Introduction
The anterior cruciate ligament (ACL) plays an
important role in the stability of the knee joint. If it fails to
heal after complete rupture, premature osteoarthritis and
disability can result (1). At
present, ACL reconstruction is considered as the standard treatment
following injury. Allogeneic bone-patellar tendon-bone (B-PT-B) for
ACL reconstruction is one of the most important ligaments (2). Furthermore, it is widely known that
the healing process of the grafted tendon involves four main
stages: necrosis, angiogenesis, cell repopulation and final
maturation, among which, angiogenesis is an essential step in the
process of tendon healing and graft remodeling in which
revascularization prompts delivery of inflammatory cells,
fibroblasts and growth factors to the wound site (3). A clinical case report suggests that
the tendon grafts do not recover to physiological levels even 18
months after surgery (4).
Therefore, we tried to develop a new strategy by
enhancing angiogenesis to accelerate the remodeling of tendon
graft. Vascular endothelial growth factor (VEGF) plays an essential
role in angiogenesis, regulating the activation, migration and
proliferation of endothelial cells in various pathological
conditions (5). VEGF is easily
decomposed in vivo, however, which makes it easy to lose its
biological effects. If there was a method to make VEGF release
slowly, consequently, it would be able to promote revascularization
and improve the quality of graft survival.
Sodium hyaluronate (SH), a derivative of hyaluronate
acid (HA), was discovered in bovine vitreous humour by Meyer and
Palmer in 1934 (6). It is
well-tolerated, safe and efficacious and has been commonly used as
a growth scaffold in surgery, wound healing and embryology
(7). In addition, administration
of purified high-molecular-weight SH into orthopedic joints can
restore the desirable rheological properties and alleviate some of
the symptoms of osteoarthritis. Previous studies have shown that SH
could be used as a carrier of drugs (8), delaying the drug release rate.
Based on these two points, we hypothesized that an
application of VEGF mixed with SH enhances angiogenesis of a graft
by prolonging the action time of VEGF in ACL reconstruction, and
the application does not affect the mechanical characteristics of
the ACL graft. The aim of this study was to test these hypotheses
by a rabbit ACL reconstruction model using the B-PT-B graft.
Materials and methods
Experimental design
VEGF 165, 1 and 2.3% SH were mixed to yield SH
formulations containing VEGF 165 in concentrations of 5–10 μg/ml.
Non-cumulative release into phosphate-buffered saline (PBS) was
first measured spectrophotometrically over 1–4 days to detect the
release kinetics of VEGF. Allogeneic B-PT-B was then soaked in the
VEGF/SH formulations and implanted in the rabbit model to
regenerate ACL. Briefly, 45 skeletally mature female New Zealand
rabbits (90 limbs) weighing 3.0–3.5 kg supplied by the Animal
Centre of the Third Military Medical University (Chongqing, China)
were then randomly divided into 5 groups (groups A–E). From groups
A–D, ACL was transected at each hind limb, and then replaced by
allogeneic B-PT-B. In group A (n=9), B-PT-B allografts soaked in
VEGF 165 and SH were transplanted into the knee joints. In group B
(n=9), B-PT-B allografts soaked in VEGF 165 were transplanted. In
group C (n=9), B-PT-B allografts soaked in SH were transplanted. In
group D (n=9), B-PT-B allografts soaked in PBS were transplanted.
In group E (n=9), no treatment was applied but an incision of the
capsule was performed. Six limbs were harvested from each group at
2, 4 and 8 weeks after surgery for biomechanical analysis, 3 of
which were then used for immunohistological evaluations for VEGF,
and the other 3 for CD31, which is a marker for vascular
endothelial cells. All studies involving animals were approved by
the Institute’s Animal Care and Use Committee.
Release kinetics of VEGF
VEGF (VEGF 165; Peprotech, Inc., Rocky Hill, NJ,
USA) at concentrations of 5 or 10 μg/ml, respectively, and 1 or
2.3% SH (Sigma, USA), respectively, were mixed. A total of 0.2 ml
of the gel was placed into 0.2 ml of receiver fluid of PBS. The
samples were incubated at 37°C. Release kinetics were measured as
non-cumulative release (8), with
measurements at 3, 6, 12, 18, 24, 48 h and 4 days. Because the
receiver fluid was not replaced, each container was measured only
once. For every single concentration and time point at least eight
samples were examined. VEGF 165 concentration in the supernatants
was assayed spectrophotometrically at 239 nm (Ultrospec 1000;
Pharmacia Biotech). To determine whether the system of SH/VEGF
represents a diffusion-controlled or a membrane-limited release
system, the initial release was examined carefully by preparing a
large amount of samples as described above and taking measurements
at 5 min intervals (each single sample was measured only once). The
release of VEGF was plotted as a function of the square root of
time.
