Introduction
Malignant pleural mesothelioma (MPM) is an
aggressive malignant tumor of mesothelial origin associated with
asbestos exposure (1–3). Although asbestos usage has recently
been banned in Western countries and Japan, the incidence of MPM is
expected to markedly increase over the next few decades since there
is a long latency period (20–40 years) between asbestos exposure
and tumor development (4). MPM
shows limited response to conventional chemotherapy and
radiotherapy. Although multi-targeted anti-folate pemetrexed has
been approved as a first-line agent in combination with cisplatin
for MPM treatment, overall survival remains very poor (5) with median survival durations of 8–18
months (6). In several centers,
potentially curative surgery combined with some form of adjuvant
therapy has been performed. Such early therapeutic intervention
appears to be more beneficial than late intervention. Diagnosing
MPM is critical (1) since the
general conditions of these patients such as a poor performance
status may hinder adequate therapy. However, diagnosis may be
extremely difficult in histological studies.
Computed tomography (CT) plays a role in identifying
the location and dissemination of malignant pleural tumors
(7), however, it is not always
able to differentiate between malignant and benign pleural lesions
(8). Pleural biopsies such as
video-assisted thoracic surgery (VATS) are required to enable
definite diagnosis of MPM. However, these are invasive procedures;
therefore, new non-invasive techniques for assessment of MPM are
required to judge whether those procedures should be practiced for
diagnosis of MPM.
Currently, 18-fluorodeoxyglucose (FDG) positron
emission tomography (PET) is an important imaging tool for the
diagnostic assessment of patients with cancer (9). PET is useful for detecting malignant
lung nodules (10,11). There have been several reports
concerning the uptake of FDG in MPM and clinical assessment such as
diagnostic and prognostic information of MPM using PET (12–15).
However, its clinical utility in MPM has not been fully
investigated. In this study, we evaluated the diagnostic and
prognostic role of PET in Japanese MPM patients.
Materials and methods
Patients
This study was performed using 76 patients who
presented at the Department of Respiratory Medicine of Hyogo
College of Medicine Hospital from September 2009 to April 2011.
Forty-seven individuals had malignant plural mesothelioma (MPM); 43
individuals were diagnosed using histopathological samples and 4
were diagnosed using cytological samples by pathologists skilled in
the diagnosis of MPM. Thirty-two patients had a documented history
of asbestos exposure. All 47 patients were classified using the
staging system of the International Mesothelioma Interest Group
(IMIG) (16). Patients with MPM
were treated according to our therapeutic guidelines; combination
chemotherapy including multi-target anti-folate pemetrexed or
pemetrexed alone was administered to patients with PS 0–1, and the
best supportive care was chosen for the remaining patients.
Surgical and radiation treatment was performed on 3 patients in the
present study. Twenty-nine individuals, including 3 cases with
benign asbestos pleurisy, had non-malignant pleural effusion. We
verified asbestos exposure by interview. Thirteen patients had a
documented history of asbestos exposure. Informed consent was
obtained from all patients. This study was approved by our ethics
committee in accordance with the 1975 Declaration of Helsinki.
FDG-PET imaging
18F-FDG PET/CT imaging was performed at the PET
Center of Hyogo College of Medicine Hospital. All patients fasted
for at least 6 h before PET/CT examination and their blood glucose
concentrations were measured. Patients were scanned on a PET/CT
scanner (GEMINI GXL 16; Philips Medical System, Eindhoven, The
Netherlands) 60 min after injection of 18F-FDG depending on the
patient’s weight. CT was performed from head to foot at a 5.0-mm
slice thickness. For semi-quantitative assessment, regions of
interest (ROIs) were examined. The maximum SUV (SUVmax) of 18F-FDG
was measured from ROI according to the standard formula. PET and CT
datasets were reported by two independent readers.
Statistical analysis
The nonparametric Mann-Whitney U-test was used to
compare two groups. In all tests, a p-value <0.05 was considered
to indicate statistical significance. In order to estimate the
significance of SUVmax, receiver operating characteristic (ROC)
curves, area under the ROC curves (AUC), and their 95% confidence
intervals (95% CI) were calculated using standard techniques. To
obtain appropriate SUVmax level cut-off values, we calculated the
total sensitivity and specificity for each cut-off value and then
chose the cut-off values that maximized the sum of sensitivity plus
1-specificity. Estimates of the probability of survival were
calculated using the Kaplan-Meier method and compared using the
log-rank test. In order to evaluate SUVmax prognostic significance
on the survival of patients with MPM, Cox’s proportional hazards
regression analysis (backward) was carried out as multivariate
analysis.
