Introduction
Recent advances in obesity research have led to the
recognition that adipose tissue is an active endocrine organ that
secretes multiple bioactive factors, termed adipokines (1). Adipokines are involved in diverse
biological functions, including energy homeostasis, insulin
sensitivity, lipid metabolism, inflammation and immunity (2). Accompanying insulin resistance,
β-cell dysfunction is another significant pathophysiological change
associated with type 2 diabetes mellitus (T2DM). Following the
discovery of receptors for several adipokines, including leptin
(3), tumor necrosis factor-α
(4) and interleukin-6 (5) in pancreatic β cells, the possible
involvement of these factors in β-cell dysfunction has been
proposed. Although not extensively investigated, several
observations indicate that adipokines influence β-cell function and
play a role in the development of β-cell dysfunction in T2DM
(6).
Diabetes mellitus is strongly associated with
oxidative stress, which may be a consequence of either the
increased production of free radicals or reduced antioxidant
defenses. α-lipoic acid (LA) is a potent antioxidant which
scavenges free radicals and recycles other antioxidants to reduce
oxidative stress. As a result, it is widely used in the treatment
of diabetic neuropathy and has the potential for treating several
aspects of diabetes pathology since it prevents β-cell destruction
(a cause of T1DM) (7) and enhances
glucose uptake in T2DM (8).
Recently, R-LA (dextrogire) was found to have protective effects on
MIN6 and isolated rat islets chronically exposed to oleic acid
(9). These observations prompted
us to explore the detailed mechanism by which LA acts on islet cell
dysfunction.
To investigate the integrated effects of adipocytes
on rat islet cells and the potential of LA to mediate a protective
effect, we established a co-culture system comprising
differentiated 3T3L1 adipocytes and islet cells. The effects of the
adipocytes and LA on islet cell function were monitored by
assessing the changes in insulin secretion and gene expression and
the protein levels of factors involved in insulin secretion,
signaling and oxidative stress under co-culture conditions and in
the presence or absence of LA.
Materials and methods
Reagents
3T3L1 cells were provided by Dr Tang Qiqun (Shanghai
Medical College of Fudan University, Shanghai, China). Dulbecco’s
modified Eagle’s medium (DMEM), penicillin/streptomycin and fetal
bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA,
USA). Dexamethasone, isobutylmethylxanthine, LA and protein A/G
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Insulin was
purchased from Eli Lilly (Shuzhou, China). Antibodies were obtained
as follows: insulin receptor-β (IR-β) and insulin receptor
substrate-1 (IRS-1) antibodies (Cell Signaling Technology, Danvers,
MA, USA); and anti-phospho-tyrosine antibody, clone 4G10 (Upstate
Biotechnology, Inc., Lake Placid, NY, USA). Membrane inserts for
6-well culture dishes with a pore size of 0.4 μm and insert
companion plates were supplied by Corning (New York, NY, USA).
Collagenase P was purchased from Roche Diagnostics GmbH (Mannheim,
Germany). Ficoll 400 was purchased from GE Healthcare (Uppsala,
Sweden).
Animals
Sprague-Dawley rats (10–12 weeks old, 300–350 g)
were purchased from the Chinese Academy of Sciences (Shanghai,
China). They were given free access to tap water and standard
pelleted chow following the regulations of the State Science and
Technology Commission for the care and use of laboratory
animals.
3T3L1 cell culture and
differentiation
3T3L1 pre-adipocytes were grown to confluence in an
incubator equilibrated with 5% CO2 at 37°C on membrane
inserts in DMEM containing 10% FBS. At 2 days post-confluence (day
0), differentiation was induced by the addition of
isobutylmethylxanthine (0.5 mmol/l), dexamethasone (1
μmol/l) and insulin (0.17 μmol/l) in DMEM containing
25 mmol/l glucose and 10% FBS. After 2 days, the
isobutylmethylxanthine and dexamethasone were removed and the cells
were incubated in DMEM with insulin for an additional 2 days. On
day 4, the medium was switched back to DMEM (without insulin
supplementation) plus 10% FBS and replenished every 2 days
(10). The cells were ready for
use after 10 days of differentiation.
Rat islet isolation and purification
The isolation method was a modification of the
method of Sakata et al (11). Pancreatic islets were isolated from
anesthetized Sprague-Dawley rats following distention of the
pancreas by perfusion via the common bile duct with collagenase P
solution (1.5 mg/l). The distended pancreas was removed and
incubated at 37°C for 14 min to promote collagenase digestion. The
incubated mixture was filtered through a 600-μm nylon screen
and the filtrate was washed by performing 3 cycles of
centrifugation and resuspension in Hanks’ solution. Finally, the
islets were purified by Ficoll-mediated discontinuous gradient
centrifugation. The islets were further tested for purity by
dithizone (DTZ) staining (12) and
for viability with acridine orange and propidium iodide (13). The purified islets were maintained
in DMEM (containing 5.6 mmol/l glucose and 10% FBS) for 2–3 h
before co-culturing with adipocytes. For each individual
experiment, islet cells were seeded in 6-well culture dishes at a
density of approximately 200 islets per well.
