Introduction
Under the influence of steroid from the ovaries, the
endometrium undergoes periodical changes. Thus, the embryos can
implant in the endometrium only during the proper phase (1). Blastocyst implantation is shared by
all mammals in nature and usually occurs between 3 and 6 days after
fertilization, which corresponds to ∼Days 21 to 24 or ∼5 to 8 days
after the peak in luteinizing hormone (LH) levels. That is to say,
the embryos enter the uterus in the implantation window. The
embryos and the endometrium secrete several related proteins and
cytokines in a strictly spatial-temporal sequence. These proteins
and cytokines recognize each other and cooperate, leading to
implantation (2). These bioactive
cytokines and proteins are known as markers of endometrial
receptivity.
Tubal factors are the main causes of female
infertility and account for ∼40% of all causes. Furthermore, the
hydrosalpinx accounts for ∼10 to 30% of the tubal factors causing
infertility. In vitro fertilization-embryo transfer (IVF-ET)
was initially applied in women with tubal factor infertility.
However, numerous studies have shown that the hydrosalpinx can
reduce the implantation and pregnancy rates (3). The mechanisms underlying the impact
of hydrosalpinx on IVF-ET are poorly understood. There is evidence
that the influence of hydrosalpinx on the endometrial receptivity
is one of the mechanisms (4). In
the present study, the expression of integrin αvβ3 in the
endometrium during the implantation window was compared between
hydrosalpinx patients and those with fallopian tube obstruction,
and the expression of integrin αvβ3 in the endometrium during the
implantation window in hydrosalpinx patients were also compared
before and after surgery. Our results may be helpful to elucidate
the cause of poor outcome of hydrosalpinx patients following
IVF-ET.
Materials and methods
Patients
A total of 60 patients with hydrosalpinx and 30
patients with fallopian tube obstruction were recruited from April
2010 to December 2010 from the Center for Reproductive Medicine of
the First Affiliated Hospital of Sun Yat-Sen University (Guangdong,
China).
All patients were aged <40 years and had a
regular menstrual cycle. Endocrine examinations revealed normal
levels and the basal body temperature was biphasic. Hormones were
not administered within 6 months before the start of the study.
Cases presenting with endometriosis, uterine fibroids, polycystic
ovary syndrome, ovarian cancer, infertility of unknown causes,
immune infertility, chronic systemic disease, sexually transmitted
disease and trophoblastic disease, and cases with a positive status
for smoking and drinking were excluded from the study. Cases
associated with a spouse presenting with male infertility were also
excluded.
Diagnosis
Bilateral or unilateral hydrosalpinx was diagnosed
by hysterosalpingography (HSG) or laparoscopy (LAP) and
untrasonography. Fallopian tube obstruction was diagnosed by HSG or
LAP, and ultrasonography was performed to exclude the presence of
hydrosalpinx.
Surgical intervention of
hydrosalpinx
Vaginal ultrasound-guided hydrosalpinx aspiration,
laparoscopic salpingostomy, laparoscopic proximal tubal ligation or
laparoscopic salpingectomy was performed.
Sample collection and processing
The LH peak was measured by using LH strip from Day
10 of the menstrual cycle. In addition, transvaginal
ultrasonography and the test of serum sex hormones were also
performed. At Days 7–8 after ovulation, the endometrium was
collected at the bottom of the uterus by using a curette, and the
samples were washed in normal saline to remove blood. Samples were
fixed in fixation solution, embedded in paraffin and sectioned.
Pathological examination was carried out to confirm that the
endometrium was in the secretory phase. For patients with
hydrosalpinx, the endometrium was collected during the implantation
window before and after surgery; collection of endometrium was
performed once in patients with fallopian tube obstruction.
Immunohistochemistry
Mouse anti-human integrin αvβ3 monoclonal antibody
(Abcam) (1:80) was used for immunohistochemistry which was
performed according to the manufacturer’s instructions.
Determination of findings
Five fields were randomly selected from each section
at magnification ×4,000, and the integrated optical density (IOD)
was determined by using the Image-Pro Plus 5.1 Chinese
software.
Statistical analysis
The IOD was expressed as means ± standard deviation
(SD). Statistical analysis was performed with SPSS version 13.0. A
value of two-tailed P<0.05 was considered statistically
significant.