Graft preservation
A total of 45 B-PT-B were harvested from donor New
Zealand white rabbits (from other experiments). Each graft was
separated into at least two bundles of ligament and each bone was
cut 4-mm wide and 10-mm long. All the grafts were enclosed in tubes
and placed in dry ice in a container for γ irradiation by cobalt-60
(Co-60) for 9 h yielded at a dose of 2.5 Mrad. The tendon grafts
were then stored at −80°C for >3 months for later use.
Preoperative preparation of B-PT-B
allografts
Thirty minutes before surgery, the graft for group A
was soaked in 1% SH formulations containing VEGF 165 at a
concentration of 5 μg/ml as described previously (9) with 10 ml PBS for 30 min; the graft
for group B was soaked in recombinant human VEGF (5 μg/ml) with 10
ml PBS for 30 min; the graft for group C was soaked in SH (100
μg/ml) with 10 ml PBS for 30 min. In group D, the graft was soaked
in 10 ml PBS for 30 min.
Surgical procedure
The experimental animal was anesthetized with
intravenous pentobarbital sodium (0.03 g/kg). Using a sterile
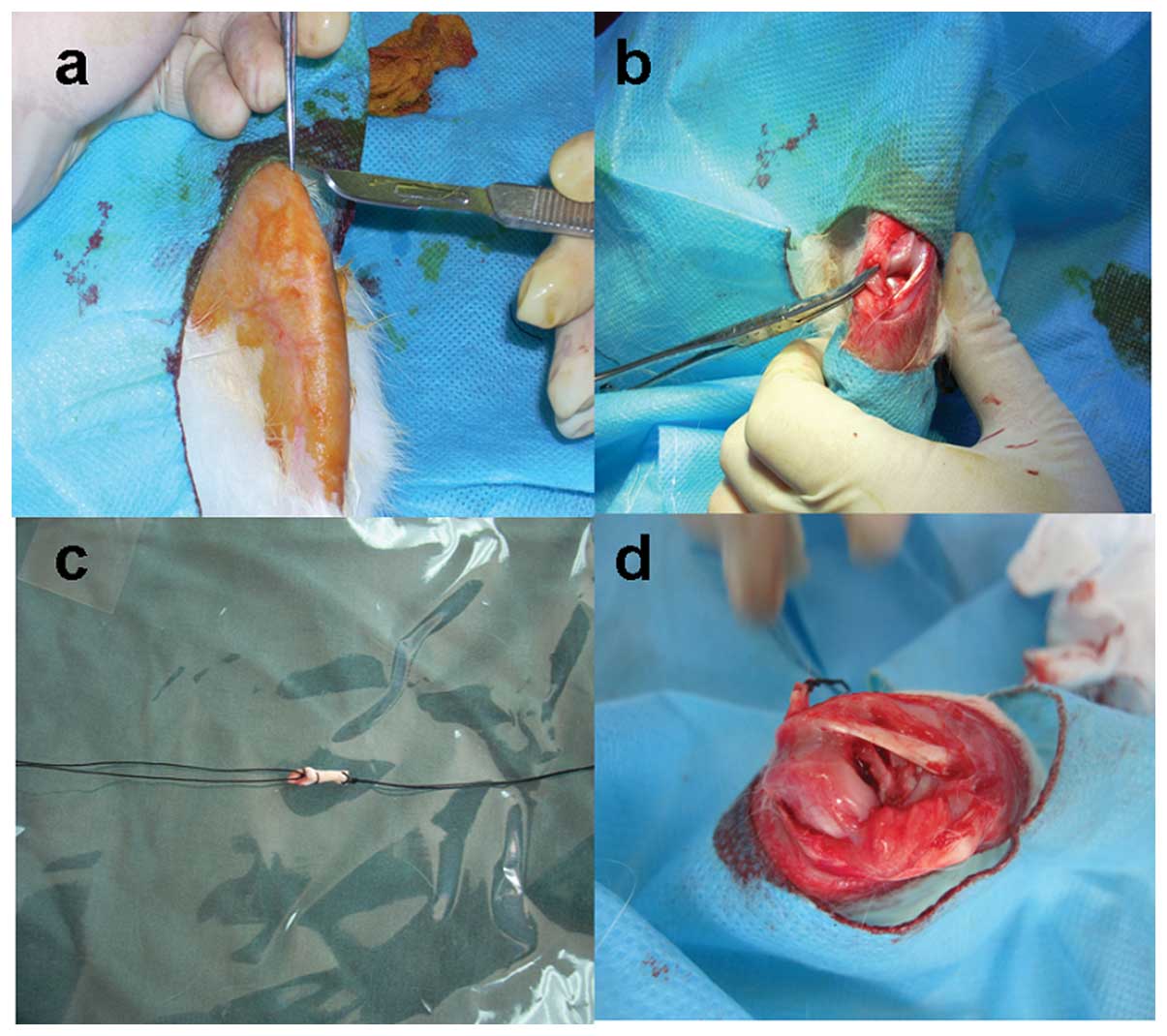
technique, the ACL was exposed through a medial parapatellar
incision (Fig. 1a) and transected
with a retrograde knife at its femoral attachment under visual
control (Fig. 1b). The bone
tunnels in the femur and tibia were made at the centers of the
insertion sites of the ACL. The tibial tunnel was reamed to 2 mm
over a guide pin. For the femoral tunnel, a 2-mm cannulated drill
bit was inserted over a guide pin from the inside of the knee
joint. The graft (Fig. 1c) was
then inserted via the holding suture from the tibial and femoral
tunnels at the same time slowly. Each end of the graft was sewed to
a screw inserted into the bone. Then the incision was routinely
closed in layers (Fig. 1d). After
surgery, all the animals were allowed to move freely in their
cages.