Results
SUVmax levels in patients with MPM and
patients with non-MPM
We recruited a total of 76 subjects. Of them, 47 had
confirmed MPM and 29 had non-malignant pleural effusion. Their
characteristics are shown in Table
I. Of the 47 patients with MPM, 31 were of epithelioid
histology, 6 sarcomatoid, 4 biphasic, 1 desmoplastic and 5
unknown.
 | Table I.Characteristics of the study
groups. |
Table I.
Characteristics of the study
groups.
| A, MPM patients
(n=47) |
|
| Patient
characteristics | n (%) |
|
| Age (mean age ± SD),
in years | 65.2±9.6 |
| Gender | |
| Male | 38 (80.9) |
| Female | 9 (19.1) |
| Histology | |
| Epithelioid | 31 (66.0) |
| Sarcomatoid | 6 (12.8) |
| Biphasic | 4 (8.5) |
| Desmoplastic | 1 (2.1) |
| Unknown | 5 (10.6) |
| Stage | |
| I | 9 (19.2) |
| II | 10 (21.3) |
| III | 9 (19.2) |
| IV | 19 (40.3) |
|
| B, Non-malignant
patients (n=29) |
|
| Patient
characteristics | n (%) |
|
| Age (mean age ± SD),
in years | 70.1±11.1 |
| Gender | |
| Male | 22 (75.9) |
| Female | 7 (24.1) |
| CT findings | |
| Benign asbestos
pleurisy | 3 (10.3) |
| Plaque | 5 (17.2) |
| Tuberculosis (TB)
pleurisy | 1 (3.5) |
| Infectious (non-TB)
pleurisy | 1 (3.5) |
| Chronic
pleurisy | 6 (20.7) |
| Pleural
thickening | 12 (41.3) |
| Pleural
effusion | 1 (3.5) |
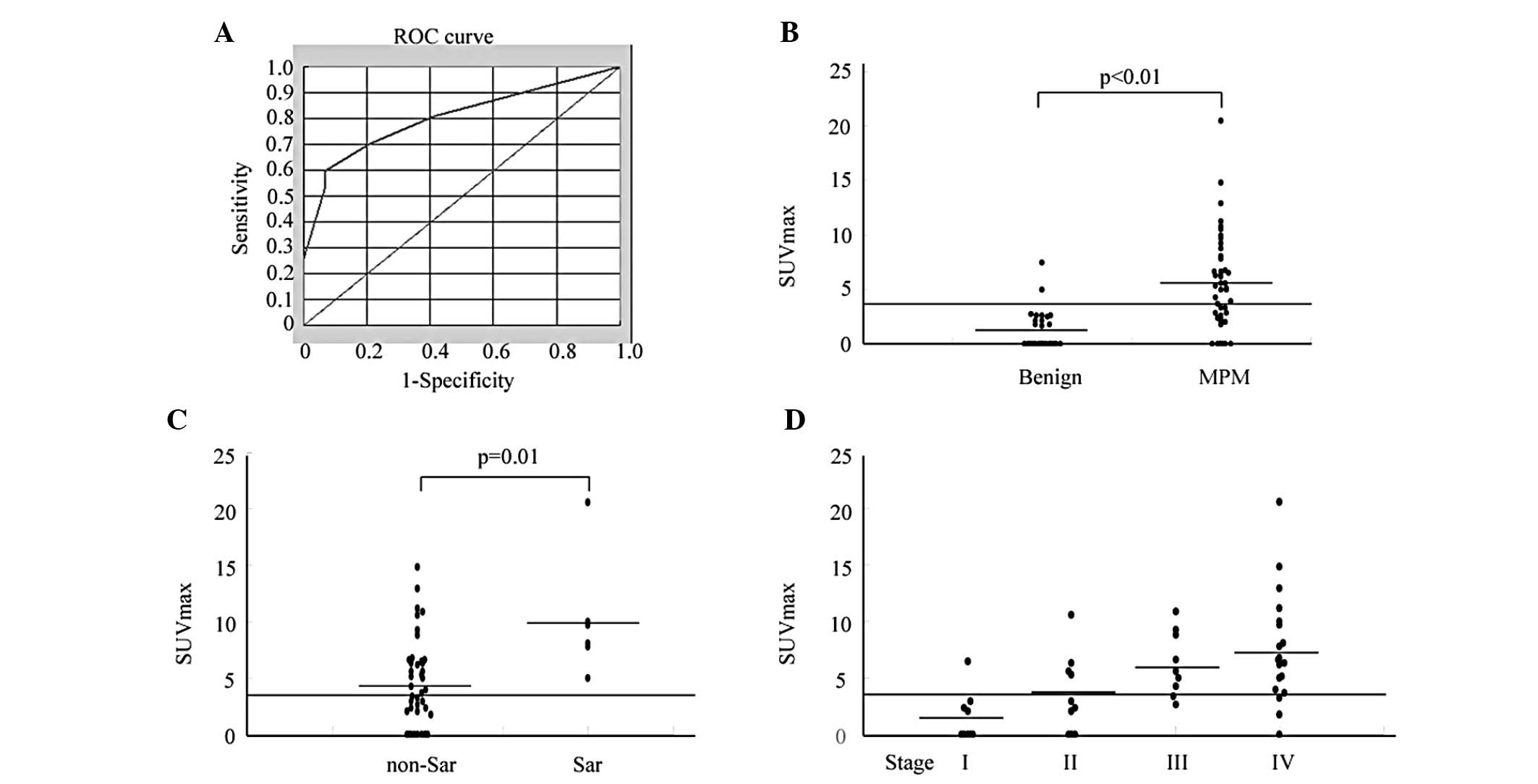
The ROC curves for SUVmax levels demonstrated that
patients with MPM had an AUC of 0.803 which differed from those
with non-MPM (95% CI, 0.722–0.885). At the optimal cut-off value of
3.5, the diagnostic sensitivity was 59.6%, and the specificity was
93.1% (Fig. 1A). The positive
predictive value (PPV) was 93.3%, and the negative predictive value
