When there was a ≥25% reduction in serum
CEA, the sensitivity and specificity were 70.5 and 68.7%,
respectively. Therefore, a ≥60% reduction in CYFRA21-1 and a ≥25%
reduction in CEA were defined as ‘serum marker response’.
Univariate and multivariate survival
analysis
During the study period, 79 of 98 (80.6%) patients
with advanced NSCLC died. The median survival of all patients was
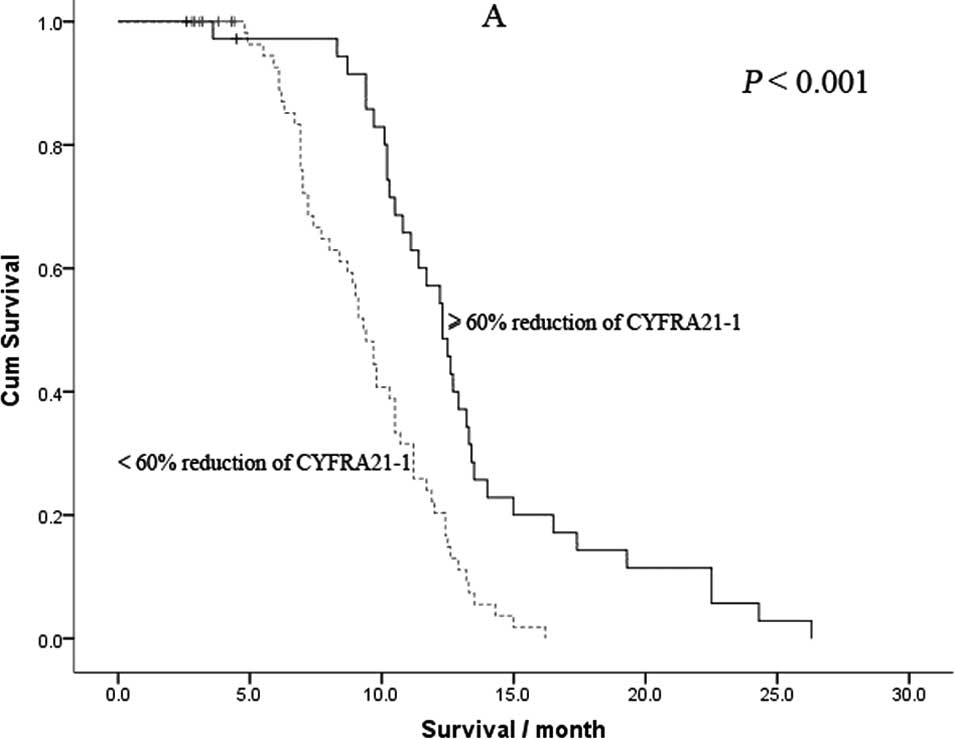
10.2 months (range 2.6–26.3). As shown in Fig. 3, the median OS time in patients
with a ≥60% reduction in CYFRA21-1 was significantly longer
compared to those with a <60% reduction (11.6 vs. 9.3 months,
P<0.001). In addition, the median OS time in patients with a
≥25% reduction in CEA was also significantly longer compared to
those with a <25% reduction (11.2 vs. 8.9 months,
P<0.001).
Univariate survival analysis showed that ECOG PS
score, radiologic OR, a ≥60% reduction in CYFRA21-1 and a ≥25%
reduction in CEA were significant prognostic factors for better OS
(Table II). However, age, gender,
clinical stage, histological type and baseline levels of CYFRA21-1
and CEA were not related to prognosis. Results from the Cox
regression analysis are shown in Table
III. In this analysis, the independent prognostic roles of ECOG
PS score, a ≥60% reduction in CYFRA21-1 and a ≥25% reduction in CEA
were confirmed, while radiologic OR was not an independent
prognostic factor.
 | Table II.Univariate survival analysis. |
Table II.
