Introduction
It has been reported that reactive oxygen species
(ROS) are involved in several vascular diseases, including
endothelial dysfunction and atherosclerosis (1). Uncoupling protein 2 (UCP2) belongs to
the mitochondrial anion carrier family and is expressed in several
types of cells, including vascular smooth muscle cells (SMCs)
(2). UCP2 is known to be involved
in the uncoupling of the proton electrochemical gradient, resulting
in the reduced production of ROS. A previous study demonstrated
that LDLR−/− mice with UCP2-ablated macrophages had
significantly larger aortic atherosclerotic lesions than the
controls despite lower cholesterol levels, indicating a protective
role for UCP2 in atherosclerosis (3). Lombard reported that UCP2 knockout
enhanced the high salt diet-induced increase in superoxide
production and decrease in nitric oxide (NO) bioavailability,
eliciting a consequent elevation of blood pressure and markedly
enhancing the impairment of vasodilation, suggesting that UCP2
plays an important role in the development of salt-related
hypertension and vascular dysfunction (4). These studies strongly support the
theory that UCP2 is closely related to the development of vascular
diseases.
Although the UCP2 is highly conserved between mouse
and human, there are species differences over the protein sequence.
Other researchers have created transgenic mice that overexpressed
UCP2 in hepatocytes (5) and
hypocretin neurons (6) for the
study of obesity. However, the effect of the overexpression of
human UCP2 (hUCP2) specifically in vascular SMCs has not been
addressed. In the present study, to facilitate the functional study
of hUCP2, we report the establishment of transgenic mice carrying
the hUCP2 gene in vascular SMCs. This model provides a tool that
may be used to define the role of SMC-derived hUCP2 in the
development of vascular disease.
Materials and methods
Animal care
Handling of animals and all experimental procedures
were approved by the Institutional Animal Care and Use Committee of
the General Hospital of PLA Chengdu Military Area Command.
Transgenic founder mice were generated on an FVB background. The
cDNA from hUCP2 under the control of the rabbit smooth muscle
myosin heavy chain promoter was cloned into the pRP.Des2d vector
(Cyagen Biosciences Inc., Guangzhou, China) and verified by
sequencing. The linearized pRP.Ex2d-SMHC>UCP2 was purified from
agarose gel using a QIAquick Gel Extraction kit (Qiagen,
Chatsworth, CA, USA), adjusted to a final concentration of 1 mg/ml
in Tris-EDTA (TE) buffer and used as a DNA solution for
microinjection. The female FVB mice were hormonally superovulated
and mated with male FVB mice. Next morning the fertilized one-cell
eggs were collected from the oviducts. The eggs were microinjected
with the DNA solution under a microscope. The injected fertilized
eggs were transplanted into the oviducts of pseudo-pregnant FVB
mice. Transgenic mice were identified by PCR with the forward and
reverse primers 5′-GGAGATACCAAAGCACCGTCAA and
5′-CATAGGTCACCAGCTCAGCACA, respectively. The internal control was
identified by PCR with the forward and reverse primers 5′-TCT TAG
CTC TGC TCT CCG GT and 5′-CAC TGG CTG AGG AAG GAG AC,
respectively.
Gene expression analysis
The RNA from the aorta was prepared using the RNeasy
mini kit (Qiagen) and reverse transcription and PCR were performed
using a QuantiTect SYBR Green one-step RT-PCR kit (Qiagen)
according to the manufacturer’s specifications. A standard curve
was prepared for each assay by the serial dilution of cDNA
synthesized from pooled RNA. The samples and standards were
analyzed in triplicate (7).
Dihydroethidium assay
To assess the levels of superoxide production, the
fresh frozen aortae were cut into 20-μm thick sections. Following
incubation in Krebs solution for 30 min, the samples were incubated
in the dark with dihydroethidium (DHE; Sigma-Aldrich, St. Louis,
MO, USA) diluted in Krebs solution (40 μmol/l) for 30 min at 37°C
followed by a 15-min wash in DHE-free Krebs. To quantitate the DHE
fluorescence, the glass slides were placed under a Leica DM LB2
Fluorescent Microscope (Leica, Wetzlar, Germany) fitted with a
rhodamine filter set (8).
NO bioavailability assay
NO levels in the freshly isolated aortae were
assessed using 4,5-diaminofluorescein diacetate (DAF-2 DA;
Sigma-Aldrich, St Louis, MO), as described previously. Aortae were
prepared in the manner described for the DHE assay and vessel
sections were loaded with 5 μmol/l DAF-2 DA in the dark for 45 min.
When the dye loading was complete, the vessels were rinsed three
times with fresh Krebs solution. To quantitate the DAF-2 DA
fluorescence, the glass slides were placed under the Leica DM LB2
fluorescent microscope fitted with a fluorescein isothiocyanate
filter set (8).