(NPV) was 58.7%. The SUVmax level of patients with MPM was
significantly higher (5.3±4.4) than that of the non-MPM patients
including those with benign asbestos pleurisy (1.2±1.8) (p<0.01)
(Fig. 1B). Differences in SUVmax
levels between the various MPM histological groups were significant
(non-sarcomatoid, 4.6±3.9 and sarcomatoid, 10.2±5.4, respectively)
(p=0.01) (Fig. 1C). Moreover,
scatter plots of SUVmax levels in MPM demonstrated tendencies to
increase with increasing stage (stage I, 1.5±2.2; stage II,
3.5±3.4; stage III, 6.3±2.8; and stage IV, 7.6±4.9) (p<0.01 by
the nonparametric Kruskal-Wallis test followed by the Mann-Whitney
U-test) (Fig. 1D). However, there
were no significant differences in SUVmax levels by gender (male,
5.2±4.4 and female, 5.5±4.7) and age (≤65, 5.5±4.3 and >65
years, 5.1±4.7). There were no significant differences in SUVmax
levels between patients with benign asbestos pleurisy and those
with non-MPM (1.8±1.5 and 1.1±1.8, respectively).
Relationship between SUVmax levels and
overall survival
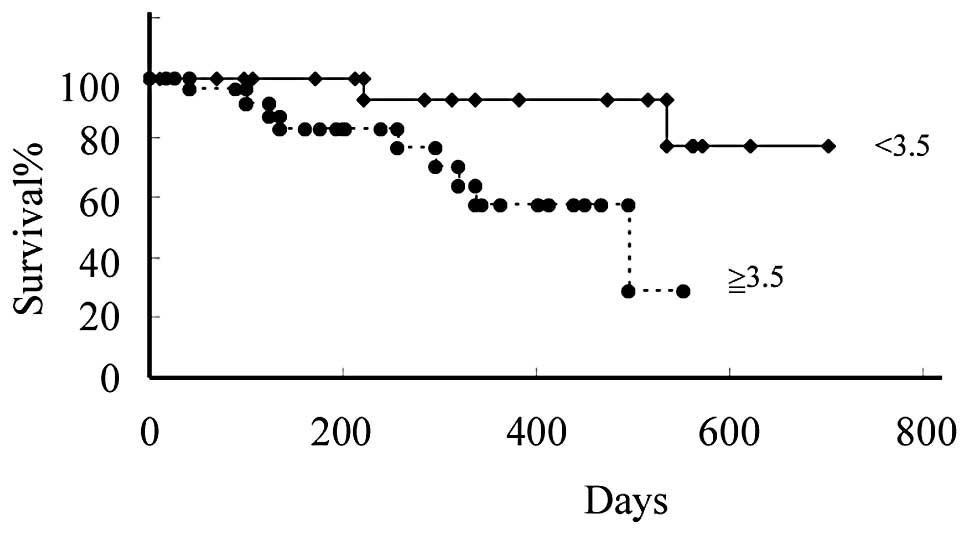
We were able to closely monitor all MPM patients for
700 days. To study the relationship between SUVmax levels and
patient clinical course, we divided patients based on their SUVmax
levels at the time of the first measurement. The first group
included patients with SUVmax levels <3.5, the cut-off value
that was used. In this group of 19 patients, the mean SUVmax level
was 1.4±1.4. The other group included the remaining 28 patients
with SUVmax levels ≥3.5, whose mean SUVmax level was 8.0±3.7. The
difference in overall survival between the groups with SUVmax
levels lower and higher than 3.5 was significant (p=0.02) (Fig. 2).
