Introduction
The optic nerve is a part of the central nervous
system and is commonly used to study central nerve damage and
regeneration due to its anatomical particularity. Approximately 95%
of rat retinal ganglion cell (RGC) fibers project to the superior
colliculus (SC) and can be labeled via injection of a fluorescent
tracer into the rat bilateral SC (1,2).
Retrograde labeling of RGCs with
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
(DiI) and examining the retinas using fluorescence microscopy
following periods of survival, is effective and reliable for the
observation of dynamic changes in RGCs.
An optic nerve lesion not only causes typical
morphological change but is able to activate signal transduction
which may reduce neuronal loss and promote intrinsic axonal
regeneration (3). Partial optic
nerve crush (PONC), an experimental procedure of a standardized and
reproducible incomplete axotomy of the RGCs, mimics the key
pathological progress which is related to RGC apoptosis (4). In the present study, we evaluated the
reliability of the PONC model by retrograde labeling of RGCs by
DiI.
Materials and methods
Animals
All experiments were performed in compliance with
guidelines for animal care of the Association for Research in
Vision and Ophthalmology. A total of 46 adult male Sprague-Dawley
(SD) rats with a body weight of 150–200 g were used in the study.
During experimentation, animals were housed under a 12:12-h
light/dark cycle, with water and food available ad
libitum.
SC retrograde DiI labeling
The DiI suspension was prepared by mixing 3 mg DiI
in 1 ml saline containing 1–3% Triton X-100. Sonication and
repeated agitation produced a mixture of dissolved DiI and small
DiI crystals in suspension. The animals were anesthetized via
intraperitoneal injection of 20% chloral hydrate (420 mg/kg) prior
to surgery and were fixed in a stereotaxic apparatus. Following
skin removal, the cranium was exposed and pierced by a 50-ml
injector needle. The position of the SC was located at the point
behind the fonticuli minor 6.4 mm apart from the mid-line 1.5 mm
and inserting needle 4.0 mm. Using a micro-injector, 1.5 μl 10% DiI
was injected at each point. To compare the effect of the bilateral
and unilateral SC retrograde labeling, six rats were divided into
two groups at random (three rats per group). Another 40 rats with
bilateral SC retrograde labeling underwent partial optic nerve
crush.
PONC model
The animals were anesthetized via intraperitoneal
injection of 20% chloral hydrate (420 mg/kg) after SC retrograde
DiI labeling for three days. The optic nerve of the right eye in
all groups was exposed by opening the meninges of the optic nerve
with the sharp tips of forceps, followed by blunt dissection. The
exposed optic nerve was then partially crushed 1 mm behind the
globe for 5, 10 and 30 sec (mild, moderate and severe crush,
respectively) each with 40 gram power (n=10). A sham-operated
control group (n=10) was treated in the same way on the right eye,
but without closing of the the forceps, to check for any falsifying
influence of surgery on the treatment effects. In all cases, the
retinal blood supply remained grossly intact, as judged on the
basis of a direct microscopic inspection during and after the
procedure.
Quantification of RGCs
The rats were anesthetized via intraperitoneal
injection of 20% chloral hydrate (420 mg/kg) following DiI
application for six days and perfused transcardially with saline
and 4% paraformaldehyde (PFA) for 30 min. Following enucleation,
the eyes were postfixed for 1 h in 4% PFA solution. The retinas
were dissected, vitreal side-up flat-mounted on gelatin-coated
slides and RGC counts were performed immediately using laser
confocal fluorescence microscope. The cell count was performed in
an area approximately the same distance from the optic disc; 1/6,
3/6 and 5/6 of the retinal radius. Three fields were selected where
images were obtained using a digital imaging system (ImagePro 6.0)
and the average number of RGCs/mm2 was calculated.
Statistics
Data were analyzed using SPSS 19.0 software (SPSS,
Chicago, IL, USA). Data were tested for statistical significance
with the independent samples t-test or by analysis of variance
(one-way ANOVA). A P-value <0.05 was considered to indicate a
statistically significant result.
