Introduction
Submucosal injection assisted endoscopic mucosal
resection (EMR) and endoscopic submucosal dissection (ESD) have
been widely used in the removal of benign and early malignant
lesions of the gastrointestinal tract (1). EMR, ESD and peroral endoscopic
myotomy (POEM) (2) have inspired
endoscopists and endoscopic surgeons to identify an optimal
submucosal injection solution, which is not only safe and
cost-effective but has a unique lifting ability with endoscopic
submucosal cushion and aids the early healing of artificial ulcers
(3,4).
In clinical practice, a number of cushioning agents
are used for endoscopic submucosal injection (5–7). At
present, normal saline is the most popular agent used during EMR,
ESD and POEM. However, since normal saline absorbs too quickly
(8,9), repeated injections during surgical
procedures may be necessary to maintain effective lifting. One of
the problems is that more injections may result in a greater cost
of anesthesia and nursing. The most significant issue is that there
is no report regarding an understanding of injection-caused
submocosal tissue damage, which may play a key role in the early
healing of artificial ulcers.
Hyaluronic acid, glycerol, hydroxypropyl
methylcellulose and sodium alginate appear to have the most durable
cushioning effect (5,7,10–12).
These agents with durable cushioning effects are not only quite
expensive but are also difficult to inject (13). Additionally, a concern with
hyaluronic acid is the stimulation of tumor cells (14,15),
and hydroxypropyl methylcellulose has the ability to cause a local
inflammatory reaction and could potentially give rise to antigenic
reactions at high doses (11,16).
Previous human and animal studies (13,17,18)
demonstrated that autologous whole blood produced the most durable
cushion compared with standard agents. The autologous blood was
noted to have the advantages of being readily available and
cost-free. However, there were no systemic studies on tissue damage
following ESD or EMR using whole blood and plasma, since the plasma
may have different advantages for submucosal injection.
Notably, although the risk factors for bleeding and
artificial ulcer healing following ESD using normal saline as a
cushioning agent in patients with gastrointesintal neoplasm have
been studied in previous studies (19), tissue damage during therapy has not
been analyzed as an independent factor. In the present study we
assumed that submucosal injection with normal saline induced tissue
damage, and may affect the artificial ulcer healing following ESD
and EMR. Therefore, the authors designed the animal and human
studies to investigate the endoscopic and microscopic
characteristics of a bood solution as a cushioning agent and
provide a more extensive understanding of submucosal
injection-caused tissue damage. In order to demonstrate the
advantages of autologous whole blood or plasma solution as a
submucosal injection agent, the most comonly used normal saline was
used as the control for comparison.
Materials and methods
Animals, patients and materials
Four minipigs (Wuzhishan minipig, mean weight 15 kg)
were obtained from the Jiangsu Academy of Agricultural Science
(Nanjing, China). The animal preparation prior to endoscopy and the
management of the animals after the endoscopic procedure were
carried out by trained veterinarians. General anesthesia with
endotracheal intubation was administered. The endoscopy was carried
out with standard endoscopes (GIF-Q240 and CF-260, Olympus, Tokyo,
Japan) in the animal laboratory of the Institute of Digestive
Endoscopy at the Second Affiliated Hospital (Nanjing Medical
University, Nanjing, China) and the animal study was approved by
the institutional ethics board.
A total of 38 patients (age 54.37±13.27 years; 25
male, 13 female; 35 with polyps, 2 with early cancer and 1 with
cystic gastritis; diameter of neoplasms 12.24±7.1 mm; 4 EMRs in
esophagus, 9 in stomach, 1 in duodenum and 24 in large intestine)
consented to the EMR procedure of the colon for polyps (between
June 2011 and October 2011; Medical Center for Digestive Diseases,
Second Affiliated Hospital, Nanjing Medical University, Nanjing,
China). Of 38 patients, 7 accepted autologous whole blood
submucosal injection for EMR. Of 38 patients, 31 acted as a sham
control group and accepted normal saline injection. The human study
was approved by the ethics committee of the Second Affiliated
Hospital, Nanjing Medical University, Nanjing, China.