Co-culture of 3T3L1 adipocytes and islet
cells
After differentiating in vitro on membrane
inserts for 10 days, the 3T3L1 adipocytes were transferred to
culture plates containing purified islets. The two cell types
shared the same culture medium (DMEM containing 5.6 mmol/l glucose
and 10% FBS), but were separated by the membrane inserts.
Co-culturing was conducted for 48 h. The integrity of the cells was
routinely checked at the end of the co-culture period by light
microscopy. There were three groups: a control group (islets
alone), a co-culture group and a co-culture plus LA group. For the
latter group, the islets were exposed to 0.4 mmol/l LA during the
co-culture period.
Insulin secretion and insulin
content
After co-culturing for 48 h, the adipocytes were
removed and the islets were washed twice with Krebs-Ringer-HEPES
buffer (KRBH: 115 mmol/l NaCl, 5.4 mmol/l KCl, 2.38 mmol/l
CaCl2, 0.8 mmol/l MgSO4, 1 mmol/l
Na2HPO4, 10 mmol/l HEPES, 0.5% BSA, pH 7.35)
containing 2.8 mmol/l glucose. Eight purified islets of
approximately the same size were selected and preincubated for 1 h
in KRBH containing 2.8 mmol/l glucose. They were subsequently
incubated for 1 h in KRBH containing either 2.8 or 22 mmol/l
glucose (14). The supernatant was
collected and assayed for insulin secretion using a rat insulin
radioimmunoassay kit (Linco Research, St. Charles, MO, USA)
according to the manufacturer’s instructions. The total cellular
insulin content was extracted using 75% ethanol containing 1.5%
(vol/vol) HCl for 24 h at 4°C (15) and was normalized by cellular
protein content.
RNA extraction and real-time RT-PCR
RNA was isolated from islets using TRIzol
(Invitrogen). cDNA was synthesized from 1 μg total RNA using
AMV Reverse Transcriptase (Promega, Madison, WI, USA) in a
20-μl reaction volume. The PCR products were quantified
fluorometrically using SYBR® Premix Ex Taq™ (2X) (Takara
Bio, Inc., Shiga, Japan) according to the manufacturer’s
instructions. The cycling parameters were 95°C for 2 min, then 45
cycles of 94°C for 2 sec and 60°C for 30 sec. The primer sequences
used are presented in Table I.
GAPDH expression in each sample was used as a control. All
reactions were performed in triplicate and the data were normalized
to values of GAPDH, and evaluated using the 2−ΔΔCT
method. Expression levels are presented as the fold changes of the
transcripts of the respective genes relative to the controls.
 | Table IPrimer sequences used in real-time
RT-PCR amplification of cDNA prepared from rat islet cells of three
different groups. |
Table I
Primer sequences used in real-time
RT-PCR amplification of cDNA prepared from rat islet cells of three
different groups.
| Rat genes | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| GLUT2 |
CTCATAGTCACACCAGCACATACG |
CAAGCCACCCACCAAAGAACG |
| GCK |
CCTGGGCTTCACCTTCTCCTTC |
CCTCACATTGGCGGTCTTCATAG |
| Kir6.2 |
CCTGGCCATCCTTATTCTGA |
CTTTTTCGGAGGTCCCCTAC |
| SOD |
CTTGGGCAAAGGTGGAAATGAAG |
ACAGTTTAGCAGGACAGCAGATG |
| CAT |
AGATACCTGTGAACTGTCCCTACC |
CGTATAGAATGTCCGCACCTGAG |
| GAPDH |
ACTCCCATTCTTCCACCTTTGATG |
TCCACCACCCTGTTGCTGTAG |
Immunoprecipitation and western
blotting
The cell lysates were extracted with lysis buffer
(Cell Signaling Technology), 1% PMSF and a complete protease
inhibitor cocktail. Lysis was carried out with gentle rotation at
4°C for 20 min. The lysate was then centrifuged at 12,000 × g for 5
min at 4°C to remove the insoluble materials. The protein
concentrations were determined using the BCA Protein Assay kit
(Novagen, Madison, WI, USA). For immunoprecipitation, the
supernatant was incubated with Protein A or G agarose beads for 1 h
at 4°C for pre-cleaning. Subsequently the pre-cleaned supernate was
incubated with Protein A or G agarose beads carrying a pre-absorbed
antibody (IR-β or IRS-1, diluted 1:50) for 5 h at 4°C with gentle
rotation. The resulting immunopellet was collected by
centrifugation and washed 5 times with the lysis buffer. The cell
lysates or immunoprecipitates were boiled with 2X loading buffer
for 8 min and centrifuged, and the complete supernatant was used
for SDS-PAGE analysis. The proteins were transferred from gel to
nitrocellulose sheets and blocked with 5% fat-free milk. The blots
were probed with various primary antibodies (IR-β, IRS-1 and 4G-10,
diluted 1:500, β-actin diluted 1:2000). The proteins were detected
by enhanced chemiluminescence using horseradish peroxidase-labeled
secondary antibodies (diluted 1:5000, Millipore, Billerica, MA,
USA).