Results
SC retrograde DiI-labeled normal
RGCs
The majority of RGCs were labeled with bilateral SC
retrograde DiI injection and there were more RGCs adjacent to the
optic nerve area than compared with the number in the retina
perimeter; no RGCs were labeled in the blood vessel area (Fig. 1a). The majority of RGCs were not
labeled following unilateral SC retrograde DiI injection (Fig. 1b).
Bilateral SC retrograde DiI-labeled RGCs
in PONC model
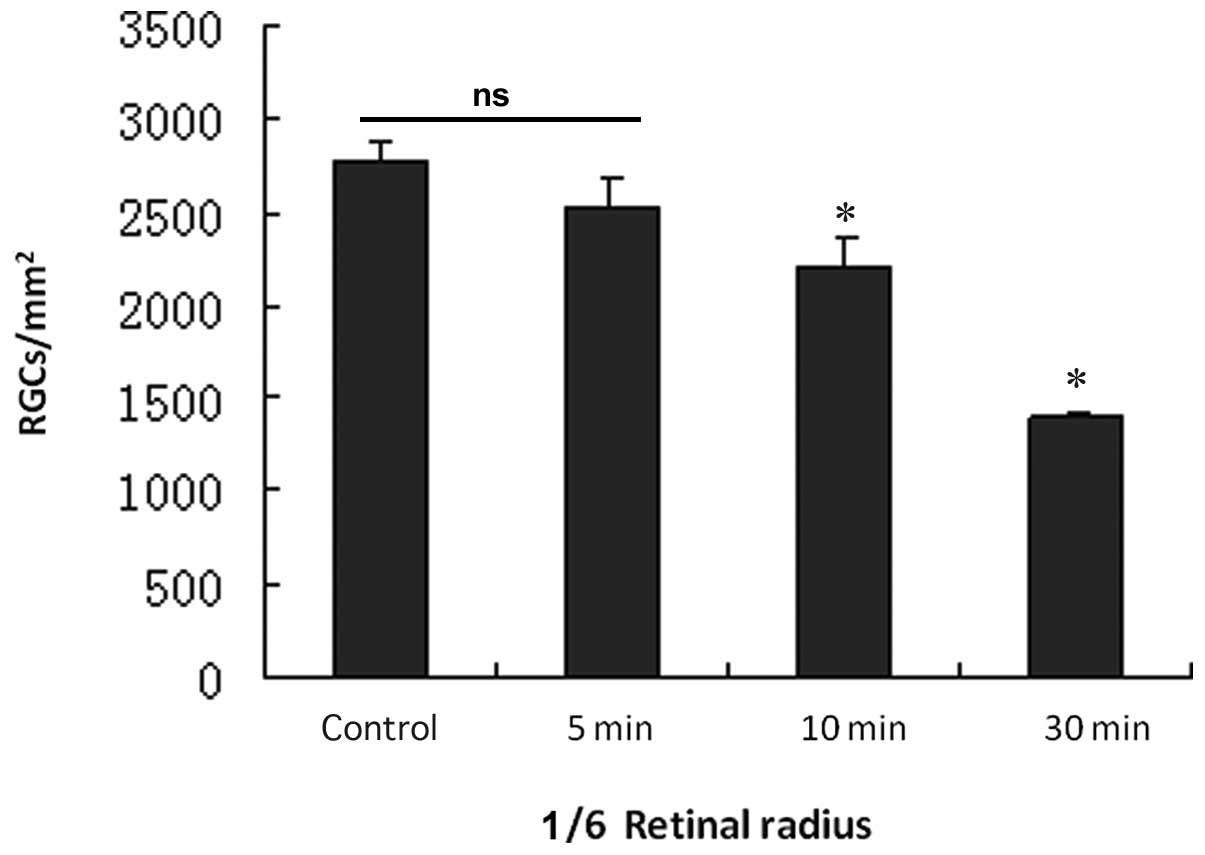
The effects of the various PONC extents (5, 10 and
30 sec) on the numbers of labeled RGCs are shown in Figs. 2–5
and Table I. In the 5 sec PONC
group the majority of RGCs were labeled (Fig. 2a). The RGC densities in the regions
1/6, 3/6 and 5/6 of the retinal radius compared to the
sham-operated control group were not significantly different
(P-values = 0.734, 0.461, 0.273; Figs.