Injection solutions and endoscopic
procedures
The normal saline soution for submucosal injection
in the animal and human studies was mixed with 1% epinephrine and
0.5% methylene blue. Whole blood was extracted using a vacuum tube,
which had 3.2% sodium citrate for anticoagulation (Improve Medical
Instruments Company, Guangzhou, China). Using a 23-gauge
sclerotherapy needle (Boston Scientific Microvasive, Natick, MA,
USA), at least 3 ml of whole blood plus 0.5% methylene blue was
injected into the mucosa immediately after it was taken out from
the vein. With regard to the experiments in animals, after
centrifugation of whole blood at 3,000 rpm for 2 min, the plasma
was suctioned from the vacuum tube and mixed with equal volumes of
normal saline. A total of 50% pig plasma solution (volume ratio of
plasma:normal saline is 1:1) plus 1% methylene blue was prepared
for submucosal injection. The volume of plasma solution or normal
saline solution for each group was 3 ml in pigs.
The pig colons were used as the organ for evaluation
of the lifting effects of the plasma solution and normal saline.
The injections in pig colons were administered 4 cm apart, starting
above the transverse colon and moving proximally towards the
sigmoid colon. Each site of blood injection was closely followed
with a normal saline injection for comparison on closer
observation. If the mucosa did not elevate after 0.5 ml of the
injection, the needle was repeatedly reinserted at different angles
until a visible elevation of mucosa was created (13). The time for the mucosal cushion to
disappear was recorded. Subsequent injections at the next separate
site were performed after the disappearance of the cushion.
The pig stomachs were chosen as the targeting organ
for ESD. The mucosal dissections in pig stomachs were performed
every 3 cm, starting from the gastric antrum and moving proximally
towards the gastric fundus. The ESD procedures were performed for
each mucosal dissection (20 mm diameter) and the procedure time
from the inception of the submucosal injection to the end of
managing the wound surface was recorded. In total, 2 ESDs by the
injection of plasma solution and 2 ESDs by the injection of normal
saline were performed in each pig stomach. The ESD techniques were
performed using a conventional method. In brief, a flex-knife
(KD-630L; Olympus, Japan) was used as the main knife for mucosal
cutting (dry cut mode, 40 W). All ESD procedures were performed by
a single endoscopist. The specimens for haematoxylin and eosin
(H&E) staining were placed in a formalin solution immediately
after ESD. Endoscopy for follow-up was performed one month
later.
Histological scoring evaluation for
tissue damage
A sufficient histological scoring system for
evaluating tissue damage following submucosal injection does not
exist. In order to evaluate the characteristics of the tissue
damage of samples by EMR and ESD, the authors established the
histological scoring method for the evaluation of tissue damage
following submucosal injection (Table
I).
 | Table IHistological score (H-score) for
gastrointestinal tissue damage evaluation. |
Table I
Histological score (H-score) for
gastrointestinal tissue damage evaluation.
| H-score for tissue
damage evaluation
|
|---|
| Parameters | Score | Description |
|---|
| Degree of
hydrops | 1 | Without or only with
hydrops in observed SM |
| 2 | Marked hydrops in SM
and LMM |
| 3 | Marked hydrops in SM,
LMM and ML |
| Degree of tears | 1 | Tears exist in
<1/3 area of observed SM |
| 2 | Tears exist in
1/3–2/3 area of observed SM |
| 3 | Tears exist in
>2/3 area of observed SM |
Statistical analysis
The data were analyzed with statistical software
(SPSS v.11.5; SPSS Corp., Chicago, USA). The Mann-Whitney U test
was used for nonparametric data of histological scores. The
Student’s t-test was used for analysis of the lifting time and
procedure time. P<0.05 was considered to indicate a
statistically significant result.