Assay of malondialdehyde (MDA)
levels
MDA concentrations were determined by
spectrophotometric assays according to the manufacturer’s
instructions (Nanjing Jianchen Tech, Nanjing, China).
Statistical analysis
Data are expressed as the mean ± SE. Statistical
analysis was performed by one-way ANOVA followed by the
least-significant difference test or Tamhane’s T2 test. P<0.05
was considered to indicate a statistically significant result.
Results
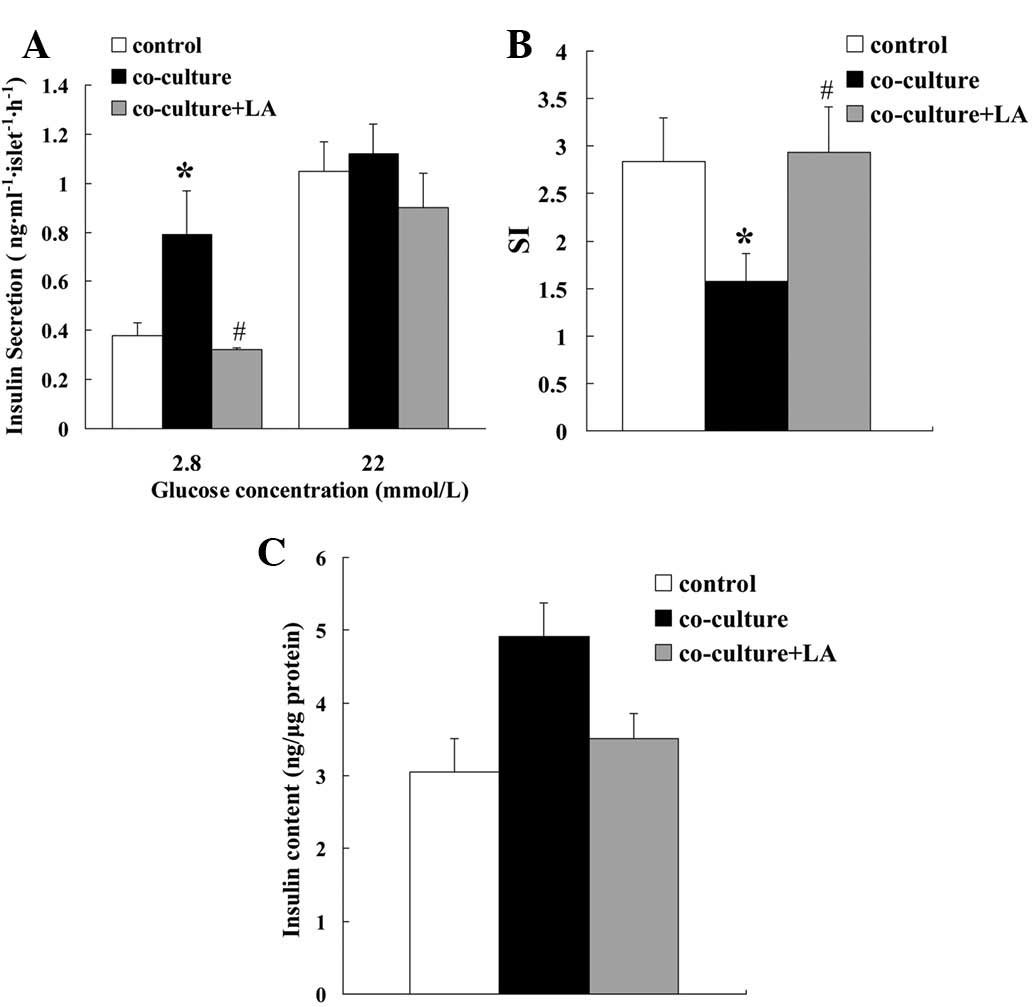
Effect of LA on insulin secretion by rat
islets co-cultured with 3T3L1 adipocytes
To examine the effects of adipocytes and LA on islet
cell function, we first evaluated insulin secretion levels. The
islets were isolated and incubated in KRBH containing either 2.8 or
22 mmol/l glucose. At the lower glucose level, insulin secretion
levels were higher for the islets that had been co-cultured with
adipocytes (co-culture) than those that had not (control). The
presence of LA (co-culture plus LA) eliminated this increase in
secretion. At the higher glucose level, the insulin secretion
levels were similar for the three differently treated islet groups
(Fig. 1A). The stimulation index
(SI, insulin release at high glucose/low glucose) of the
co-cultured islets was lower than that of the control islets, but
was restored by the addition of LA (Fig. 1B). The cellular insulin content was
similar for the three groups (Fig.
