Introduction
Gastric cancer is the second most common cause of
cancer-related mortality and morbidity worldwide, accounting for
almost 12% of cancer-related mortality and 1.6% of total mortality
(1–3). Postoperative chemotherapy is used as
a supplementary treatment to prevent tumor recurrence and
metastasis (4,5). Several retrospective studies have
reported that supplementary chemotherapy may improve the quality of
life and total survival (6–9).
However, the emergence of drug resistance, particularly multidrug
resistance (MDR), has prevented successful treatment in a large
proportion of patients (10). The
two main forms of MDR are intrinsic resistance, in which the
previously untreated cancer cells are inherently insensitive to
chemotherapeutic drugs, and acquired resistance, in which the
cancer cells become insensitive as a result of chemotherapy
(11,12). The most commonly reported mechanism
for the acquisition of resistance to a broad range of anticancer
drugs is the expression of one or more energy-dependent
transporters, including P-glycoprotein (P-gp), multidrug
resistance-associated proteins (MRPs), lung resistance protein
(LRP) and breast cancer resistance protein (BCRP/MXR/ABCG2)
(12–19). These transporters mediate drug
efflux from tumor cells and may further cause cross-resistance to
multiple drugs with diverse chemical structures and curative
efficacies. According to previous studies, MDR is observed in the
majority of gastric cancers during treatment and is an significant
cause of treatment failure (19,20,21).
Glutathione (GSH) is the tripeptide thiol
L-γ-glutamyl-L-cysteinyl-glycine, a ubiquitous endogenous
antioxidant. Its main functions are the protection of the
intracellular environment from oxidative stress and the
detoxification of cells by inactivation of xenobiotics (22). As the predominant cellular thiol,
intracellular GSH concentrations may exceed 10 mM (23). Levels of GSH are reported to be
elevated in various tumor cells, for example in bone marrow,
breast, ovary, colon, larynx and lung cancer cells (24–29).
Elevated levels of GSH are often associated with an increased
resistance to cancer chemotherapeutic agents due to the protective
conjugation and detoxification effects of GSH (30). Similarly, other studies have shown
that cross-resistance to a number of drugs, including
cyclophosphamide, melphalan, mechlorethamine, platinum-containing
compounds and sulfhydryl-reactive chemotherapeutic drugs,
correlates with increased levels of intracellular GSH (31–33).
Conversely, the depletion of intra-cellular GSH has been revealed
to decrease the resistance of cancer cells to multiple
chemotherapeutic agents (30).
However, to date there have been few studies comparing the levels
of GSH in multidrug resistant cancer cell lines with those in the
parent cancer cell lines and it remains unclear whether the
alteration of intracellular GSH levels generates different effects
on the cross-resistance of multidrug resistant and parent cell
lines.
In the present study, we monitored the levels of
intracellular GSH in a gastric adenocarcinoma cell line with
resistance to cisplatin (CDDP) and in its parent cell line. A GSH
synthesis inhibitor and/or stimulator were used to change
intracellular GSH levels in order to evaluate the effects of GSH on
the chemosensitivities of the two cell lines.
Materials and methods
Cell culture and treatment
The human gastric adenocarcinoma cell line SGC7901
and its MDR subline SGC7901/DDP were purchased from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China). The cells were cultured in
RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with
10% (v/v) fetal bovine serum, penicillin (100 U/ml) and
streptomycin (100 μg/ml) at 37°C in an incubator containing
a humid atmosphere of 95% air and 5% CO2. To maintain
the MDR phenotype, CDDP (final concentration 800 ng/ml) was added
to the medium for the SGC7901/DDP cells. The cells were propagated
according to the instructions provided by the American Type Culture
Collection.
Cell viability assays
The survival ratios of the cells were determined
using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) colorimetric assay. The cells were seeded in 96-well
microplates at a density of 2x103 cells per well. Then
the cells were treated with the following methods. i) Cells were
treated with various concentrations of CDDP, fluorouracil (5-FU) or
mitomycin (MMC) for 36 h; ii) The cells were pretreated with
various nontoxic concentrations of L-buthionine-(S,R)-sulfoximine
(BSO, Sigma, St. Louis, MO, USA) for 24 h, washed 3 times with PBS
before replacing fresh medium with various concentrations of 5-FU
or MMC combined with or without 5 mM N-acetylcysteine (NAC, Sigma)
for 36 h; iii) The cells were treated with various concentrations
of 5-FU or MMC combined with 5 mM NAC for 36 h. Then the medium was
replaced with fresh medium, allowing the cells to be continuously
grown for up to 72 h. The MTT (Sigma) dye was added to a final
concentration of 50 mg/ml and the cells were subsequently incubated
for another 4 h at 37°C. The medium containing residual MTT dye was
carefully aspirated from each well and 150 μl DMSO was added
to dissolve the reduced formazan dye. The effect of 5-FU or MMC on
the growth of the cells was determined from the differences in
absorbance. The fraction of viable cells was calculated by
comparing the optical absorbance of the 5-FU-or MMC-treated culture
with that of the untreated control. The 50% inhibitory
concentration (IC50) value was calculated on the basis
of the fraction of viable cells.