Statistical analysis
Data are the mean ± SEM. The statistical differences
in mean values were assessed by the Student’s t-test. Two-sided
p-values <0.05 were considered to indicate a statistically
significant result.
Results
Establishment of hUCP2 transgenic
mice
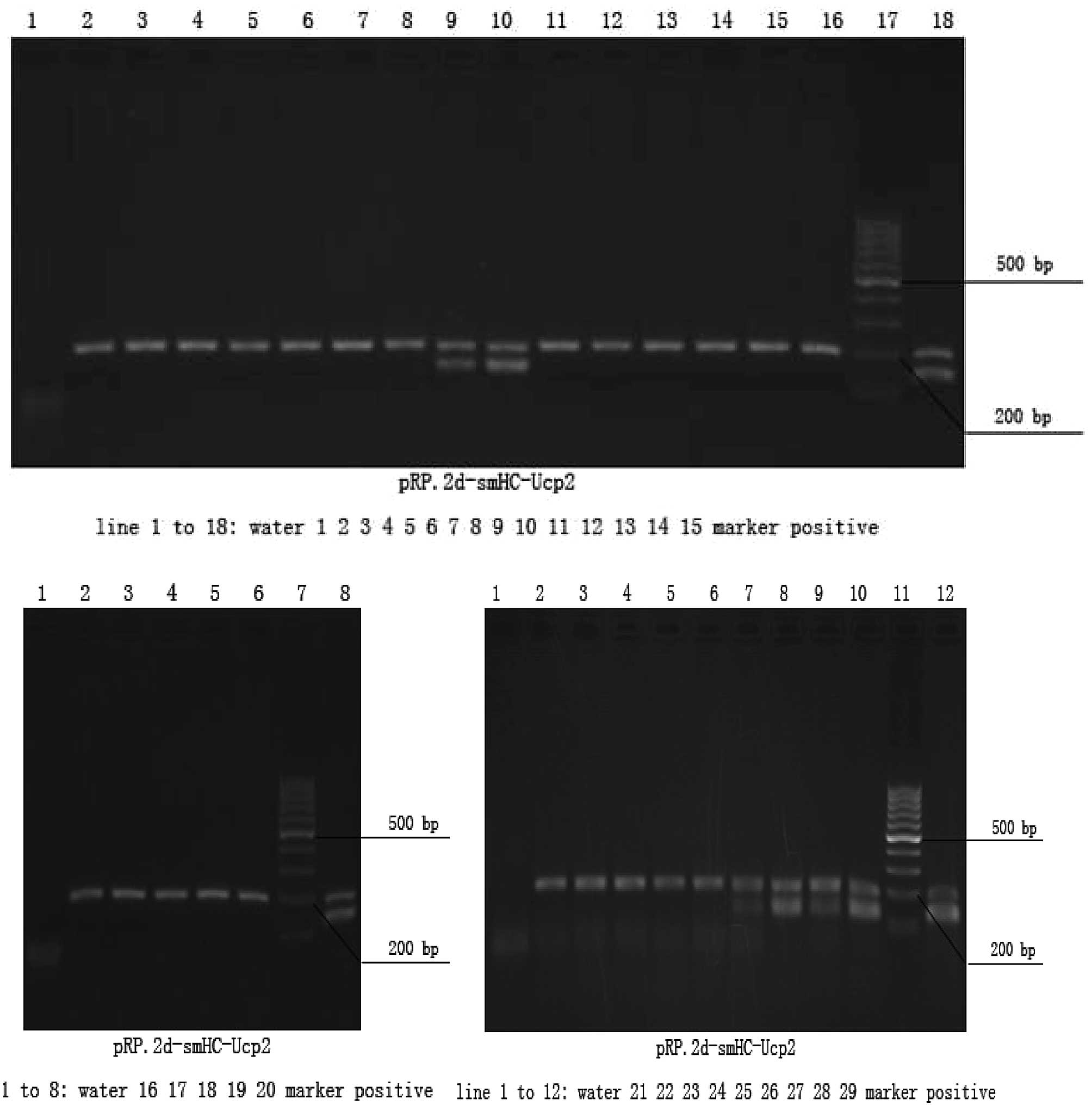
The transgenic fragments containing the full length
rabbit smooth muscle myosin heavy chain promoter and hUCP2 cDNA
were microinjected into the male pronuclei of 220 fertilized
oocytes of FVB mice. A total of 160 injected eggs were implanted
into the oviducts of 6 pseudo-pregnant foster mothers, which gave
birth to 29 offspring. Six offspring mice were identified to be
carrying the hUCP2 cDNA by PCR analysis (Fig. 1).