Cox’s regression analysis was carried out for all
MPM patients for whom data on age, gender, histology, performance
status, and SUVmax levels were available. An independent
significant prognostic effect on survival of age (≤65 versus
>65; HR, 2.4; 95% CI, 1.07–5.23; p=0.03) was found.
Discussion
Mesothelioma is a malignant transformation caused by
the exposure of mesothelial cells to asbestos, and has a limited
response to conventional therapy, and a very poor prognosis. The
lifetime risk of mesothelioma is associated with occupational
and/or environmental asbestos exposure history. Due to the long
latency period (typically longer than 30 years) between first
asbestos exposure and the onset of the disease, the diagnosis of
mesothelioma remains difficult with an increasing incidence
worldwide (1–3).
Current imaging tools lack the ability to accurately
detect the distribution of MPM. Previous reports have shown that CT
and magnetic resonance imaging (MRI) provide anatomic information
that is not precise in the preoperative staging and resectability
of MPM (17,18).
PET is a very useful imaging tool for the clinical
assessment of cancer patients (9).
The uptake of 18F-FDG is commonly higher in most types of cancer
than that in most normal organs. Bénard et al examined
whether PET helps to distinguish between benign and malignant
pleural disease and revealed that PET was a sensitive tool in 22
MPM patients. The main results of their study were that
differentiation of benign lesions from malignant pleural diseases
still required pathological confirmation and that PET did seem to
be useful in guiding surgical biopsy (12). In the next report, they performed a
survival analysis of 17 MPM patients (13), however, its clinical utility in MPM
was not fully investigated. In this study, we examined the
diagnostic and prognostic role of PET in 47 Japanese MPM patients.
Our current study was more than double the size of the prior study
in a single institution. 18F-FDG uptake levels are
semi-quantitatively expressed as SUVmax levels in general clinical
institutions. Similarly to Bénard et al, we found that
patients with MPM had significantly higher SUVmax levels than the
non-MPM population containing patients with a history of asbestos
exposure and a group that had never been exposed to asbestos. The
difference in SUVmax levels between the population with or without
a history of asbestos exposure was not significant, suggesting that
not only asbestos exposure, but also MPM growth is required for
elevations in SUVmax levels. Furthermore, we analyzed SUVmax levels
of MPM patients using histology and staging systems and we found
significant differences in SUVmax levels among MPM histological
groups and staging. Although pleural biopsies such as VATS are
often critical to the diagnosis of MPM, they are invasive
procedures. Therefore, new non-invasive techniques for assessment
of MPM are required to judge whether those procedures should be
practiced for diagnosis of MPM.
Although the diagnostic sensitivity and NPV of
SUVmax levels for MPM measured on a ROC curve were not high (59.6
and 58.7%, respectively), its specificity and PPV was fairly high
(93.1 and 93.3%, respectively), suggesting that high SUVmax levels
are supportive of a differential diagnosis of MPM, which is
extremely difficult to obtain for individuals with pleural
disease.
Moreover, we also demonstrated a significant
correlation between SUVmax levels and survival in most MPM patients
using the Kaplan-Meier method, which suggested their usefulness as
a tool to estimate prognosis. Since there is no clinical useful
imaging system of MPM and early distinction of MPM patients from
those with benign asbestos-related diseases is necessary, we
propose that measuring SUVmax levels is a useful imaging tool for
the clinical management of MPM.