Univariate survival analysis.
| Prognostic
factors | No. of patients | MST (months) | 95% CI | P-value |
|---|
| Age (years) | | | | 0.504 |
| ≤65 | 60 | 10.1 | 9.4–10.8 | |
| >65 | 38 | 11.3 | 9.9–12.5 | |
| Gender | | | | 0.170 |
| Male | 65 | 11.2 | 10.3–12.1 | |
| Female | 33 | 10.2 | 9.3–10.1 | |
| Clinical stage | | | | 0.761 |
| III | 37 | 10.6 | 9.1–10.3 | |
| IV | 61 | 10.9 | 9.7–11.3 | |
| ECOG PS score | | | | 0.001 |
| 0 | 59 | 11.7 | 9.9–12.3 | |
| 1–2 | 39 | 9.6 | 8.3–10.9 | |
| Histology | | | | 0.088 |
| Squamous cell
carcinoma | 28 | 11.2 | 9.5–12.9 | |
| Adenocarcinoma | 61 | 9.7 | 9.0–10.4 | |
| Adenosquamous
carcinomas | 9 | 10.4 | 9.2–11.1 | |
| Radiologic OR | | | | 0.034 |
| Yes | 55 | 11.3 | 10.4–13.0 | |
| No | 53 | 9.8 | 9.0–10.6 | |
| CYFRA21-1 baseline
level | | | | 0.401 |
| Normal | 22 | 10.7 | 9.1–10.5 | |
| >3.2 ng/ml | 76 | 11.1 | 9.4–11.6 | |
| CEA baseline
level | | | | 0.683 |
| Normal | 16 | 10.7 | 9.7–12.7 | |
| >3.4
ng/ml | 82 | 10.2 | 9.5–10.5 | |
| ≥60% reduction in
CYFRA21-1 | | | | <0.001 |
| Yes | 36 | 11.6 | 11.5–12.9 | |
| No | 62 | 9.3 | 8.8–9.8 | |
| ≥25% reduction in
CEA | | | | <0.001 |
| Yes | 40 | 11.2 | 10.9–13.1 | |
| No | 58 | 8.9 | 9.1–9.9 | |
 | Table III.Multivariate survival analysis. |
Table III.
Multivariate survival analysis.
| Prognostic
factors | HR | 95% CI | P-value |
|---|
| ECOG PS | | | <0.0001 |
| 0 | 1.000 | | |
| 1–2 | 2.904 | 1.763–4.784 | |
| Radiologic OR | | | 0.1910 |
| Yes | 1.000 | | |
| No | 1.563 | 0.807–2.924 | |
| ≥60% reduction
in | | | 0.0010 |
| CYFRA21-1 | | | |
| No | 1.000 | | |
| Yes | 0.254 | 0.110–0.588 | |
| ≥25% reduction in
CEA | | | 0.0380 |
| No | 1.000 | | |
| Yes | 0.417 | 0.182–0.954 | |
Discussion
CYFRA21-1 is an acidic protein of 40 kDa that is
part of the cytoskeleton of epithelial cells (12). CYFRA21-1 is a specific and
reproducible negative-prognostic marker for NSCLC (13). Many studies have confirmed that
CYFRA21-1 is both a sensitive and specific tumor marker for NSCLC
and especially for squamous cell carcinoma (14). It appears more sensitive and more
specific than other tumor markers, such as CEA and NSE, and
slightly better than squamous cell carcinoma-antigen (SCC) in
squamous cell carcinoma (15). CEA
is a glycoprotein expressed during early fetal life, and is the
product of the CEACAM5-gen (16).
CEA is an oncofetal protein attached to epithelial cell apical
membrane via its C-terminal glycosylphosphatidylinositol anchor, a
member of the immunoglobulin superfamily of cell adhesion molecules
(17). Usually, CEA is
overexpressed in a variety of neoplasms, such as colorectal,
breast, bladder, gastric, pancreatic and lung carcinomas (16). CEA is a good monitoring marker for
conventional chemotherapy. High serum CEA levels have been
associated with disease progression and relapse in patients with
advanced NSCLC (18). Several
reports have been published concerning the prognostic value of
serum tumor markers in patients with advanced NSCLC, for example
CYFRA21-1, CEA, NSE and SCC (19).