Intracellular GSH assay
Following the treatment of triplicate samples of
106 cells with various reagents, the intra-cellular
levels of GSH were measured using the glutathione
reductase/5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) recycling
assay kit (Beyotime Institute of Biotechnology, Haimen, China),
following the methods recommended by the manufacturer. Briefly, GSH
was determined using a reaction mixture containing 50 μl
cell lysates, 50 μl 2.4 mM DTNB and 50 μl 10.64
mU/μl glutathione reductase in an assay buffer (pH 7.5)
containing 153 mM sodium phosphate and 8.4 mM EDTA. After a 5-min
incubation at 25°C, the reaction was initiated by the addition of
50 μl NADPH solution (0.16 mg/ml) in assay buffer. The
standard and test sample cuvettes were placed into a dual-beam
spectrophotometer and the absorbance at 412 nm was followed as a
function of time.
Statistical analysis
Data are reported as the means ± SEM of three
separate experiments. Statistical significance was measured by the
independent samples t-test and analysis of variance. A value of
p<0.05 was considered to indicate a statistically significant
result.
Results
Selection of the sublethal concentration
of BSO
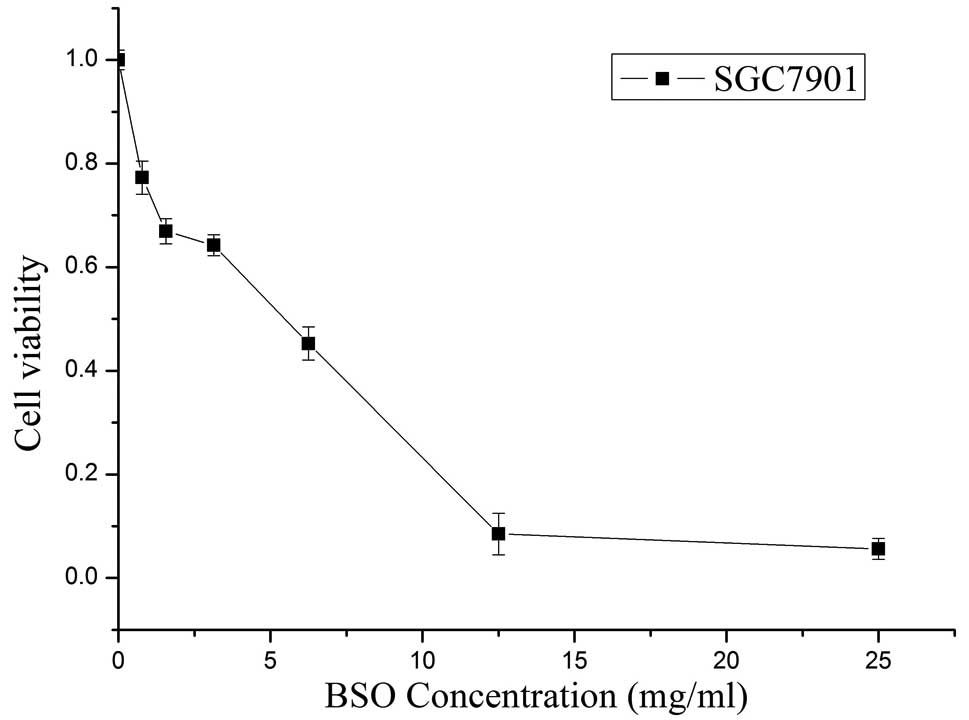
The cytotoxicity of BSO (Sigma), a specific
inhibitor of GSH biosynthesis, was evaluated by MTT assay. As shown
in Fig. 1, the cell viability
gradually reduced concomitantly with the increase in BSO
concentration. The IC50 value of BSO in the SGC7901
cells, calculated using the Hill equation, was 3.43 mg/ml. There
were non-significant reductions in the viabilities of the cells
exposed to the various concentrations of BSO below 20%
IC50 (p>0.05). Hence, the concentrations of BSO were
set at 20% IC50 (0.686 mg/ml), 10% IC50
(0.343 mg/ml) and 5% IC50 (0.172 mg/ml) in subsequent
studies.