1C).
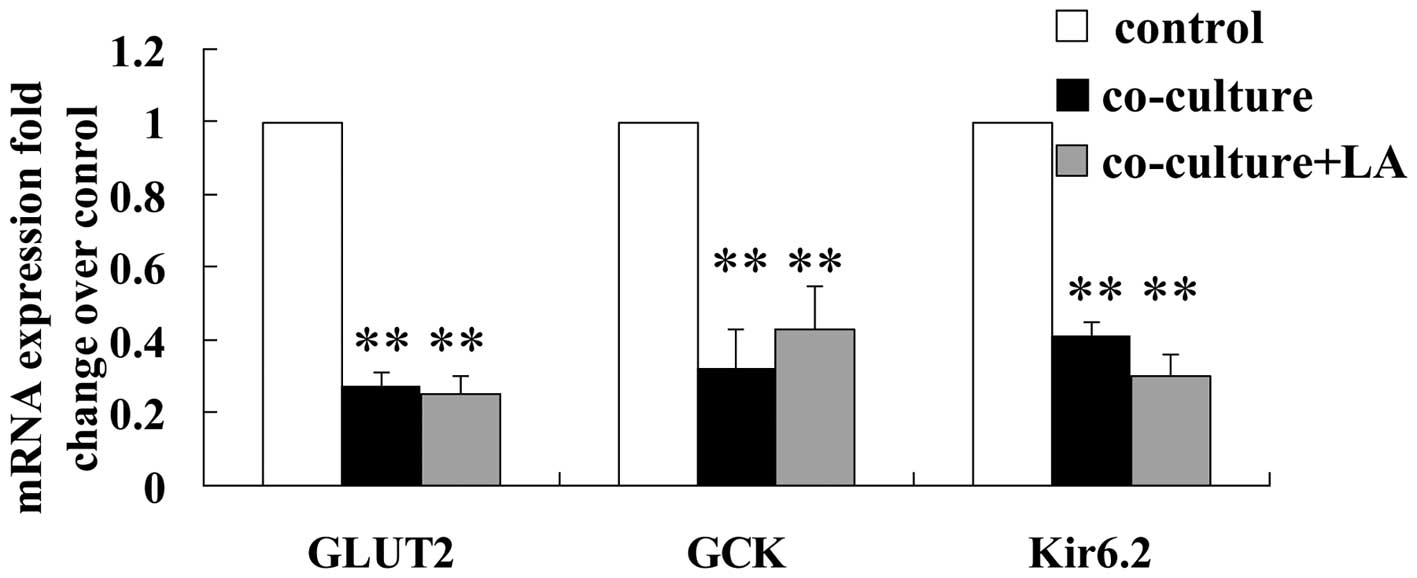
Effect of LA on glucose transporter 2
(GLUT2), glucokinase (GCK) and Kir6.2 mRNA levels in rat islets
co-cultured with 3T3L1 adipocytes
Glucose enters the β cells through GLUT2. Elevated
concentrations of glucose within the β cells enhance glucose
metabolism resulting in an increased cellular adenosine
triphosphate/adenosine diphosphate (ATP/ADP) ratio and subsequently
leads to the closure of KATP channels, membrane depolarization, an
influx of extracellular calcium and exocytosis of insulin granules.
GLUT2, GCK and Kir6.2 are the key genes involved in the process of
glucose-stimulated insulin secretion (GSIS). The mRNA levels of
these factors were examined in the three groups of islets following
48 h of culturing to assess whether the presence of adipocytes and
LA altered their expression. The expression levels in the
co-cultured islets were lower than in the control islets
(downregulated to 27, 32 and 40%, respectively). Meanwhile, the
mRNA levels of these three factors were not altered by the addition
of LA (Fig. 2).