Results
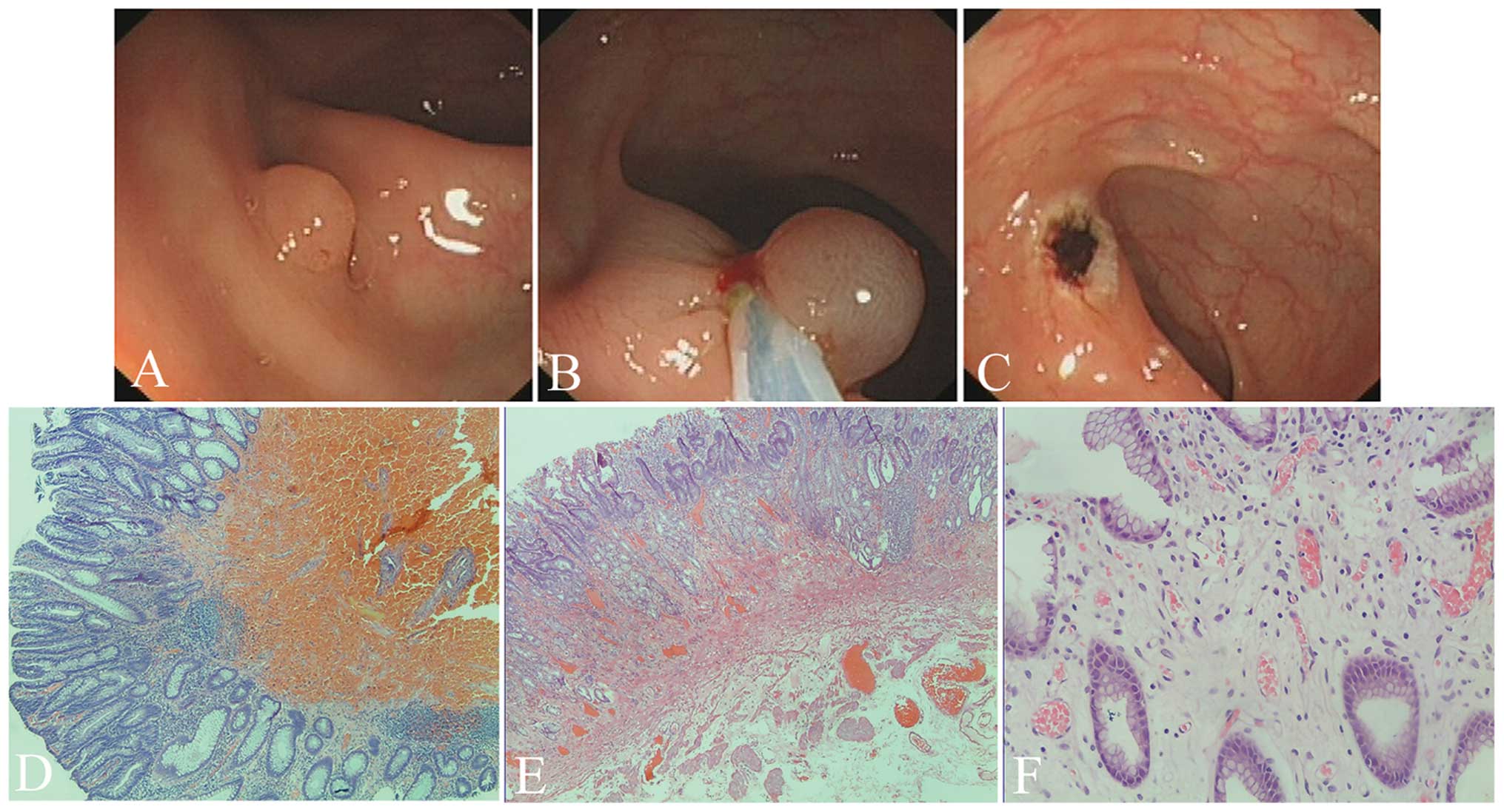
Surgical procedure
Submucosal injection in animal colons, ESDs in
animal and EMRs in patients were performed easily and no
complications were observed. No blood clotting was observed prior
to or during injection, but the sclerotherapy needle was partially
obstructed 5 min later during whole blood injection. Authors
presumed that it was the plasma, not the blood cells, that played
the key role in producing effective submucosal seperation and
reducing tissue hydrops. The animal experiments were designed to
demonstrate the endoscopic and microscopic characteristics of
plasma as a lifting agent (Fig.