Effects of BSO on the chemosensitivity of
cells
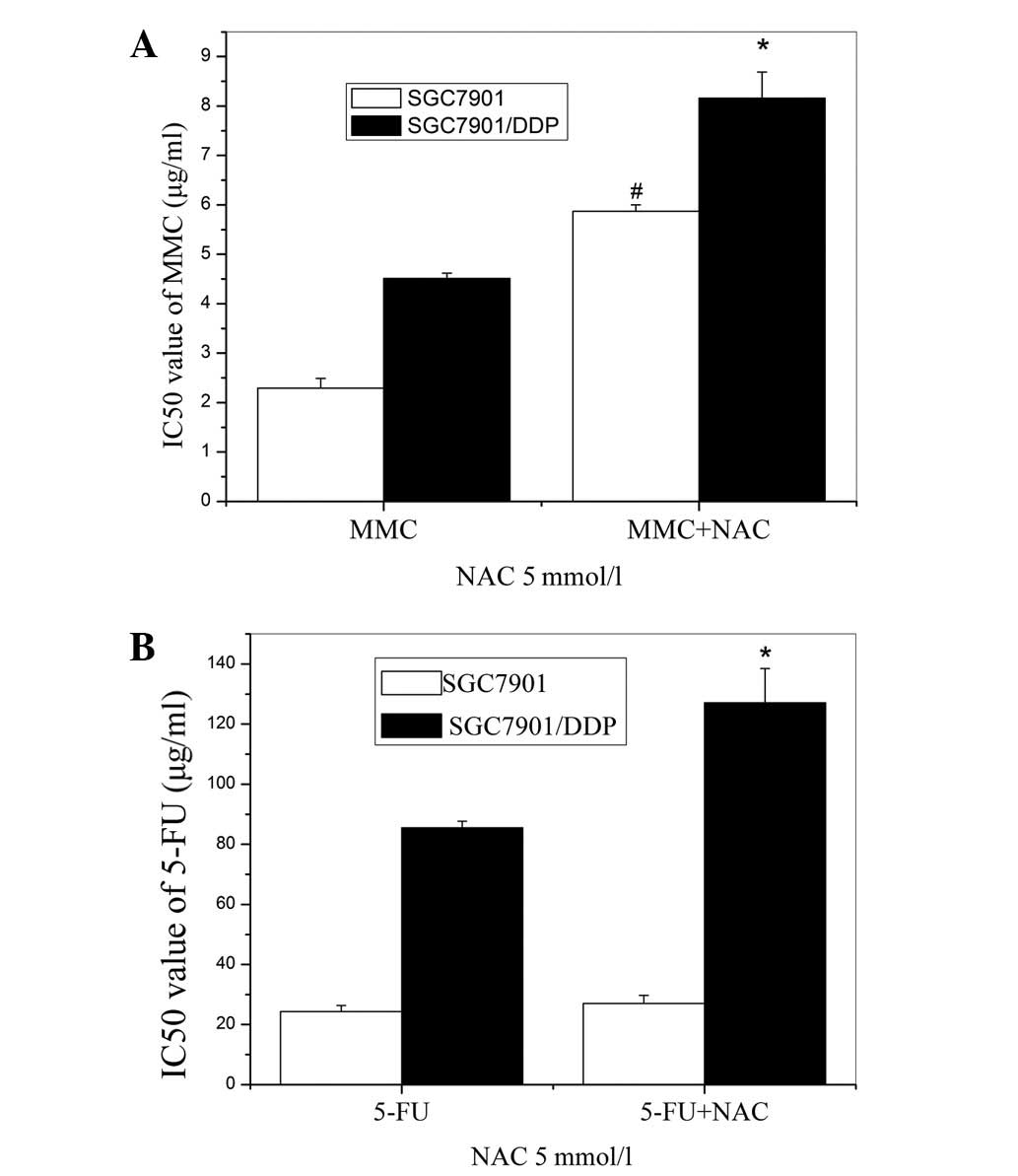
The sensitivities of the tumor cells to CDDP, MMC
and 5-FU are shown in Fig. 2. The
SGC7901/DDP cells exhibited stronger resistance than the parent
cells to the three drugs; 6.20-fold for CDDP, 3.49-fold for 5-FU
and 1.97-fold for MMC (p<0.01). These results indicate that
SGC7901/DDP cells are cross-resistant to other chemotherapeutic
drugs.