Effect of LA on protein expression and
tyrosine phosphorylation of IR-β and IRS-1 in rat islets
co-cultured with 3T3L1 adipocytes
Insulin has an significant autocrine action on β
cells, which is necessary for maintaining normal secretion. We
investigated certain factors that participate in the
insulin-signaling pathway. As depicted in Fig. 3, the protein levels of IR-β and
IRS-1 were altered by the presence of the adipocytes. The protein
levels of IR-β and IRS-1 in the co-cultured islets decreased to ∼77
and 75%, respectively, of their values in the control islets
(Fig. 3A). The tyrosine
phosphorylation levels of these proteins were reduced to ∼45 and
67%, respectively, of their values in the control islets (Fig. 3B and C). The addition of LA
upregulated the IR-β and IRS-1 protein expression levels 1.49- and
1.28-fold, respectively, and increased their tyrosine
phosphorylation levels 2.68-and 1.79-fold, respectively, compared
with the co-cultured islets (Fig.
3).
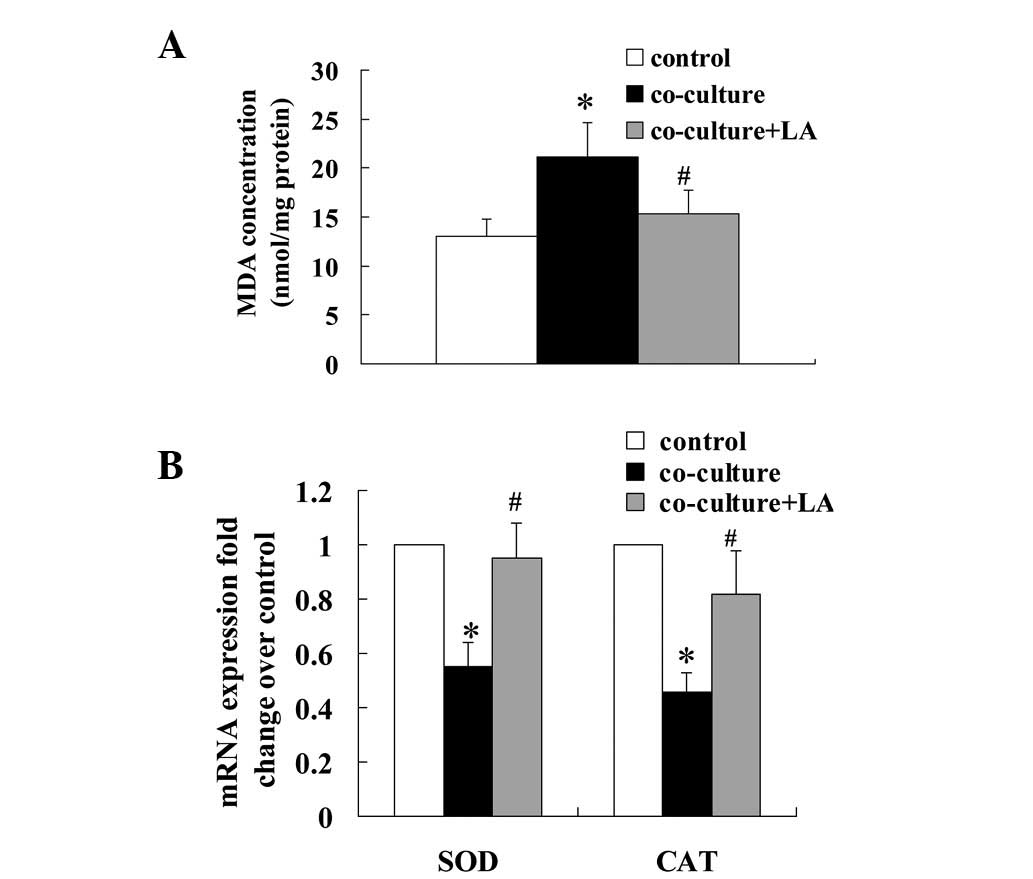
Effect of LA on oxidative stress
biomarkers in rat islets co-cultured with 3T3L1 adipocytes
Since LA is a potent antioxidant, we examined the
cellular content of the lipid peroxidation factor MDA and the mRNA
levels of the antioxidant enzymes superoxide dismutase (SOD) and
catalase (CAT). MDA levels were increased in the co-cultured islets
compared with the control islets (21.08±12.36 vs. 13.03±5.98
nmol/mg; Fig. 4A). This increase
was inhibited by the addition of LA. The mRNA levels of SOD and CAT
in the co-cultured islets were significantly decreased to 55 and
46%, respectively, of their levels in the control islets and this
reduction was blocked by treatment with LA (Fig. 4B).