1).
Lifting time
The injections with the plasma solution in the
submucosa of each pig colon (n=4) were paired with normal saline
injections (n=4). The lifting time of the plasma solution
(18.25±5.44 min) in pig colons was significantly longer than that
in the normal saline group (6.5±2.38 min; t=3.96, P<0.007).
However, there was no difference in ESD surgery time between the
groups injected with plasma and normal saline in the pig stomachs
(7.0±2.12 vs. 5.25±0.96 min, P>0.05; Table II, figures are not shown).
 | Table IITissue damage evaluation for tissues
dissected by ESD in the pig stomachs. |
Table II
Tissue damage evaluation for tissues
dissected by ESD in the pig stomachs.
| | H-score of hydrops
| H-score of tears
|
|---|
| Group | n | 1 | 2 | 3 | P-valuea | 1 | 2 | 3 | P-valuea |
|---|
| Normal saline | 4 | 0 | 0 | 4 | 0.011 | 4 | 0 | 0 | 0.008 |
| Plasma solution | 4 | 3 | 1 | 0 | | 0 | 0 | 4 | |
Animal hydrops and tearing
The hydrops in the normal saline group were more
extensive than those in the plasma solution-injected group
(P=0.011). Additonally, the tearing in the normal saline group was
less than that in the plasma-injected group (P=0.008; Table II and Fig. 1). The animals that underwent ESDs
survived and the endoscopy one month later showed the stomach with
four well-healed scars (figures not shown).
Patient hydrops and tearing
In order to further demonstrate the endoscopic
characteristics of autologous blood injection and the tissue damage
following submucosal injection in humans, 38 patients who underwent
EMR were studied with the comparison of autologous blood and normal
saline. As shown in Fig. 2,
Table I and Table III, the hydrops in the group with
the normal saline injection were more extensive than those in the
group with whole blood (P<0.001). Additonally, the tearing in
the group injected with normal saline was less than that in the
group injected with blood (P<0.001).
 | Table IIITissue damage evaluation for tissues
dissected by EMR in humans. |
Table III
Tissue damage evaluation for tissues
dissected by EMR in humans.
| | H-score of hydrops
| H-score of tears
|
|---|
| Group | n | 1 | 2 | 3 | P-valuea | 1 | 2 | 3 | P-valuea |
|---|
| Normal saline | 31 | 0 | 3 | 28 | <0.001 | 29 | 2 | 0 | <0.001 |
| Blood solution | 7 | 7 | 0 | 0 | | 0 | 1 | 6 | |
Discussion
Since the first description in the 1950s of a
submucosal injection to assist polypectomy (20), several attempts have been made to
find the optimal agent. Such an optimal agent for submucosal
injection should be cost-effective, readily available, easy to
inject and provide a durable cushion with minimal damage of the
surrounding tissues at the site of injection. Previous studies have
shown that the use of hyaluronic acid, hydroxypropyl
methylcellulose or glycerol provides dextrose water, albumin and
sodium alginate, and have a lasting elevating effect (6,7,10,11,21,22).
A recent study reported that carbon dioxide (CO2) could
be a satisfactory submucosal injection agent during ESD (21). The drawbacks of these agents are
that they are expensive and difficult to prepare, store or inject
(1,3,6,7,10,11,21,23).