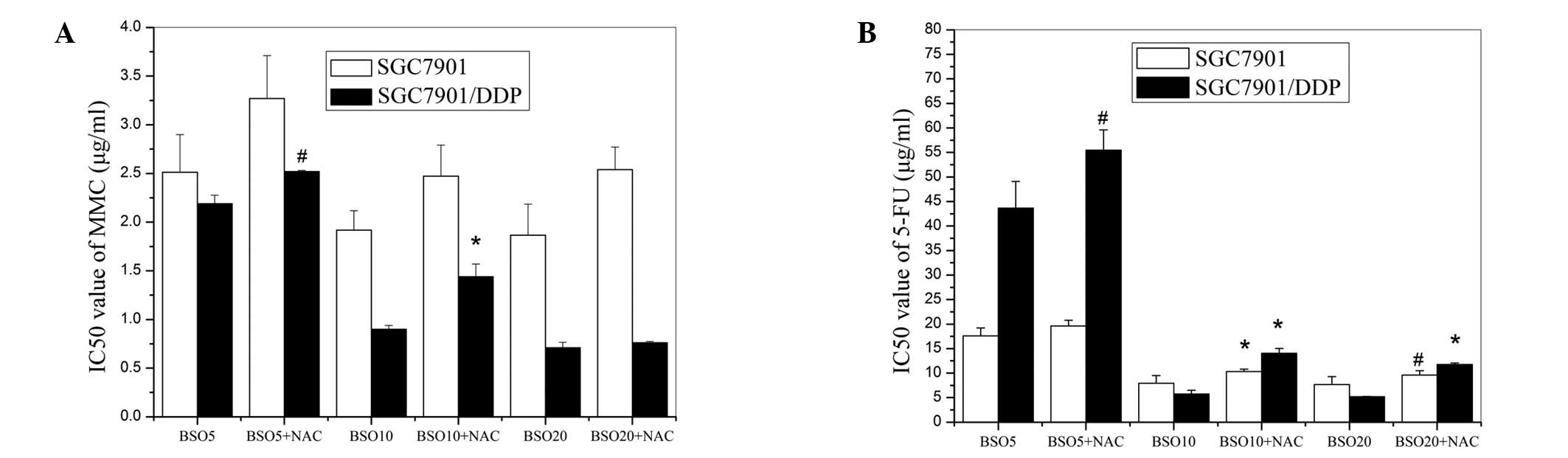
Following the pretreatment of the cells with various
concentrations of BSO, the IC50 values of 5-FU were
significantly decreased in the two cell lines (Fig. 3B). BSO also increased the
chemosensitivity of the SGC7901/DDP cells to MMC. Although BSO
slightly reduced the viability of the MMC-treated SGC7901 cells,
the difference between the chemosensitivities of the cells with and
without BSO pretreatment was not statistically significant
(Fig. 3A). These results also
revealed that, following BSO treatment, the SGC7901/DDP cells were
more sensitive than the SGC7901 cells to 5-FU and MMC.
Effects of N-acetylcysteine (NAC) on the
chemosensitivity of cells
A GSH precursor, NAC (5 mM), was used to further
investigate alterations in the chemosensitivities of the cells.
Marked increases in the IC50 values of MMC and 5-FU in
the SGC7901/DDP cells were observed (Fig. 4). The resistance of the SGC7901
cells to MMC was also elevated by the NAC pretreatment, but the
resistance to 5-FU was unchanged.
Effects of combining NAC with BSO on the
chemosensitivity of cells
To further examine the responses of the multidrug
resistant and parent cells to changes in intracellular GSH levels,
NAC was added to the media of the cells pretreated with various
concentrations of BSO. The effects of MMC and 5-FU on those cells
were then detected and their IC50 values are shown in
Fig. 5. The results reveal that
NAC partly reversed the inhibitory effect of BSO on the
chemoresistance of the two cell lines to 5-FU and reduced the
inhibitory effect of BSO on the chemoresistance of SGC7901/DDP
cells to MMC. However, no statistically significant effects on the
response of the BSO-pretreated SGC7901 cells to MMC were observed,
as shown in Fig. 5A. Therefore,
the SGC7901/DDP cells appear to be more sensitive than the parent
cells to the alteration of cellular GSH.