Discussion
Adipocytes produce adipokines, which travel to
distant sites (including the pancreas) where they may exert
deleterious effects. To analyze the metabolic effects of
adipokines, most studies have investigated the effects of
recombinant proteins in vitro. However, this approach may
not reflect what is happening in vivo, for example the
extensive crosstalk between bioactive factors (16). The co-culture system
(differentiated 3T3L1 adipocytes and islet cells) characterized in
this study may be a new and potentially more physiologically
relevant tool for investigating the effects of adipokines on rat
islet dysfunction.
In this study, we found that at a low glucose
concentration (2.8 mmol/l), co-culturing the islets with adipocytes
resulted in increased insulin secretion levels. However, at high
glucose levels (22 mmol/l), the co-cultured and control islets
secreted similar levels of insulin resulting in a decreased SI.
Zhao et al have previously established a co-culture system
of differentiated 3T3L1 adipocytes and MIN6 cells (17). In contrast to our findings, they
found that after seven days of co-culturing, insulin secretion by
the co-cultured MIN6 cells decreased at low glucose levels, and
tolbutamide (a KATP blocker) stimulated a significant increase in
insulin secretion levels in the control but not the co-cultured
MIN6 cells (17). The difference
between the findings of the two studies may result from a
difference in culture time (2 days in the current study compared
with 7 days for the earlier study) and/or in cells (we used
isolated rat islets while the earlier study used MIN6 cells).
Tolbutamide was used in the earlier study since the MIN6 cells did
not respond normally to high glucose levels. In support of our
findings, the previous study revealed that pancreatic islets
exposed to free fatty acid (FFA) for 48 h exhibited enhanced basal
insulin secretion and an impaired response of the β cells to
glucose stimulation (18). Taken
together, it is expected that short-term exposure (2 days) to
adipocytes may overactivate the islet cells, resulting in an
augmentation of basal insulin release and a reduced susceptibility
to high glucose levels, whereas longer exposure (7 days) to
adipocytes may result in exhaustion of the insulin secretion by the
β cells. Moreover, the findings in these studies are consistent
with the pathophysiological process of hyperinsulinemia at the
early stage of T2DM and the progressive impairment of insulin
secretion over time.
In agreement with the findings of Zhao et al,
we found that the cellular insulin contents were comparable for the
co-cultured and control islets. These findings suggest that the
adipokines do not increase insulin secretion by enhancing insulin
synthesis. The decreases in the mRNA levels of GLUT2, GCK and Kir
6.2 suggest that adipokines downregulate the mRNA levels of GSIS
factors prior to a drop in insulin secretion levels.
Insulin-producing pancreatic β cells are targets for
insulin action. Insulin affects a variety of cellular processes,
including transcription, translation, glucose and lipid metabolism,
ion flux, cell proliferation, cell size and β-cell apoptosis
(19). Insulin resistance, in
addition to peripheral targets including liver, muscle, fat and
brain, may also affect β cells. In our study, the protein and
tyrosine phosphorylation levels of IR-β and IRS-1 decreased in the
co-cultured islets, which suggests that the adipocytes downregulate
the ability of insulin to signal to rat β cells. An increasing body
of evidence suggests that β-cell insulin resistance is coupled with
β-cell dysfunction and apoptosis (20). Furthermore, β-cell insulin
resistance may add to the deterioration of β-cell function and
therefore accelerate the progression of T2DM (19).
The beneficial effects of LA on the co-cultured
islets may result from its ability to act as a direct mitochondrial
antioxidant, phase 2 antioxidant enzyme inducer, energy enhancer or
enzyme cofactor (21). In our
study, the addition of LA to the co-culture reduced insulin
secretion at low glucose levels whereas at high glucose levels it
had no significant affect on insulin secretion. These findings
indicate that LA suppresses the adipocyte-induced increase in
insulin release when glucose levels are low and promotes insulin
secretion when they are high.
In a previous study, it was found that the
administration of LA to obese rats resulted in increased
insulin-stimulated glucose disposal in the whole body and in
skeletal muscle (22). LA has also
been reported to directly activate tyrosine kinase in 3T3L1 cells
(23). The upregulated expression
of factors involved in the insulin signaling pathway following LA
supplementation in our study indicates that LA reinforces the
insulin sensitivity of islet cells in addition to that of
peripheral insulin targets. Since the insulin-signaling pathway may
affect β-cell insulin release, its activation may contribute to the
restored islet secretion of the co-cultured islets treated with
LA.