An additional concern discouraging the use of hyaluronic acid is
its potential to stimulate tumor growth, both in vivo and
in vitro (14,15), and the concern with regard to
hydroxypropyl methylcellulose is its potential to cause a local
inflammatory reaction and give rise to antigenic reactions at high
doses (11,16).
The osmolality of the cushioning agents is also
another significant determinant in the properties of the injected
solutions. Fujishiro et al (23) demonstrated that the use of
hypertonic solutions (3.75% NaCl and dextrose water at
concentrations of 20%) caused significant tissue damage that was
not limited to the superficial layers but also extended into the
muscularis propria. Bures et al (3) reported that viscous or hypertonic
solutions for submucosal injection, outperform normal saline and
perform as well as sodium hyaluronate in porcine stomachs in
vitro.
Previous human and animal studies (13,17,18)
demonstrated that autologous blood produces the most durable
cushion compared with standard agents. Autologous blood also has
the advantages of being readily available and without cost. The
endoscopic findings further confirmed the durability of whole blood
and further demonstrated the durability of plasma solution. The
H&E staining in the present study showed whole blood or plasma
solution injection within loose submucosa caused separation of the
mucosa and muscularis propria layer, but without tissue damage in
the mucosa above the hematoma. The current histological supporting
evidence from the in vivo model demonstrated that blood
solution as a cushioning agent causes less tissue damage than
normal saline.
The established in vivo model is one of the
strengths of the present study. The majority of previous studies
are based on ex vivo studies (3,5,17).
The frequently used model for evaluation of injection-induced
tissue damage is to inject a solution into the isolated stomach or
to resect the targeted stomach wall following injection, instead of
gathering tissue samples following the in vivo ESD
procedure. The disadvantage of an ex vivo study is that the
time for the submucosal fluid to resolve is prolonged due to the
absence of perfusion and active absorption (8). In order to establish a more useful
in vivo animal model and reduce the number of animals used,
due to the high perforation rate of ESD in the colon (19), the colon was used for the
evaluation of elevation duration following submucosal injection and
stomachs were used for ESD.
The major finding in the present study was that the
results demonstrated evidence of tissue damage following submucosal
injection with normal saline and blood solution. The fundamental
concern of tissue damage is the healing of artificial ulcers and
bleeding following mucosa removal (3,4). A
tissue damage scoring system was developed based on
injection-induced hydrops and tears for the evaluation of tissue
damage. The results showed that the blood solution produced a
marked blunt dissection and tears of the mucosa from the muscularis
propria layer as compared to normal saline. The fundamental
principle of submucosal injection for EMR, ESD, POEM and submucosal
tunneling endoscopic resection (STER) (2,24,25)
is the elevation of targeted tissue or separation of deeper layers
in the gastrointestinal tract wall prior to resection, dissection
or seperation of targeted tissue. Whole blood and plasma solutions
induced tears and may be effective in avoiding possible
unpredictable devices assisting separation or sharp dissection
causing deeper tissue damage, vessel damage and perforation. This
type of effective submucosal tear was first reported by Sumiyama
et al (26) in 2007 with a
cap-fitted endoscope. This technique of blunt dissection/dilation
was highlighted in application of natural orifice translumenal
endoscopic surgery (NOTES) (27).
Due to the fact that normal saline is rapidly absorbed, the
injected saline diffuses into the surrounding area of the injection
sites and causes serious hydrops and hyperemia, which would delay
the early healing of the artificial ulcers and even increase the
risk of post-ESD or post-EMR bleeding. Our findings indicate that
an ideal submucosal injection solution or plasma-like gel should be
transparent, easily injected, nonabsorbing or less absorbable and
easily inducing blunt dissection.
During the experiments using the blood solution, we
noted that a 23-gauge sclerotherapy needle showed no difficulty in
the injection of blood solution, despite the higher injection
pressures required, compared with normal saline. Additionally,
since gathering blood and centrifugation for plasma are necessary
steps for preparing plasma solution, it would take 3 to 5 min more
to prepare the injection solution in clinical practice, compared
with using normal saline.