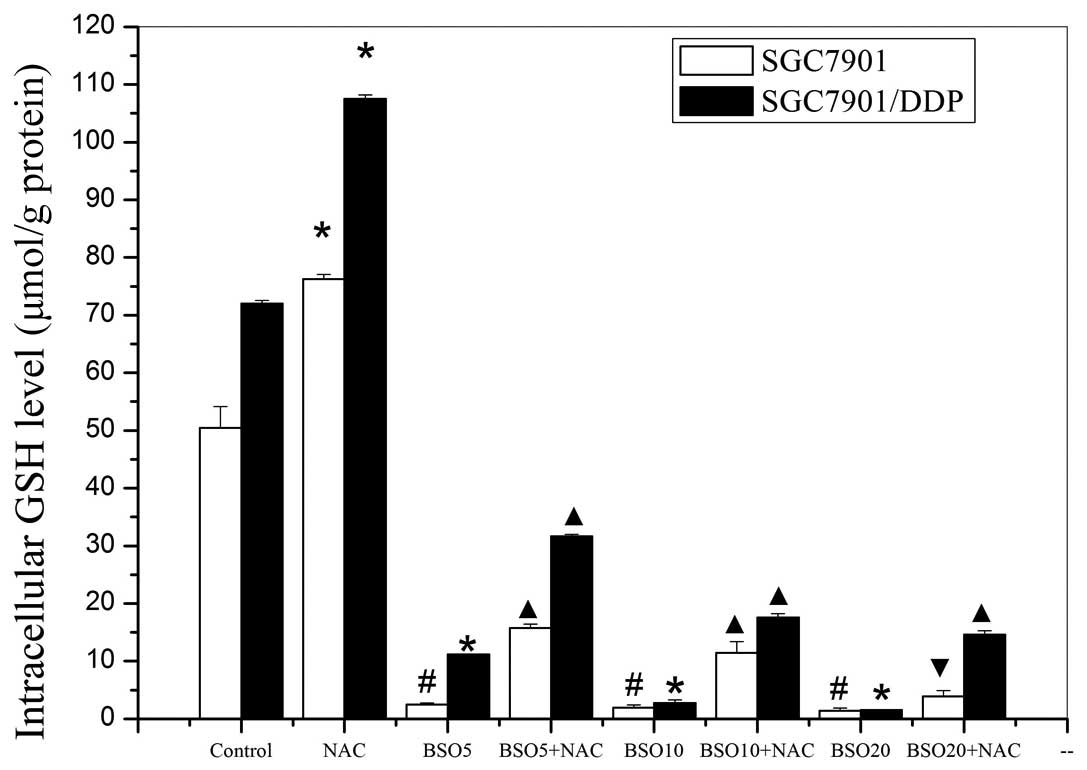
Alteration of intracellular GSH
The intracellular GSH levels in the two cell lines,
after various pretreatments, are shown in Fig. 6. The SGC7901/DDP cells had higher
GSH levels than the SGC7901 cells and treatment with NAC alone
increased the levels of GSH in the two cell lines. Conversely, in
the two cell lines treated with various concentrations of BSO,
significant reductions of intracellular GSH levels occurred in a
concentration-dependent manner. Subsequent experiments revealed
that the inhibition of intracellular GSH by BSO treatment was
partially reversed by the addition of NAC. The trends in the
alteration of intracellular GSH levels were consistent with those
in chemoresistance of the SGC7901/DDP cells but not those in the
SGC7901 cells.
Discussion
The resistance of cancer cells to a single drug is
usually accompanied by resistance to other chemotherapeutic drugs
(34–36). It is well known that CDDP acts on
multiple cellular targets representing various signal transduction
pathways. It is therefore conceivable that multiple mechanisms are
correlated with the generation of cross-resistance by CDDP,
including detoxification of cells and increased DNA repair
(37,38). Evidence indicates that
intracellular GSH content is a determinant of the sensitivity of
tumor cells to chemotherapeutic agents (39). BSO inhibits GSH biosynthesis by
irreversible inhibition of γ-glutamyl cysteine synthase and NAC is
a thiol antioxidant and cysteine source for GSH synthesis. In the
present study, SGC7901/DDP cells were shown to have higher basal
GSH levels than the parent cells and this positively correlated
with increased cross-resistance to 5-FU and MMC. Modification of
the GSH levels in the SGC7901/DDP cells by treatment with NAC or
BSO resulted in changes in the sensitivities of the cells to the
drugs. Following the inhibition of GSH synthesis by BSO,
SGC7901/DDP cells lost their resistance to 5-FU and MMC, whereas
NAC supplementation increased the resistance of the SGC7901/DDP
cells to 5-FU and MMC treatment. Similar results have been
described previously in other cell lines overexpressing MRP1
(40,41). It therefore appears that the
cross-resistance of tumor cells depends on their levels of GSH.
According to previous studies, GSH is important for promoting the
refractory response of tumor cells to cytotoxic drugs via increased
expression of P-gp and MRPs (42–44).
Intracellular GSH levels are closely correlated with MRP-mediated
multi-drug resistance since GSH interacts directly with MRP and
this interaction causes a change in MRP structure that facilitates
the binding and transport of anticancer drugs (45). Alternatively, GSH and anticancer
drugs may spontaneously form a complex that behaves as an MRP
substrate. Unlike the SGC7901/DDP cells, the SGC7901 cells treated
with BSO did not undergo a reduction in IC50 value for
MMC, although they were sensitized to 5-FU. Interestingly, the
sensitivities of the SGC7901 cells to MMC were upregulated
following NAC pretreatment, but the resistance of the cells to 5-FU
was not significantly altered by the same treatment. It is
therefore possible that the changes of intracellular GSH levels
have a greater effect on the chemo-sensitivity of the resistant
cells than on that of the parent cells.