3–5 and Table I). In the 10 sec PONC group, the
numbers of labeled RGCs were less than that in the sham-operated
control group (Fig. 2b), however,
the 10-sec procedure led to a significant decrease in RGC numbers
in regions 1/6, 3/6 and 5/6 of the retinal radius compared to RGC
numbers in the sham-operated control group (P-values = 0.000,
0.000, 0.000; Figs. 3–5 and Table
I). In the 30 sec PONC group, the number of labeled RGCs were
significantly decreased (Fig. 2c);
some cells in the region 1/6 retinal of the radius were labeled.
However, fewer cells in the region 3/6 of the retinal radius were
labeled and almost no cells were labeled in the region 5/6 of the
retinal radius. The 30 sec PONC led to a significant decrease in
RGCs in 1/6 and 3/6 retinal radius regions compared to these values
in the the sham-operated control group (P-values = 0.000, 0.000;
Figs. 3–4 and Table
I).
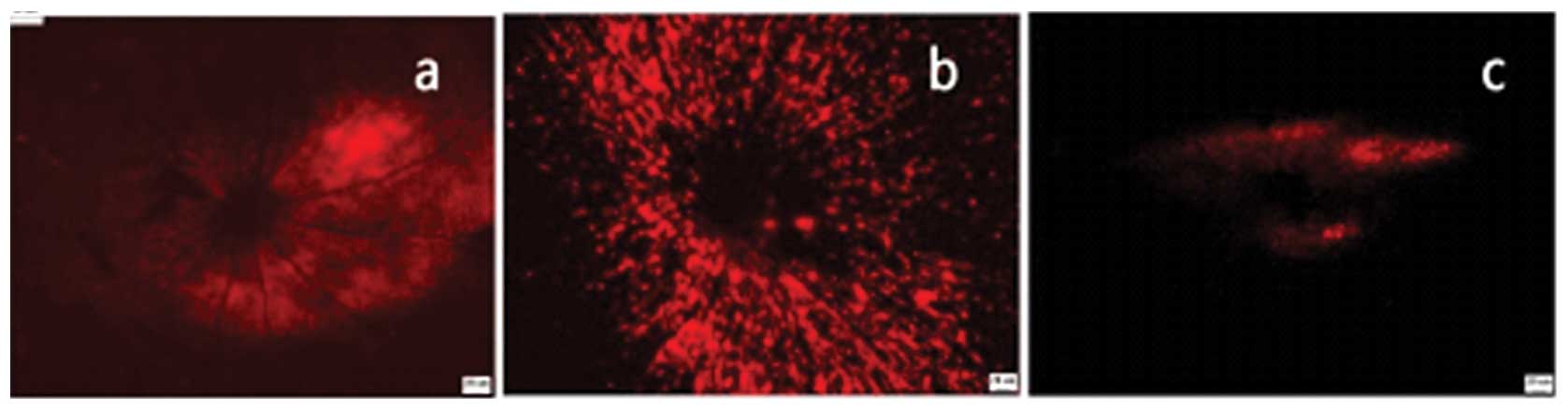 | Figure 2Bilateral SC retrograde DiI-labeled
RGCs of various PONC extents: (a) 5 sec group (mild), (b) 10 sec
group (moderate) and (c) 30 sec (severe) group. Bar, 20 μm. SC,
superior colliculus; RGCs, retinal ganglion cells; PONC, partial
optic nerve crush; DiI,
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine
perchlorate. |
 | Table IRGC densities as a result of different
PONC processes (n=10, mean ± SD). |
Table I
RGC densities as a result of different
PONC processes (n=10, mean ± SD).