Unlike the procedures for varied human lesions, the
time of ESD operation in pigs for both groups was short due to the
fact that the stomach is normal, instead of in early cancer stages.
There was no significant difference between the two groups of ESDs
in pig stomachs, but we suggest that the potential difference
should be significant in human, since shorter injecting times
during ESD would benefit from the much longer lifting time when
using plasma solution, instead of normal saline.
With regard to the limitations of this study, a
number of hypertonic solutions should be included as control
groups, although previous research revealed that the blood provides
the longest duration of the submucosal cushion compared with
hydroxypropyl methylcellulose, albumin and normal saline (13).
In conclusion, this is the first study using
autologous plasma solution as a gastrointestinal submucosal
injection agent. The in vivo animal and human study
demonstrated that whole blood or plasma solution may outperform
normal saline due to its unique lifting ability, less tissue damage
and marked effective submucosal blunt dissection. The study
highlighted that normal saline used as a submucosal injection
caused tissue damage, which may affect the early healing of
artificial ulcers and be associated with bleeding following mucosal
therapies. Our comparative studies are ongoing in order to confirm
the clinical benefits of using autologous blood solution for
patients with submucosal injection procedures.
Acknowledgements
The study was partially supported by
grants from the National Natural Science Foundation of China (No.
30900667), the Natural Science Foundation of Jiangsu Province
(BK200910511) and Innovation Team Programe of Jiangsu Health
Department (Zhining Fan). We thank Dr Vandhana Kiswani and Dr
Venkata Sandeep Akshintala at Johns Hopkins Hospital
Gastroenterology division for their insightful suggestions and
editorial assistance.
References
|
1.
|
Rösch T, Sarbia M, Schumacher B, et al:
Attempted endoscopic en bloc resection of mucosal and submucosal
tumors using insulated-tip knives: a pilot series. Endoscopy.
36:788–801. 2004.PubMed/NCBI
|
|
2.
|
Inoue H, Minami H, Kobayashi Y, et al:
Peroral endoscopic myotomy (POEM) for esophageal achalasia.
Endoscopy. 42:265–271. 2010. View Article : Google Scholar
|
|
3.
|
Bures J, Kopácová M, Kvetina J, et al:
Different solutions used for submucosal injection influenced early
healing of gastric endoscopic mucosal resection in a preclinical
study in experimental pigs. Surg Endosc. 23:2094–2101. 2009.
View Article : Google Scholar
|
|
4.
|
Goto O, Fujishiro M, Kodashima S, et al:
Short-term healing process of artificial ulcers after gastric
endoscopic submucosal dissection. Gut Liver. 5:293–297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Fujishiro M, Yahagi N, Kashimura K, et al:
Comparison of various submucosal injection solutions for
maintaining mucosal elevation during endoscopic mucosal resection.
Endoscopy. 36:579–583. 2004. View Article : Google Scholar
|
|
6.
|
Eun SH, Cho JY, Jung IS, et al:
Effectiveness of sodium alginate as a submucosal injection material
for endoscopic mucosal resection in animal. Gut Liver. 1:27–32.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Uraoka T, Fujii T, Saito Y, et al:
Effectiveness of glycerol as a submucosal injection for EMR.
Gastrointest Endosc. 61:736–740. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Polymeros D, Kotsalidis G, Triantafyllou
K, Karamanolis G, Panagiotides JG and Ladas SD: Comparative
performance of novel solutions for submucosal injection in porcine
stomachs: An ex vivo study. Dig Liver Dis. 42:226–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hirao M, Masuda K, Asanuma T, et al:
Endoscopic resection of early gastric cancer and other tumors with
local injection of hypertonic saline-epinephrine. Gastrointest
Endosc. 34:264–269. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Yamamoto H, Yube T, Isoda N, et al: A
novel method of endoscopic mucosal resection using sodium
hyaluronate. Gastrointest Endosc. 50:251–256. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Feitoza AB, Gostout CJ, Burgart LJ,
Burkert A, Herman LJ and Rajan E: Hydroxypropyl methylcellulose: A
better submucosal fluid cushion for endoscopic mucosal resection.