To verify this hypothesis, we evaluated the changes
of the GSH levels in the cells following various treatments. The
results reveal that NAC increased the GSH levels in the two cell
lines but did not significantly reverse the inhibitory effect of
BSO. Due to the irreversible effects of BSO, the addition of NAC
did not increase the GSH levels immediately, although it had a
significant (p<0.05) effect in the cells compared with treatment
with BSO alone. The GSH levels were reduced from their control
levels by the BSO treatment more markedly in the resistant cells
than in the parent cells. NAC increased the GSH levels whereas BSO
markedly reduced GSH synthesis in the two cell lines. When compared
with the SGC7901 cells, the IC50 values of the resistant
cells were more markedly affected by the variation of GSH levels.
We propose that, upon addition of BSO, GSH depletion contributes to
the substantial increase in the drug cytotoxicity in the resistant
cells. However, NAC appears to have the opposite effect. The
results of our study confirm the hypothesis that intracellular GSH
levels have a close correlation with MDR. With reference to
previous studies, we speculate that the mechanism involves the
following: i) The depletion of GSH with BSO treatment, resulting in
the downregulation of MRP1 expression, correlates with apoptosis in
various cell lines. Therefore, GSH plays an important role in the
inhibition of apoptosis (43,46,47);
ii) GSH is important for the metabolic detoxification of drugs
together with increased activities of glutathione S-transferases,
which may also protect cells from cytotoxic drugs, so the
SGC7901/DDP cells exhibited more resistance than the control
(48); iii) the redox-regulating
capacity of GSH leads to detoxification in cells expressing high
levels of MRP1 (49). Consistent
with our results, certain previous studies using human MCF7 cells
and A549 cells have shown that changes in intracellular GSH levels
give rise to clear alterations in multidrug resistance (50,51).
As shown in earlier studies of BSO and in the current study, BSO is
capable of suppressing intracellular GSH levels and increasing
chemosensitivity. The sensitization of resistant cells may be a
promising strategy for overcoming drug resistance in cancer
patients, particularly those in whom drug resistance occurs as a
result of high GSH levels.
Our results demonstrate that NAC increases multidrug
resistance in SGC7901/DDP cells and that this effect may relate to
GSH synthesis. Additionally, BSO appears to play a vital role in
enhancing the sensitivity to chemotherapeutic drugs via GSH
depletion. In summary, our study suggests that the alteration of
the intracellular micro-environment redox state changes the
multidrug resistance in vitro. This primary research may
provide a promising strategy for anticancer therapy.
Acknowledgements
The authors thank the Teaching and
Research Section of Nuclear Medicine, Anhui Medical University for
its support.
References
|
1.
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Estimates of the worldwide mortality from 25 cancers in 1990. Int J
Cancer. 83:870–873. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Alberts SR, Cervantes A and van de Velde
CJ: Gastric cancer: epidemiology, pathology and treatment. Ann
Oncol. 14(Suppl 2): ii31–ii36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Shibuya K, Mathers CD, Boschi-Pinto C,
Lopez AD and Murray CJ: Global and regional estimates of cancer
mortality and incidence by site: II. Results from the global burden
of disease 2000. BMC Cancer. 2:372002. View Article : Google Scholar
|
|
4.
|
Meads MB, Hazlehurst LA and Dalton WS: The
bone marrow microenvironment as a tumor sanctuary and contributor
to drug resistance. Clin Cancer Res. 14:2519–2526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Parmar K, Mauch P, Vergilio JA, Sackstein
R and Down JD: Distribution of hematopoietic stem cells in the bone
marrow according to regional hypoxia. Proc Natl Acad Sci USA.
104:5431–5436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Glimelius B, Ekström K, Hoffman K, et al:
Randomized comparison between chemotherapy plus best supportive
care with best supportive care in advanced gastric cancer. Ann
Oncol. 8:163–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hill ME and Cunningham D: Medical
management of advanced gastric cancer. Cancer Treat Rev.
24:113–118. 1998. View Article : Google Scholar
|
|
8.
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
J Med. 345:725–730. 2001. View Article : Google Scholar
|
|
9.
|
Cunningham D, Allum WH, Stenning SP, et
al: Perioperative chemotherapy versus surgery alone for resectable
gastroesophageal cancer. N Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Petty RD, Nicolson MC, Kerr KM,
Collie-Duguid E and Murray GI: Gene expression profiling in
non-small cell lung cancer: from molecular mechanisms to clinical
application. Clin Cancer Res. 10:3237–3248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Stavrovskaya AA: Cellular mechanisms of
multidrug resistance of tumor cells. Biochemistry (Moscow).