Biomechanical research
At 2, 4 and 8 weeks following surgery, 6 limbs were
harvested from each group. The hind limbs were removed at the hip
and cut 3 cm above and below the knee joint line. Next,
femur-graft-tibia specimens were prepared, removing all
periarticular and intra-articular soft tissues, respectively.
RGT-5KN microcomputer control electron omnipotent biomechanical
testing machine (Model RGT-5KN; Shenzhen Shenke Medical Instrument
Technical Development, Co., Ltd., China) was used for biomechanical
testing of the graft and the normal ligament at each time point.
The femur-graft-tibia complex was placed vertically between two
clamps of the tensiometer. When the tension reached 2.0 N/m, we
measured the length of the graft 3 times, taking its average as
ligament length. Before the tensile test, the specimen was
preconditioned with a static preload of 5.0 N for 10 min, followed
by 10 cycles of loading and unloading with a strain of 0.5% at the
crosshead speed of 50 mm/min. Subsequently, the complex underwent
tensile testing at the crosshead speed of 50 mm/min until the
complex failed. Applied maximum load was recognized as the maximum
force and stress withheld before wound rupture.
Histological and immunological
observation
The femur-graft-tibia specimens were embedded in
paraffin and cut into 5-μm thick sections longitudinal to the bony
tunnels. The slides were stained with hematoxylin and eosin
(H&E) and with CD31 mouse monoclonal antibody (Neomarkers,
Fremont, CA, USA) to assess the endothelial cells. Three
independent observers, who were blinded to the treatment groups,
enumerated the microvessels of the graft under a fluorescent
microscope, as described previously (10). In brief, five consecutive CD31
immunofluorescent sections were prepared for every sample. One
observer selected microvessel-dense areas in every section under
final magnification ×100, and randomly chose five fields under
final magnification ×200. The microvessels of each randomly chosen
field were counted by three independent observers, respectively.
The mean of the three independent counts by the observers was
considered the final counting value for each counting field. All
observers followed prescheduled rules as following. Under the
microscope, each luminal structure composed of endothelial cells,
each separate solitary brownish-yellow endothelial cell or each
cell mass within bone tissue was counted as a vessel. Vessels with
a thick muscle layer or those with a luminal diameter >8 red
blood cells were not counted. Areas of bleeding and fibrosis were
also disregarded.
Statistical analysis
All data were expressed as mean values ± standard
deviation (SD). The statistical significance of differences in
parameters was assessed by one-way analysis of variance (ANOVA)
using SPSS software (SPSS v12.0; IBM, New York, NY, USA). If
statistical differences between periods were found, Fisher’s PLSD
tests for post hoc multiple comparisons were used. For all data
collection, the investigators were blinded to the identity of the
groups. The significance limit was set at P=0.05.