UCP2 expression in aorta
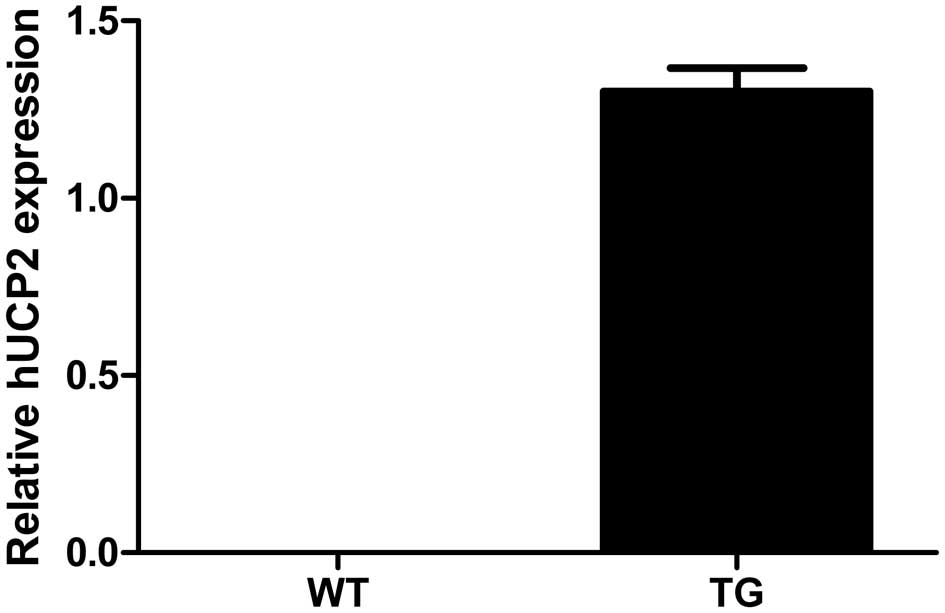
Real-time PCR analysis confirmed that the transgenic
construct was abundantly expressed in the aortae of the transgenic
mice (TG) and that the UCP2 transcript was undetectable in the
wild-type (WT) animals (Fig.
2).
Superoxide level
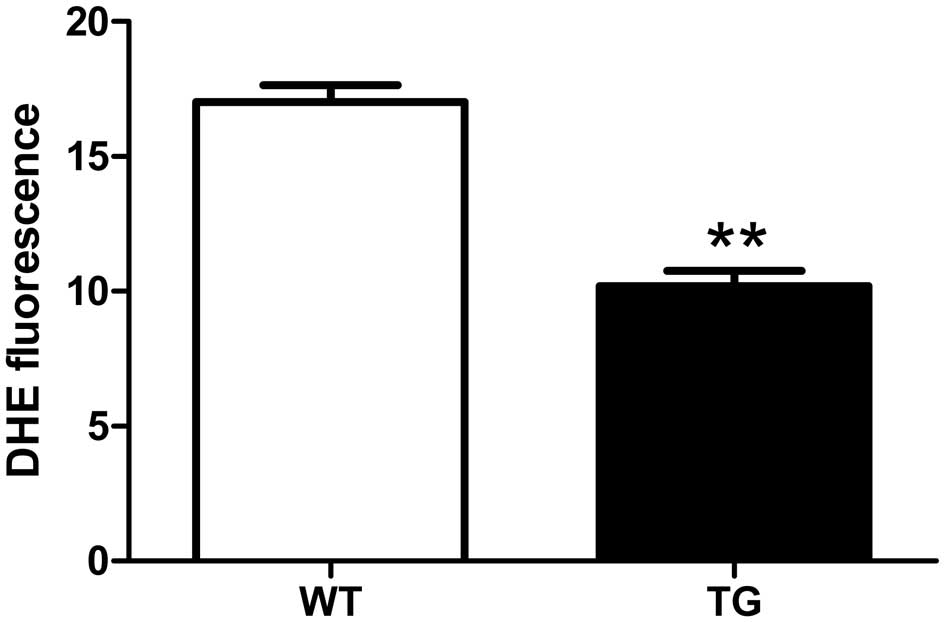
Superoxide anion production in the aorta was
assessed by DHE staining. We found that the DHE fluorescence was
significantly lower in the aortae from the UCP2 TG mice than in
those from their WT littermates (p<0.01; Fig. 3).