65:95–106. 2000.PubMed/NCBI
|
|
12.
|
Banerjee D, Mayer-Kuckuk P, Capiaux G,
Budak-Alpdogan T, Gorlick R and Bertino JR: Novel aspects of
resistance to drugs targeted to dihydrofolate reductase and
thymidylate synthase. Biochim Biophys Acta. 1587:164–173. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Larsen AK, Escargueil AE and Skladanowski
A: Resistance mechanisms associated with altered intracellular
distribution of anticancer agents. Pharmacol Ther. 85:217–229.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ross DD: Novel mechanisms of drug
resistance in leukemia. Leukemia. 14:467–473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Litman T, Druley TE, Stein WD and Bates
SE: From MDR to MXR: New understanding of multidrug resistance
systems, their properties and clinical significance. Cell Mol Life
Sci. 58:931–959. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Narasaki F, Oka M, Nakano R, Ikeda K,
Fukuda M, Nakamura T, Soda H, Nakagawa M, Kuwano M and Kohno S:
Human canalicular multispecific organic anion transporter (cMOAT)
is expressed in human lung, gastric, and colorectal cancer cells.
Biochem Biophys Res Commun. 240:606–611. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nakamura T, Oka M, Aizawa K, Soda H,
Fukuda M, Terashi K, Ikeda K, Mizuta Y, Noguchi Y, Kimura Y, et al:
Direct interaction between a quinoline derivative, MS-209, and
multidrug resistance protein (MRP) in human gastric cancer cells.
Biochem Biophys Res Commun. 255:618–624. 1999. View Article : Google Scholar
|
|
18.
|
Tomonaga M, Oka M, Narasaki F, Fukuda M,
Nakano R, Takatani H, Ikeda K, Terashi K, Matsuo I, Soda H, et al:
The multidrug resistance-associated protein gene confers drug
resistance in human gastric and colon cancers. Jpn J Cancer Res.
87:1263–1270. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hao Z, Li X, Qiao T, Du R, Hong L and Fan
D: CIAPIN1 confers multidrug resistance by upregulating the
expression of MDR-1 and MRP-1 in gastric cancer cells. Cancer Biol
Ther. 5:261–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Maeda S, Sugiura T, Saikawa Y, Kubota T,
Otani Y, Kumai K and Kitajima M: Docetaxel enhances the
cytotoxicity of cisplatin to gastric cancer cells by modification
of intracellular platinum metabolism. Cancer Sci. 95:679–684. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tang XQ, Bi H, Feng JQ and Cao JG: Effect
of curcumin on multidrug resistance in resistant human gastric
carcinoma cell line SGC7901/VCR. Acta Pharmacol Sin. 26:1009–1016.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Chen Y, Ji L, Wang H and Wang Z:
Intracellular glutathione plays important roles in pyrrolizidine
alkaloids-induced growth inhibition on hepatocytes. Environ Toxicol
Pharmacol. 28:357–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kosower NS and Kosower EM: The glutathione
status of cells. Int Rev Cytol. 54:109–160. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Markman M: Antineoplastic agents in the
management of ovarian cancer: current status and emerging
therapeutic strategies. Trends Pharmacol Sci. 10:515–519. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Muggia F: Platinum compounds 30 years
after the introduction of cisplatin: implications for the treatment
of ovarian cancer. Gynecol Oncol. 112:275–281. 2009.PubMed/NCBI
|
|
26.
|
Lustberg MB and Edelman MJ: Optimal
duration of chemotherapy in advanced non-small cell lung cancer.
Curr Treat Options Oncol. 8:38–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Matthews GM, Howarth GS and Butler RN:
Nutrient and anti-oxidant modulation of apoptosis in gastric and
colon cancer cells. Cancer Biol Ther. 5:569–572. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Podder S, Chattopadhyay A, Bhattacharya S,
Ray MR and Chakraborty A: Fluoride-induced genotoxicity in mouse
bone marrow cells: effect of buthionine sulfoximine and
N-acetyl-L-cysteine. J Appl Toxicol. 31:618–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Beketić-Oresković L, Osmak M and Jaksić M:
Human larynx carcinoma cells resistant to
cis-diamminedichloroplatinum(II): mechanisms involved in the
resistance. Neoplasma. 41:163–169. 1994.
|
|
30.
|
Balendiran GK, Dabur R and Fraser D: The
role of glutathione in cancer. Cell Biochem Funct. 22:343–352.
2004. View
Article : Google Scholar
|
|
31.
|
Akiyama SI, Chen ZS, Sumizawa T and
Furukawa T: Resistance to cisplatin. Anticancer Drug Des.