Results
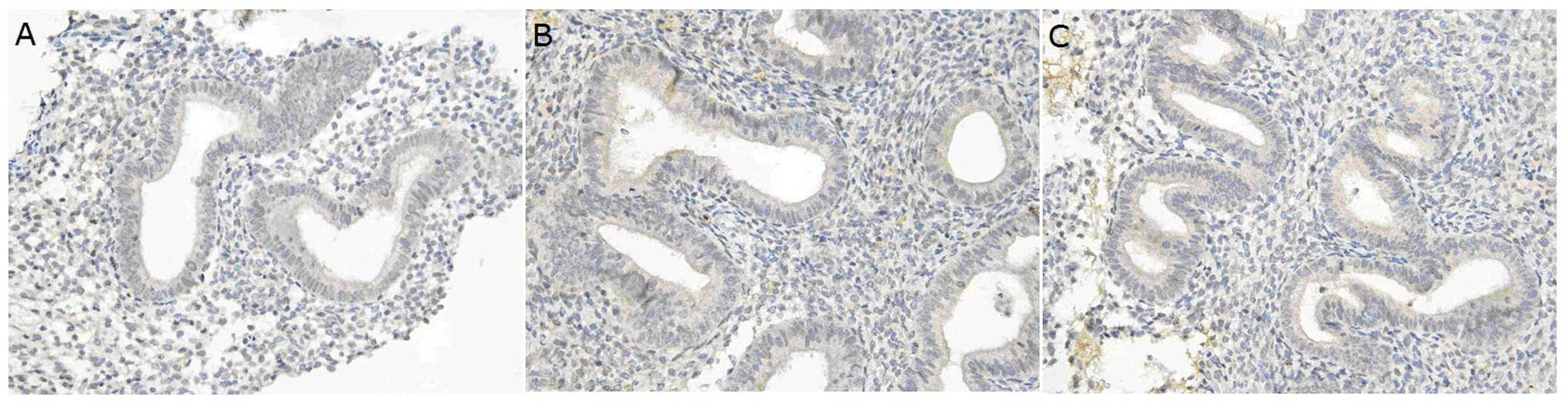
Under light microscopy, integrin αvβ3 was mainly
expressed on the membrane and in the cytoplasm of the endometrial
gland epithelial cells, while the endometrial interstitium had weak
integrin αvβ3 expression. In hydrosalpinx patients, integrin αvβ3
expression levels were significantly different at the times before
and after surgery (P<0.05). Before surgery, integrin αvβ3
expression in the endometrium of the hydrosalpinx patients
(Fig. 1A) was markedly lower than
that in the controls (Fig. 1C)
(P<0.05). However, no dramatic difference was found in the
integrin αvβ3 expression in the endometrium between hydrosalpinx
patients after surgery and control patients (P>0.05) (Fig. 1B and Table I).
 | Table IExpression of endometrial integrin
αvβ3 in hydrosalpinx patients and fallopian tube obstruction
patients. |
Table I
Expression of endometrial integrin
αvβ3 in hydrosalpinx patients and fallopian tube obstruction
patients.
| Hydrosalpinx patients
| Fallopian tube
obstruction patients (n=30) | |
|---|
| Before surgery
(n=60) | After surgery
(n=60) | |
|---|
| Integrin αvβ3 | 0.29±0.10 | 0.58±0.17 | 0.55±0.11 |
Discussion
The histologically normal endometrium dos not always
have a normal function and does not always reflect normal
receptivity. Currently, indicators for the evaluation of the
endometrium are limited. There are numerous cytokines and molecules
that are being applied to evaluate the successful implantation of
embryos. In the present study, we employed integrin αvβ3 as a
marker of emdometrial receptivity. To date, few studies have been
conducted to investigate the effect of hydrosalpinx on integrin
αvβ3 expression in the endometrium.
Biochemical characteristics of
integrins
Integrins are a type of cellular adhesion molecule
and are expressed on the cell membrane. They are receptors shared
by the extracellular matrix and heterodimers consisting of subunits
α and β in a non-covalent manner. A total of 14 α subunits and 9 β
subunits have been identified and can form >20 integrins. Both
subunits α and β are composed of extracellular, transmembrane and
intracellular domains. The α subunit is 120–180 kDa and is
indispensable for the integrin function. The β subunit is 90–110
kDa. Different combinations of α and β subunits form different
integrins. The N terminal of the heterodimers of subunits α and β
is extracellular and long and forms a spherical domain. In
addition, the N terminal also contains a divalent cation-binding
site which can specifically bind to the laminin (LN), fibronectin
(FN), vitroneetin (VN) and the Arg-Gly-Asp (RGD) in the human
complement C3. The C terminal is intracellular and short. Different
α and β subunits have distinct structures of the C terminal. Dou
et al (5) determined the
expression levelss of α2, α3, α4, α5, α6.1, α6.2, αv, β1, β2, β3
and β5 in the endometrium during the entire menstrual cycle. They
found that α2, α3 and α5 were predominantly expressed during the
proliferative phase, and α4, α6.2, αv, β1, β2, β3 and β5 were
mainly expressed during the secretory phase. However, α6.1
expression was constant during the entire menstrual cycle. In
addition, the changes in the expression of αv and β3 in the
menstrual cycle were more obvious than changes in other subunits.