NO availability
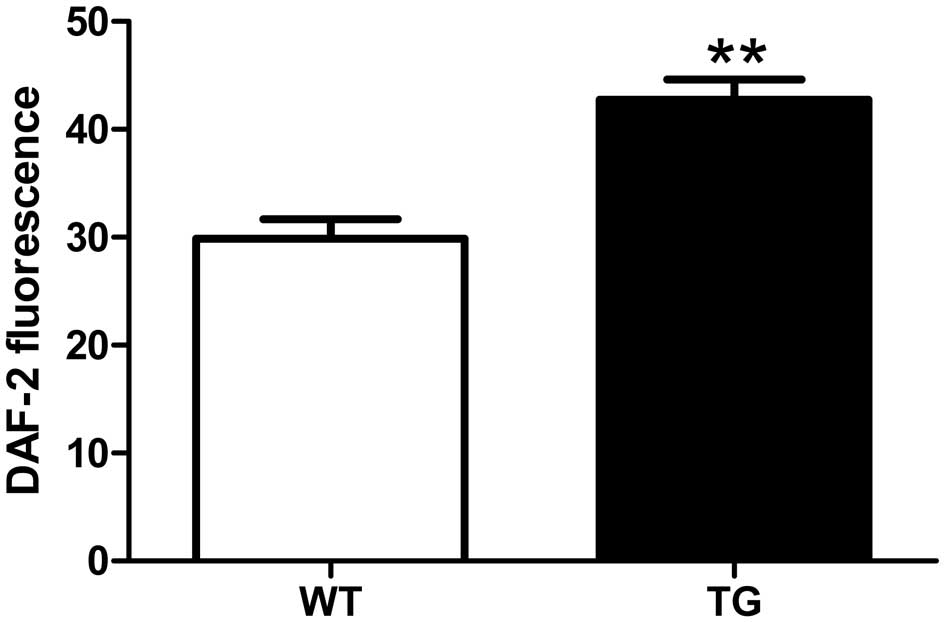
The production of NO in the aorta was assessed by
DAF-2 DA staining. We found that DAF-2 DA fluorescence was
significantly higher in the aortae from the UCP2 TG mice than in
those from their WT littermates (p<0.01; Fig. 4).
Discussion
In the present study, we report the establishment
and initial characterization of a mouse model with the transgenic
expression of human UCP2 in vascular SMCs under the control of the
smooth muscle myosin heavy chain promoter. In the hUCP2 transgenic
mouse, the hUCP2 mRNA was abundantly present in the aorta.
The aortae from the hUCP2 transgenic mice have
significantly lower levels of superoxide and markedly higher levels
of NO than the aortae from the WT mice. These characteristics may
provide certain beneficial effects on the development of vascular
dysfunction and remodeling.
In summary, we have established a transgenic mouse
model with vascular SMC-specific overexpression of hUCP2. This
model provides a novel tool for studies of the vascular effects of
SMC-derived hUCP2.
Acknowledgements
We acknowledge Cyagen Biosciences for
technical assistance and Dr Song Hu of Chengdu Medical College for
animal care. This study was supported by the Natural Science
Foundation of China (No. 81100232, to Shuangtao Ma).
References
|
1.
|
Chisolm GM and Steinberg D: The oxidative
modification hypothesis of atherogenesis: an overview. Free Radic
Biol Med. 28:1815–1826. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rousset S, Alves-Guerra MC, Mozo J, Miroux
B, Cassard-Doulcier AM, Bouillaud F and Ricquier D: The biology of
mitochondrial uncoupling proteins. Diabetes. 53(Suppl 1):
S130–S135. 2004. View Article : Google Scholar
|
|
3.
|
Blanc J, Alves-Guerra MC, Esposito B,
Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B and Mallat Z:
Protective role of uncoupling protein 2 in atherosclerosis.
Circulation. 107:388–390. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lombard JH: Uncoupling protein 2 (UCP2):
Another player in the complex drama of vascular salt sensitivity.
Am J Hypertens. 23:8162010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tsuboyama-Kasaoka N, Sano K, Shozawa C,
Osaka T and Ezaki O: Studies of UCP2 transgenic and knockout mice
reveal that liver UCP2 is not essential for the antiobesity effects
of fish oil. Am J Physiol Endocrinol Metab. 294:E600–E606. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Conti B, Sanchez-Alavez M, Winsky-Sommerer
R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S,
Henriksen S, Zorrilla EP, et al: Transgenic mice with a reduced
core body temperature have an increased life span. Science.
314:825–828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yang D, Ma S, Tan Y, Li D, Tang B, Zhang
X, Sun M and Yang Y: Increased expression of calpain and elevated
activity of calcineurin in the myocardium of patients with
congestive heart failure. Int J Mol Med. 26:159–164. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zhu J, Huang T and Lombard JH: Effect of
high-salt diet on vascular relaxation and oxidative stress in
mesenteric resistance arteries. J Vasc Res. 44:382–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|