14:143–151. 1999.
|
|
32.
|
Panasci L, Paiement JP, Christodoulopoulos
G, Belenkov A, Malapetsa A and Aloyz R: Chlorambucil drug
resistance in chronic lymphocytic leukemia: the emerging role of
DNA repair. Clin Cancer Res. 7:454–461. 2001.PubMed/NCBI
|
|
33.
|
Bredel M: Anticancer drug resistance in
primary human brain tumors. Brain Res Rev. 35:161–204. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Germann UA: P-glycoprotein - a modulator
of multidrug resistance in tumor cells (Review). Eur J Cancer.
32A:927–944. 1996. View Article : Google Scholar
|
|
35.
|
Müller M, Meijer C, Zaman GJ, et al:
Overexpression of the gene encoding the multidrug
resistance-associated protein results in increased ATP-dependent
glutathione S-conjugate transport. Proc Natl Acad Sci USA.
91:13033–13037. 1994.PubMed/NCBI
|
|
36.
|
Hipfner DR, Deeley RG and Cole SP:
Structural, mechanistic and clinical aspects of MRP1. Biochim
Biophys Acta. 1461:359–376. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Wang D and Lippard SJ: Cellular processing
of platinum anti-cancer drugs. Nat Rev Drug Discov. 4:307–320.
2005. View
Article : Google Scholar
|
|
39.
|
Kang YH, Lee E, Youk HJ, Kim SH, Lee HJ,
Park YG and Lim SJ: Potentiation by alpha-tocopheryl succinate of
the etoposide response in multidrug resistance protein 1-expressing
glioblastoma cells. Cancer Lett. 217:181–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Jin J, Huang M, Wei HL and Liu GT:
Mechanism of 5-fluorouracil required resistance in human
hepatocellular carcinoma cell line Bel(7402). World J
Gastroenterol. 8:1029–1034. 2002.PubMed/NCBI
|
|
41.
|
Xu BH and Singh SV: Effect of buthionine
sulfoximine and ethacrynic acid on cytotoxic activity of mitomycin
C analogues BMY 25282 and BMY 25067. Cancer Res. 52:6666–6670.
1992.PubMed/NCBI
|
|
42.
|
Chen HH and Kuo MT: Role of glutathione in
the regulation of Cisplatin resistance in cancer chemotherapy. Met
Based Drugs 2010. pii:430–939. 2010.
|
|
43.
|
Akan I, Akan S, Akca H, Savas B and Ozben
T: Multidrug resistance-associated protein 1 (MRP1) mediated
vincristine resistance: effects of N-acetylcysteine and Buthionine
sulfoximine. Cancer Cell Int. 5:222005. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Jin WS, Kong ZL, Shen ZF, Jin YZ, Zhang WK
and Chen GF: Regulation of hypoxia inducible factor-1α expression
by the alteration of redox status in HepG2 cells. J Exp Clin Cancer
Res. 30:612011.
|
|
45.
|
Franco R and Cidlowski JA: Apoptosis and
glutathione: beyond an antioxidant. Cell Death Differ.
16:1303–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Akan I, Akan S, Akca H, Savas B and Ozben
T: N-acetylcysteine enhances multidrug resistance-associated
protein 1 mediated doxorubicin resistance. Eur J Clin Invest.
34:683–689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Chuman Y, Chen ZS, Seto K, et al: Reversal
of MRP-mediated vincristine resistance in KB cells by buthionine
sulfoximine in combination with PAK-104P. Cancer Lett. 129:69–76.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Schafer FQ and Buettner GR: Redox
environment of the cell as viewed through the redox state of the
glutathione disulfide/glutathione couple. Free Radical Biol Med.
30:1191–1212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Commandeur JN, Stijntjes GJ and Vermeulen
NP: Enzymes and transport systems involved in the formation and
disposition of glutathione S-conjugates. Role in bioactivation and
detoxification mechanisms in xenobiotics. Pharmacol Rev.
47:271–330. 1995.PubMed/NCBI
|
|
50.
|
Benderra Z, Trussardi A, Morjani H, Villa
AM, Doglia SM and Manfait M: Regulation of cellular glutathione
modulates nuclear accumulation of daunorubicin in human MCF7 cells
overexpressing multidrug resistance associated protein. Eur J
Cancer. 36:428–434. 2000. View Article : Google Scholar
|
|
51.
|
Han YH, Moon HJ, You BR, Kim SZ, Kim SH
and Park WH: The effects of N-acetyl cysteine on the MG132
proteasome inhibitor-treated lung cancer cells in relation to cell
growth, reactive oxygen species and glutathione. Int J Mol Med.
25:657–62. 2010.PubMed/NCBI
|