MDA is one of the most frequently used indicators of
lipid peroxidation. SOD converts intracellular superoxide radicals
into hydrogen peroxide which is decomposed by CAT to form water.
SOD and CAT are major antioxidant enzymes. A number of studies
suggest that excessive concentrations of reactive oxygen species
(ROS) cause pancreatic islet β-cell dysfunction and impair the
action of insulin. A recent study revealed that oxidative stress
was involved in a FFA-induced decrease in β-cell secretory function
and that antioxidants prevented the loss of secretory function
(14). Pancreatic β cells have low
antioxidant defenses and are thus susceptible to ROS-induced
decreases in function and viability (24). As shown in Fig. 4, MDA cellular levels were increased
in the co-cultured islets and this increase was inhibited by LA.
This suggests that the adipocytes increased the peroxidation of
lipids and that the antioxidative effect of LA blocked this effect.
The mRNA expression levels of SOD and CAT were decreased in the
co-cultured islets, which suggests that the adipocytes inhibit this
antioxidative pathway. LA restored the levels of SOD and CAT,
possibly reflecting an increase in antioxidant activity. Oxidative
stress, the prevalence of oxidant factors over antioxidant
mechanisms, plays a central role in the pathogenesis and
progression of diabetes and its complications. Hence, it is
possible that a substance known to reduce oxidative stress in
vivo may reduce the progression of cell damage in clinical
diabetes (25). Our findings
suggest that LA reduces oxidative stress in islet cells by
alleviating lipid peroxidation or by increasing the mRNA levels of
antioxidant enzymes which detoxify free radicals.
In conclusion, the co-culture system of 3T3L1
adipocytes and islet cells may be a new model for investigating
islet lipotoxicity. The presence of 3T3L1 adipocytes disturbs
insulin secretion by the islet cells and may induce islet cell
dysfunction. The effects may be mediated by multiple pathways,
including the downregulation of GSIS gene expression, the
suppression of islet cell insulin signaling and the induction of
oxidative stress. LA, an antioxidant, may protect islet cells by
the activation of insulin signaling in islets and the upregulation
of the mRNA expression levels of antioxidant enzymes. Our findings
raise the possibility that supplementation with LA may be an
effective strategy for preventing β cells from lipotoxicity.
Abbreviations:
|
LA
|
α-lipoic acid;
|
|
IR-β
|
insulin receptor-β;
|
|
IRS-1
|
insulin receptor substrate-1;
|
|
SOD
|
superoxide dismutase;
|
|
CAT
|
catalase;
|
|
T2DM
|
type 2 diabetes mellitus;
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium;
|
|
FBS
|
fetal bovine serum;
|
|
KRBH
|
Krebs-Ringer-HEPES buffer;
|
|
MDA
|
malondialdehyde;
|
|
GLUT2
|
glucose transporter 2;
|
|
GCK
|
glucokinase;
|
|
GSIS
|
glucose-stimulated insulin
secretion;
|
|
FFA
|
free fatty acid;
|
|
ROS
|
reactive oxygen species
|
Acknowledgements
We are grateful to Yu Chen from Verigy
Shanghai for his long-term support and encouragement. This study
was supported by grants from the Natural Science Foundation of
Shanghai (no. 10ZR1424100) and the National Natural Science
Foundation of China (30700381 and 81070682).
References
|
1.
|
Ouchi N, Parker JL, Lugus JJ and Walsh K:
Adipokines in inflammation and metabolic disease. Nat Rev Immunol.
11:85–97. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Waki H and Tontonoz P: Endocrine functions
of adipose tissue. Annu Rev Pathol. 2:31–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Anubhuti and Arora S: Leptin and its
metabolic interactions: an update. Diabetes Obes Metab. 10:973–993.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Natalicchio A, De Stefano F, Orlando MR,
Melchiorre M, Leonardini A, Cignarelli A, et al: Exendin-4 prevents
c-Jun N-terminal protein kinase activation by tumor necrosis
factor-alpha (TNFalpha) and inhibits TNFalpha-induced apoptosis in
insulin-secreting cells. Endocrinology. 151:2019–2029. 2010.
View Article : Google Scholar
|
|
5.
|
Suzuki T, Imai J, Yamada T, Ishigaki Y,
Kaneko K, Uno K, Hasegawa Y, Ishihara H, Oka Y and Katagiri H:
Interleukin-6 enhances glucose-stimulated insulin secretion from
pancreatic beta-cells: potential involvement of the
PLC-IP3-dependent pathway. Diabetes. 60:537–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zhao YF, Feng DD and Chen C: Contribution
of adipocyte-derived factors to beta-cell dysfunction in diabetes.