However, no reports concerning the relationship of declines in
serum CYFRA21-1 or CEA with chemotheapy response and prognosis in
patients with advanced NSCLC have been previously published. To our
knowledge, this study is the first to propose that a ≥60% reduction
in CYFRA21-1 and a ≥25% reduction in CEA after 2 cycles of
chemotherapy can be regarded as possible surrogate markers of
chemotherapy response and prognosis in patients with advanced
NSCLC.
In the present study, we found that decreases in
serum CYFRA21-1 and CEA prior to and after 2 cycles of chemotherapy
were correlated with chemotherapy response. We also found that a
≥60% reduction in CYFRA21-1 and a ≥25% reduction in CEA after 2
cycles of chemotherapy were independent prognostic factors for
patients with advanced NSCLC in multivariate survival analysis,
while radiologic OR was not an independent prognostic factor.
Nisman et al (20) found
that there was no correlation between radiologic OR and survival,
while declines in serum CYFRA21-1 after 2 cycles of chemotherapy
were closely related to survival. Ardizzoni et al (21) studied 107 patients with advanced
NSCLC and also observed that declines in serum CYFRA21-1 and CEA
were closely related to chemotherapy response and survival, whereas
radiologic OR had no correlation with survival. Similar to previous
studies, our study also demonstrated that the declines in serum
CYFRA21-1 and CEA were closely related to chemotherapy response and
survival, especially related to radiologic OR. After 2 cycles of
chemotherapy, a ≥60% reduction in CYFRA21-1 and a ≥25% reduction in
CEA were the optimal cut-off levels, with best sensitivity and
specificity for the diagnosis of radiologic OR. Univariate survival
analysis showed that ECOG PS score, radiologic OR, a ≥60% reduction
in CYFRA21-1 and a ≥25% reduction in CEA were significant
prognostic factors. After 2 cycles of chemotherapy, the median OS
time in patients with a ≥60% reduction in CYFRA21-1 was
significantly longer compared to those with a <60% reduction.
Similarly, the median OS time in patients with a ≥25% reduction in
CEA was also significantly longer compared to those with a <25%
reduction. Multivariate analysis further confirmed the clinical
value of declines in serum CYFRA21-1 and CEA in the prediction of
chemotherapy response and prognosis in patients with advanced
NSCLC. In multivariate analysis, the independent prognostic roles
of ECOG PS score, ≥60% reduction in CYFRA21-1 and ≥25% reduction in
CEA were confirmed, while radiologic OR was not an independent
prognostic factor.
In conclusion, our study demonstrated that a ≥60%
reduction in CYFRA21-1 and a ≥25% reduction in CEA may be reliable
surrogate markers for the prediction of chemotherapy response and
prognosis, particularly for the diagnosis of radiologic OR. Due to
a limitation in the sample of patients, this conclusion should be
further confirmed by large case-control studies with an adequate
methodological quality and properly controlled for possible
confounds.
Acknowledgements
The authors would like to thank Jia-Li
Liu (Department of Oncology, The First People's Hospital, Yueyang,
Hunan) for his valuable contribution and revising of the
manuscript.
References
|
1.
|
Jemal A, Murray T, Samuels A, Ghafoor A,
Ward E and Thun MJ: Cancer statistics, 2003. CA Cancer J Clin.
53:5–26. 2003. View Article : Google Scholar
|
|
2.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3.
|
Lovly CM and Carbone DP: Lung cancer in
2010: one size does not fit all. Nat Rev Clin Oncol. 8:68–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ramalingam SS, Dahlberg SE, Langer CJ,
Gray R, Belani CP, Brahmer JR, Sandler AB, Schiller JH and Johnson
DH; Eastern Cooperative Oncology Group: Outcomes for elderly,
advanced-stage non small-cell lung cancer patients treated with
bevacizumab in combination with carboplatin and paclitaxel:
analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin
Oncol. 26:60–65. 2008. View Article : Google Scholar
|
|
5.