Gastrointest Endosc. 57:41–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Akagi T, Yasuda K, Tajima M, et al: Sodium
alginate as an ideal submucosal injection material for endoscopic
submucosal resection: preliminary experimental and clinical study.
Gastrointest Endosc. 74:1026–1032. 2011. View Article : Google Scholar
|
|
13.
|
Giday SA, Magno P, Buscaglia JM, et al: Is
blood the ideal submucosal cushioning agent? A comparative study in
a porcine model. Endoscopy. 38:1230–1234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Matsui Y, Inomata M, Izumi K, Sonoda K,
Shiraishi N and Kitano S: Hyaluronic acid stimulates tumor-cell
proliferation at wound sites. Gastrointest Endosc. 60:539–543.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tan B, Wang JH, Wu QD, Kirwan WO and
Redmond HP: Sodium hyaluronate enhances colorectal tumour cell
metastatic potential in vitro and in vivo. Br J Surg. 88:246–250.
2001. View Article : Google Scholar
|
|
16.
|
Obara S, Muto H, Shigeno H, et al: A
three-month repeated oral administration study of a low viscosity
grade of hydroxypropyl methylcellulose in rats. J Toxicol Sci.
24:33–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Shastri YM, Kriener S, Caspary WF and
Schneider A: Autologous blood as a submucosal fluid cushion for
endoscopic mucosal therapies: results of an ex vivo study. Scand J
Gastroenterol. 42:1369–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sato T: A novel method of endoscopic
mucosal resection assisted by submucosal injection of autologous
blood (blood patch EMR). Dis Colon Rectum. 49:1636–1641. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tajika M, Niwa Y, Bhatia V, et al:
Comparison of endoscopic submucosal dissection and endoscopic
mucosal resection for large colorectal tumors. Eur J Gastroenterol
Hepatol. 23:1042–1049. 2011.PubMed/NCBI
|
|
20.
|
Rosenberg N: Submucosal saline wheal as
safety factor in fulguration or rectal and sigmoidal polypi. AMA
Arch Surg. 70:120–122. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Uraoka T, Kawahara Y, Ohara N, Kato J,
Hori K, Okada H and Yamamoto K: Carbon dioxide submucosal injection
cushion: an innovative technique in endoscopic submucosal
dissection. Dig Endosc. 23:5–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Arantes V, Albuquerque W, Benfica E, et
al: Submucosal injection of 0.4% hydroxypropyl methylcellulose
facilitates endoscopic mucosal resection of early gastrointestinal
tumors. J Clin Gastroenterol. 44:615–619. 2010.
|
|
23.
|
Fujishiro M, Yahagi N, Kashimura K, et al:
Tissue damage of different submucosal injection solutions for EMR.
Gastrointest Endosc. 62:933–942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shi Q, Zhong YS, Yao LQ, Zhou PH, Xu MD
and Wang P: Endoscopic submucosal dissection for treatment of
esophageal submucosal tumors originating from the muscularis
propria layer. Gastrointest Endosc. 74:1194–1200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Xu MD, Cai MY, Zhou PH, et al: Submucosal
tunneling endoscopic resection: a new technique for treating upper
GI submucosal tumors originating from the muscularis propria layer.
Gastrointest Endosc. 75:195–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Sumiyama K, Gostout CJ, Rajan E, Bakken
TA, Knipschield MA and Marler RJ: Submucosal endoscopy with mucosal
flap safety valve. Gastrointest Endosc. 65:688–694. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Khashab MA and Kalloo AN: NOTES: Current
status and new horizons. Gastroenterology. 142:704–710. 2012.
View Article : Google Scholar : PubMed/NCBI
|