Moreover, different types of cells exhibit expression of different
integrins. In mammals, integrins are widely expressed on the cell
membrane. Currently, integrins have become an acceptable marker of
endometrial receptivity.
Role and regulation of integrins in
reproduction
Integrins function via binding to the corresponding
ligands. An integrin can recognize some ligands and a ligand may
recognize different integrins. Integrin αvβ3 is related to
endometrial receptivity and its ligands include osteopontin (OPN)
(6), perlecan, FN, VN, tenascin
and von Willebrand factor (vWF). During the establishment of
endometrial receptivity, OPN can recognize αvβ3, which is closely
related to the implantation window. During the proliferative phase,
the mRNA expression of OPN is weak. During the middle or later
secretory phase, the endometrial epithelial cells, lymphocytes and
endometrial secretions have high mRNA expression of OPN (7). Lessey et al (8) proposed the ‘Sandwich model’ in the
implantation of embryos, according to which the integrins expressed
on the embryos and in the endometrium can bind to the OPN, which
facilitates the adhesion of embryos to the endometrium. During the
implantation window, the expression of integrins in the endometrium
is significantly increased due to the regulation by steroids and a
series of cytokines and growth factors, and the affinity of
integrins is also elevated, which maximizes endometrial
receptivity. At the same time, trophoblast cells in the embryos
also express integrins. Thus, integrins in the endometrium and on
the trophoblast cells bind to the OPN which mediates the crosstalk
between embryos and endometrium. The integrins are expressed on the
cell membrane in a cluster manner and the individual integrin has a
low affinity to the ligands. However, the accumulated affinity of
clustered integrins significantly consolidates the binding between
the embryo and endometrium. Therefore, according to the ‘Sandwich
model’, the endometrium finally accepts the embryo leading to
endometrial receptivity. In addition, integrins may act as
activators and can activate the endometrium, increase vascular
permeability, promote the dilation of local blood vessels and
become involved in the decidualization of endometrium, which are
beneficial for the adhesion of embryos to the endometrium and
subsequent implantation.
Before endometrial receptivity is established and
after endometrial receptivity subsides, the expression of the
estrogen receptor (ER) and progesterone receptor (PR) in the
endometrial epithelial cells displays a decreasing tendency which
depends on progesterone. Failure of progesterone regulation may
significantly affect endometrial receptivity (9). Progesterone binds to the PR and then
regulates the αvβ3 and its ligands in two ways: i) by direct
regulation, where progesterone directly acts on the PR on
epithelial cells of endometrium and then promotes the expression of
αvβ3, OPN and other endometrial receptivity-related moleculaes
(such as α1β1 and α4β1) in the epithelial cells and ii) by indirect
regulation, where progesterone acts on the PR on endometrial stroma
cells which stimulates the transcription of downstream genes and
increases the expression of epithelium growth factor (EGF) or
heparin-binding EGF-like gowth factor (HB-EGF). These factors then
affect the corresponding receptors on the endometrial epithelial
cells leading to the production of αvβ3 and OPN (10). At the site where the embryos
implant, a series of cytokines are expressed and form a network,
which can coordinate the expression of various factors which
mediate endometrial receptivity. Integrins are also regulated by
these factors and thus, the endometrium can achieve receptivity at
the designated time. The embryos can secrete hCG and other
cytokines (such as IL-1), which then bind to the corresponding
receptors on the endometrium and regulate the expression of
molecules in the endometrium (such as integrins) and subsequently
endometrial receptivity. There is evidence showing that integrin
αvβ3 binds to the RGD sequence both of which are expressed on the
embryo (11). RGD may bridge the
recognition between integrin αvβ3 in the endometrium and that on
the embryo, but the specific mechanism is unclear. The findings
above show that integrin αvβ3 is critical for the implantation of
the embryo, and it is expressed not only in the endometrium but on
the embryo as well.
Effect of hydrosalpinx on integrin αvβ3
expression in the endometrium
Our results showed that integrin αvβ3 expression in
hydrosalpinx patients before surgery was markedly lower than that
in patients with fallopian tube obstruction. After surgical
intervention for hydrosalpinx, integrin αvβ3 expression was
comparable between patients in the two groups. In addition, when
compared with the level in patients before surgery, integrin αvβ3
expression was dramatically increased in the hydrosalpinx patients
after surgery. These findings suggest that hydrosalpinx inhibits
the integrin αvβ3 expression in the endometrium during the
implantation window, which is upregulated following surgical
intervention. These findings were consistent with previous studies.