In conclusion, we demonstrated that patients with
MPM had significantly higher SUVmax levels than a non-MPM
population with or without a history of asbestos exposure, and the
Kaplan-Meier method revealed a significant correlation between
SUVmax levels of MPM patients and survival. It is suggested that
SUVmax levels are a novel useful diagnostic and prognostic imaging
tool for MPM.
Abbreviations:
|
AUC
|
area under the ROC curve;
|
|
CI
|
confidence interval;
|
|
CT
|
computed tomography;
|
|
FDG
|
fluorodeoxyglucose;
|
|
MRI
|
magnetic resonance imaging;
|
|
MPM
|
malignant pleural mesothelioma;
|
|
NPV
|
negative predictive value;
|
|
PET
|
positron emission tomography;
|
|
PPV
|
positive predictive value;
|
|
ROC
|
receiver operating characteristic;
|
|
ROI
|
region of interest;
|
|
SUVmax
|
maximum SUV;
|
|
VATS
|
video-assisted thoracic surgery
|
Acknowledgements
We thank Ms. Hidemi Kitai for
providing technical assistance. This work was supported by grants
from KAKENHI, a Grant-in-Aid for Scientific Research (C) (23591167)
and Health Labour Sciences Research Grant.
References
|
1.
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Wagner JC, Sleggs CA and Marchand P:
Diffuse pleural mesothelioma and asbestos exposure in the North
Western Cape Province. Br J Ind Med. 17:260–271. 1960.PubMed/NCBI
|
|
4.
|
Selikoff IJ, Hammond EC and Seidman H:
Latency of asbestos disease among insulation workers in the United
States and Canada. Cancer. 15:2736–2740. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of pemetrexed in combination with cisplatin
versus cisplatin alone in patients with malignant pleural
mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Nowak AK, Lake RA, Kindler HL, et al: New
approaches for mesothelioma: biologics, vaccines, gene therapy, and
other novel agents. Semin Oncol. 29:82–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Leung AN, Müller NL and Miller RR: CT in
differential diagnosis of diffuse pleural disease. AJR Am J
Roentgenol. 154:487–492. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Müller NL: Imaging of the pleura.
Radiology. 186:297–309. 1993.
|
|
9.
|
Rigo P, Paulus P, Kaschten BJ, et al:
Oncological applications of positron emission tomography with
fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 23:1641–1674. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gupta NC, Frank AR, Dewan NA, et al:
Solitary pulmonary nodules: detection of malignancy with PET with
2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 184:441–444.
1992.
|
|
11.
|
Patz EF Jr, Lowe VJ, Hoffman JM, et al:
Focal pulmonary abnormalities: evaluation with F-18
fluorodeoxyglucose PET scanning. Radiology. 188:487–490. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bénard F, Sterman D, Smith RJ, et al:
Metabolic imaging of malignant pleural mesothelioma with
fluorodeoxyglucose positron emission tomography. Chest.
114:713–722. 1998.PubMed/NCBI
|
|
13.
|
Bénard F, Sterman D, Smith RJ, et al:
Prognostic value of FDG PET imaging in malignant pleural
mesothelioma. J Nucl Med. 40:1241–1245. 1999.
|
|
14.
|
Flores RM, Akhurst T, Gonen M, et al:
Positron emission tomography predicts survival in malignant pleural
mesothelioma. J Thorac Cardiovasc Surg. 132:763–768. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yildirim H, Metintas M, Entok E, et al:
Clinical value of fluorodeoxyglucose-positron emission
tomography/computed tomography in differentiation of malignant
mesothelioma from asbestos-related benign pleural disease: an
observational pilot study. J Thorac Oncol. 4:1480–1484. 2009.
View Article : Google Scholar
|
|
16.
|
Rusch VW: A proposed new international TNM
staging system for malignant pleural mesothelioma. From the
International Mesothelioma Interest Group. Chest. 108:1122–1128.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Heelan RT, Rusch VW, Begg CB, et al:
Staging of malignant pleural mesothelioma: comparison of CT and MR
imaging. AJR Am J Roentgenol. 172:1039–1047. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Patz EF Jr, Shaffer K, Piwnica-Worms DR,
et al: Malignant pleural mesothelioma: value of CT and MR imaging
in predicting resectability. AJR Am J Roentgenol. 159:961–966.
1992. View Article : Google Scholar : PubMed/NCBI
|