Int J Biochem Cell Biol. 38:804–819. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Schroeder MM, Belloto RJ Jr, Hudson RA and
McInerney MF: Effects of antioxidants coenzyme Q10 and lipoic acid
on interleukin-1 beta-mediated inhibition of glucose-stimulated
insulin release from cultured mouse pancreatic islets.
Immunopharmacol Immunotoxicol. 27:109–122. 2005. View Article : Google Scholar
|
|
8.
|
Kamenova P: Improvement of insulin
sensitivity in patients with type 2 diabetes mellitus after oral
administration of alpha-lipoic acid. Hormones (Athens). 5:251–258.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shen W, Liu K, Tian C, Yang L, Li X, Ren
J, Packer L, Head E, Sharman E and Liu J: Protective effects of
R-alpha-lipoic acid and acetyl-L-carnitine in MIN6 and isolated rat
islet cells chronically exposed to oleic acid. J Cell Biochem.
104:1232–1243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ryden M, Dicker A, van Harmelen V, Hauner
H, Brunnberg M, Perbeck L, Lonnqvist F and Arner P: Mapping of
early signaling events in tumor necrosis factor-alpha-mediated
lipolysis in human fat cells. J Biol Chem. 277:1085–1091. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sakata N, Egawa S, Sumi S and Unno M:
Optimization of glucose level to determine the stimulation index of
isolated rat islets. Pancreas. 36:417–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Latif ZA, Noel J and Alejandro R: A simple
method of staining fresh and cultured islets. Transplantation.
45:827–830. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bank HL: Rapid assessment of islet
viability with acridine orange and propidium iodide. In Vitro Cell
Dev Biol. 24:266–273. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Oprescu AI, Bikopoulos G, Naassan A,
Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG,
Rozakis-Adcock M, et al: Free fatty acid-induced reduction in
glucose-stimulated insulin secretion: evidence for a role of
oxidative stress in vitro and in vivo. Diabetes. 56:2927–2937.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Maedler K, Spinas GA, Dyntar D, Moritz W,
Kaiser N and Donath MY: Distinct effects of saturated and
monounsaturated fatty acids on beta-cell turnover and function.
Diabetes. 50:69–76. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Vu V, Kim W, Fang X, Liu YT, Xu A and
Sweeney G: Coculture with primary visceral rat adipocytes from
control but not streptozotocin-induced diabetic animals increases
glucose uptake in rat skeletal muscle cells: role of adiponectin.
Endocrinology. 148:4411–4419. 2007. View Article : Google Scholar
|
|
17.
|
Zhao YF, Feng DD, Hernandez M and Chen C:
3T3-L1 adipocytes induce dysfunction of MIN6 insulin-secreting
cells via multiple pathways mediated by secretory factors in a
co-culture system. Endocrine. 31:52–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zhou YP and Grill VE: Long-term exposure
of rat pancreatic islets to fatty acids inhibits glucose-induced
insulin secretion and biosynthesis through a glucose fatty acid
cycle. J Clin Invest. 93:870–876. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Leibiger IB, Leibiger B and Berggren PO:
Insulin signaling in the pancreatic beta-cell. Annu Rev Nutr.
28:233–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Okada T, Liew CW, Hu J, Hinault C, Michael
MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M and Kulkarni RN:
Insulin receptors in beta-cells are critical for islet compensatory
growth response to insulin resistance. Proc Natl Acad Sci USA.
104:8977–8982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Liu J and Ames BN: Reducing mitochondrial
decay with mitochondrial nutrients to delay and treat cognitive
dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr
Neurosci. 8:67–89. 2005.PubMed/NCBI
|
|
22.
|
Lee WJ, Song KH, Koh EH, Won JC, Kim HS,
Park HS, Kim MS, Kim SW, Lee KU and Park JY: Alpha-lipoic acid
increases insulin sensitivity by activating AMPK in skeletal
muscle. Biochem Biophys Res Commun. 332:885–891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yaworsky K, Somwar R, Ramlal T, Tritschler
HJ and Klip A: Engagement of the insulin-sensitive pathway in the
stimulation of glucose transport by α-lipoic acid in 3T3-L1
adipocytes. Diabetologia. 43:294–303. 2000.PubMed/NCBI
|
|
24.
|
Tiedge M, Lortz S, Drinkgern J and Lenzen
S: Relation between antioxidant enzyme gene expression and
antioxidative defense status of insulin-producing cells. Diabetes.
46:1733–1742. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Maritim AC, Sanders RA and Watkins JB III:
Effects of α-lipoic acid on biomarkers of oxidative stress in
streptozotocin-induced diabetic rats. J Nutr Biochem. 14:288–294.
2003.
|