Lessey and Castelbaum (8) found
that integrin αvβ3 expression in the endometrium during the
implantation window was decreased in hydrosalpinx patients, but
returned to normal levels after surgical treatment for
hydrosalpinx. Bildirici et al (12) also drew the same conclusion.
Bildirici et al (12)
investigated 10 patients with hydrosalpinx. Before surgery, the
HSCORE score of integrin αvβ3 expression was <0.7 during the
implantation window in 8 hydrosalpinx patients, and the mean HSCORE
score was increased by 2.1 after surgery (criterion for positivity,
0.7). Statistical analysis revealed a significant difference in
integrin αvβ3 expression in the endometrium of patients at a time
before and after surgery. We hypothesize that hydrosalpinx may
reduce endometrial receptivity via decreasing integrin expression
in the endometrium.
Taken together, the endometrium undergoes periodic
changes which vary among individuals. Accurate evaluation of
endometrial receptivity is basic for its improvement. In the
present study, we investigated the effect of hydrosalpinx on
expression in the endometrium. Our results demonstrate that
hydrosalpinx influences integrin αvβ3 expression in the endometrium
during the implantation window, reduces the receptivity of the
endometrium for the implantation of the embryo and compromises the
ability to maintain pregnancy. After surgical treatment for
hydrosalpinx, integrin αvβ3 expression in the endometrium is
increased.
Acknowledgements
This study was funded by the Guangdong
Science and Technology Program (no. 2009B030801155) and the
Guangdong Population and Family Planning Project (no. 2010243).
References
|
1.
|
Lessey BA: Endometrial integrins and the
establishment of uterine receptivity. Hum Reprod. 13:S247–S258.
1998. View Article : Google Scholar
|
|
2.
|
Taylor E and Gomel V: The uterus and
fertility. Fertil Steril. 89:1–16. 2008. View Article : Google Scholar
|
|
3.
|
Mijatovic V, Veersema S, Emanuel MH,
Schats R and Hompes PG: Essure hysteroscopic tubal occlusion device
for the treatment of hydrosalpinx prior to in vitro
fertilization-embryo transfer in patients with a contraindication
for laparoscopy. Fertil Steril. 93:1338–1342. 2010. View Article : Google Scholar
|
|
4.
|
Seli E, Kayisli UA, Cakmak H, Bukulmez O,
Bildirici I, Guzeloglu-Kayisli O and Arici A: Removal of
hydrosalpinges increases endometrial leukaemia inhibitory factor
(LIF) expression at the time of the implantation window. Hum
Reprod. 20:3012–3017. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Dou Q, Willians RS and Chegini N:
Expression of integrin messenger ribonucleic acid in human
endometrium: a quantitative reverse transcription polymerase chain
reaction study. Fertil Steril. 71:347–353. 1999. View Article : Google Scholar
|
|
6.
|
Lessey BA: Adhesion molecules and
implantation. J Reprod Immunol. 55:101–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Apparao KB, Murray MJ, Fritz MA, Meyer WR,
Chambers AF, Truong PR and Lessey BA: Osteopontin and its receptor
alphav-beta(3) integrin are coexpressed in the human endometrium
during the menstrual cycle but regulated differentially. J Clin
Endocrinol Metab. 86:4991–5000. 2001.PubMed/NCBI
|
|
8.
|
Lessey BA and Castelbaum AJ: Integrins and
implantation in the human. Rev Endocr Metab Disord. 3:107–117.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Petersen A, Bentin-Ley U, Ravn V, Qvortrup
K, Sørensen S, Islin H, Sjögren A, Mosselmann S and Hamberger L:
The anti-progesterone Org31710 inhibits human
blastocyst-endometrial interactions in vitro. Fertil Steril.
83:1255–1263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lessey BA: Two pathways of progesterone
action in the human endometrium: implications for implantation and
contraception. Steroids. 68:809–815. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bulletti C, Flamigni C and de Ziegler D:
Implantation markers and endometriosis. Reprod Biomed Online.
11:464–468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bildirici I, Bukulmez O, Ensari A, Yarali
H and Gurgan T: A prospective evaluation of the effect of
salpingectomy on endometrial receptivity in cases of women with
communicating hydrosalpinges. Hum Reprod. 16:2422–2426.
2001.PubMed/NCBI
|