| RGCs density
(cells/mm2)
|
|---|
| Group | 1/6 Retinal
radius | 3/6 Retinal
radius | 5/6 Retinal
radius |
|---|
| Normal group | 2779.80±96.45 | 2457.75±63.76 | 1709.00±119.49 |
| 5 sec group | 2522.60±159.74 | 2455.75±65.05 | 1692.00±118.67 |
| 10 sec group |
2210.00±156.17a | 1438.75±30.96a | 722.60±44.34a |
| 30 sec group | 1393.00±20.94a | 80.60±21.31a | 0 |
| F | 5.42 | 12.21 | 35.04 |
| P-value | 0.000 | 0.000 | 0.000 |
Discussion
Fluorescent substances, including DiI and
Fluorogold, are able to transfer from the SC or lateral geniculate
body or optic nerve stump to RGCs and obtain a fluorescent effect
due to retrograde axoplasmic transport. DiI was used as a tracer
due to its capacity to diffuse within the plasmalemma and label
RGCs, while the other types of cells are not stained. Other
tracers, including fast blue and nuclear yellow, diffuse easily to
other cells and are difficult to preserve. Using the traditional
histochemical technology it is difficult to distinguish RGCs from
other cells, especially amacrine cells. Compared with the fast blue
and nuclear yellow tracers, bilateral SC retrograde DiI-labeling is
a reliable and effective method to study the dynamic pathological
change of RGCs, and the method is already widely used in the fields
of RGC apoptosis and optic nerve regeneration (5).
As 95% of RGCs project to the bilateral SC and only
approximately 10% project to the unilateral SC, it is not reliable
to label RGCs via unilateral SC injection since at least 10% of
RGCs will not be labeled (1). The
trilateral injection is more reliable, however, it requires a
longer surgical time and may increase the risk of infection. The
present study found that the bilateral SC retrograde DiI injection
uniformly labeled the RGCs, that the number of RGCs among the
different quadrants was not statistically different and that the
number of RGCs from the center compared with the peripheral retina
was not significantly attenuated. Thus, we suggest that the
bilateral SC retrograde DiI injection is more efficient, improves
labeling of RGCs and is more stable.
The PONC model is widely used in the fields
concerned with RGC protection and optic nerve regeneration. The
present study found that the majority of RGCs were labeled in the 5
sec PONC group (Fig. 2a) and that
the RGC densities in 1/6, 3/6 and 5/6 retinal radius regions
compared with the sham-operated control group were not
significantly different, however, the number of labeled RGCs
significantly decreased and almost no cells were labeled in the 5/6
retinal radius area in the 30 sec PONC group. In the 10 sec PONC
group, the number of labeled RGCs was less than that in the control
group, and the process led to a significant decrease in RGCs in
1/6, 3/6 and 5/6 retinal radius regions compared to these numbers
in the sham-operated control group, which was similar to the
findings of a previous study (6).
Compared with the mild and severe optic nerve crush injury model,
the moderate crush injury model (10 sec PONC group) met the demands
of the PONC model and was more suitable for the study of optic
nerve damage and regeneration.
Acknowledgements
This study was funded by the National
Natural Science Foundation of China (nos. 81070728 and 81000373),
Shanghai Natural Science Foundation (nos. 08ZR1413900 and
11ZR1422000), Shanghai Municipal Education Committee Project (no.
10YZ38), Shanghai Leading Academic Discipline Project (no. S30205)
and Shanghai ‘Science and Technology Innovation Action Plan’ Basic
Research Key Project (nos. 11JC1407700 and 11JC1407701).
References
|
1.
|
Forrester J and Peters A: Nerve fibers in
the optic nerve of rat. Nature. 214:245–247. 1967. View Article : Google Scholar
|
|
2.
|
Yu S, Tanabe T and Yoshimura N: A rat
model of glaucoma induced by episcleral vein ligation. Exp Eye Res.
83:758–770. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kretz A, Happold CJ, Marticke JK and
Isenmann S: Erythro-poietin promotes regeneration of adult CNS
neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell
Neurosci. 29:569–579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Thaler S, Fiedorowicz M, Rejdak R, et al:
Neuroprotective effects of tempol on retinal ganglion cells in a
partial optic nerve crush rat model with and without iron load. Exp
Eye Res. 90:254–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lingor P, Tönges L, Pieper N, et al: ROCK
inhibition and CNTF interact on intrinsic signalling pathways and
differentially regulate survival and regeneration in retinal
ganglion cells. Brain. 131:250–263. 2008.PubMed/NCBI
|
|
6.
|
Lagrèze WA, Knörle R, Bach M and
Feuerstein TJ: Memantine is neuroprotective in a rat model of
pressure-induced retinal ischemia. Invest Ophthalmol Vis Sci.
39:1063–1066. 1998.PubMed/NCBI
|