|
Pujola JL, Barlesia F and Daurésa JP:
Should chemotherapy combinations for advanced non-small cell lung
cancer be platinum-based? A meta-analysis of phase III randomized
trials. Lung Cancer. 3:335–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lilenbaum RC, Langenberg P and Dickersin
K: Single agent versus combination chemotherapy in patients with
advanced non-small cell lung carcinoma: a meta-analysis of
response, toxicity, and survival. Cancer. 82:116–126. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nishino M, Jackman DM, Hatabu H, Yeap BY,
Cioffredi LA, Yap JT, Jänne PA, Johnson BE and Van den Abbeele AD:
New Response Evaluation Criteria in Solid Tumors (RECIST)
guidelines for advanced non-small cell lung cancer: comparison with
original RECIST and impact on assessment of tumor response to
targeted therapy. AJR Am J Roentgenol. 195:W221–W228. 2010.
View Article : Google Scholar
|
|
8.
|
Jin B, Huang AM, Zhong RB and Han BH: The
value of tumor markers in evaluating chemotherapy response and
prognosis in Chinese patients with advanced non-small cell lung
cancer. Chemotherapy. 56:417–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Birchard KR, Hoang JK, Herndon JE Jr and
Patz EF Jr: Early changes in tumor size in patients treated for
advanced stage non-small cell lung cancer do not correlate with
survival. Cancer. 115:581–586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Buccheri G and Ferrigno D:
Cytokeratin-derived markers of lung cancer. Expert Rev Mol Diagn.
1:315–322. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Rami-Porta R, Crowley JJ and Goldstraw P:
The revised TNM staging system for lung cancer. Ann Thorac
Cardiovasc Surg. 15:4–9. 2009.PubMed/NCBI
|
|
12.
|
Hanagiri T, Sugaya M, Takenaka M, Oka S,
Baba T, Shigematsu Y, Nagata Y, Shimokawa H, Uramoto H, Takenoyama
M, Yasumoto K and Tanaka F: Preoperative CYFRA 21-1 and CEA as
prognostic factors in patients with stage I non-small cell lung
cancer. Lung Cancer. 74:112–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ando S, Suzuki M, Yamamoto N, Iida T and
Kimura H: The prognostic value of both neuron-specific enolase
(NSE) and Cyfra21-1 in small cell lung cancer. Anticancer Res.
24:1941–1946. 2004.PubMed/NCBI
|
|
14.
|
Sung HJ and Cho JY: Biomarkers for the
lung cancer diagnosis and their advances in proteomics. BMB Rep.
41:615–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Barak V, Goike H, Panaretakis KW and
Einarsson R: Clinical utility of cytokeratins as tumor markers.
Clin Biochem. 37:529–540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Arrieta O, Saavedra-Perez D, Kuri R,
Aviles-Salas A, Martinez L, Mendoza-Posada D, Castillo P, Astorga
A, Guzman E and de la Garza J: Brain metastasis development and
poor survival associated with carcinoembryonic antigen (CEA) level
in advanced non-small cell lung cancer: a prospective analysis. BMC
Cancer. 9:1192009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Horst AK and Wagener C: CEA-related CAMs.
Handb Exp Pharmacol. 165:283–341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang CY, Huang MS, Huang MH, Lee HC and
Hsu HS: Persistently high serum carcinoembryonic antigen levels
after surgery indicate poor prognosis in patients with stage I
non-small-cell lung cancer. J Surg Res. 163:e45–e50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cho WC: Potentially useful biomarkers for
the diagnosis, treatment and prognosis of lung cancer. Biomed
Pharmacother. 61:515–519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Nisman B, Biran H, Heching N, Barak V,
Ramu N, Nemirovsky I and Peretz T: Prognostic role of serum
cytokeratin 19 fragments in advanced non-small-cell lung cancer:
association of marker changes after two chemotherapy cycles with
different measures of clinical response and survival. Br J Cancer.
98:77–79. 2008. View Article : Google Scholar
|
|
21.
|
Ardizzoni A, Cafferata MA, Tiseo M,
Filiberti R, Marroni P, Grossi F and Paganuzzi M: Decline in serum
carcinoembryonic antigen and cytokeratin 19 fragment during
chemotherapy predicts objective response and survival in patients
with advanced non-small cell lung cancer. Cancer. 107:2842–2849.
2006. View Article : Google Scholar
|