Results
Release kinetics of VEGF
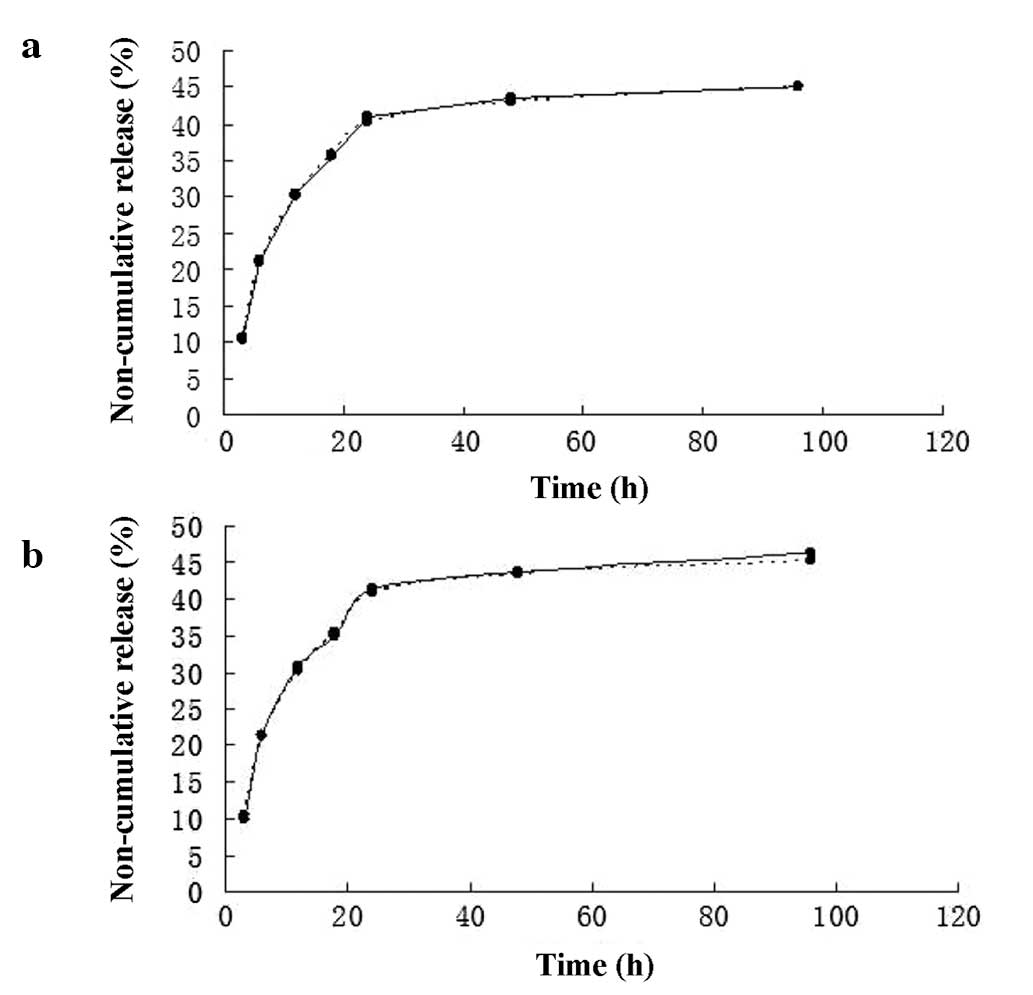
The release kinetics were well-controlled and
reproducible throughout the entire study. Steady state was
established after 48 h. The non-cumulative release rates in 1 and
2.3% SH were basically identical (Fig.
2a). A moderate initial burst effect was noted. No kinetic
differences were observed between the higher (10 μg/ml) and lower
(5 μg/ml) concentration of VEGF (Fig.
2b). The kinetics of the non-cumulative release studies did not
change when the volume of the receiver fluid was increased (data
not shown). Finally, it was observed that 1 and 2.3% SH became less
viscous and dissolved within 2 weeks.
Biomechanical evaluation
All grafts failed at the middle portion during the
ultimate failure testing and all normal ACL specimens had avulsion
fractures at the tibial insertion sites. The linear stiffness of
the FGT complex in group A was lower than that of the other groups
at 2 weeks, however, the data values were increased in group A when
compared with these values in the other groups at 4 and 8 weeks
(Fig. 3a). The stiffness values of
groups A–D were significantly lower than that of group E at every
time point (Fig. 3a). The average
ultimate failure of the group A was lower than the other groups at
2 weeks but it was significantly greater than that of the other
control groups at 4 and 8 weeks (Fig.
3b), although there were no significant differences in the
ultimate failure load between groups C and D (4 weeks, P=0.1103; 8
weeks, P=0.1302). The ultimate load values of these four groups
were significantly lower than that of the normal complex (Fig. 3b).
Histological and immunological
observation
Hematoxylin staining showed that 2 weeks after
surgery, in groups A and B, granulation was formed and
vascularization was noted. Group B obviously had a lower cell
quantity than group A. Conversely, in groups C and D most of the
host cells that invaded into the graft were inflammatory cells.
Four weeks postoperatively, endothelial cells gradually increased
from the surface into the deep portion. Furthermore, cell volumes
of the control groups were far less than groups A and B. Eight
weeks postoperatively, each group revealed that endothelial cells
had reached the depth of the graft.
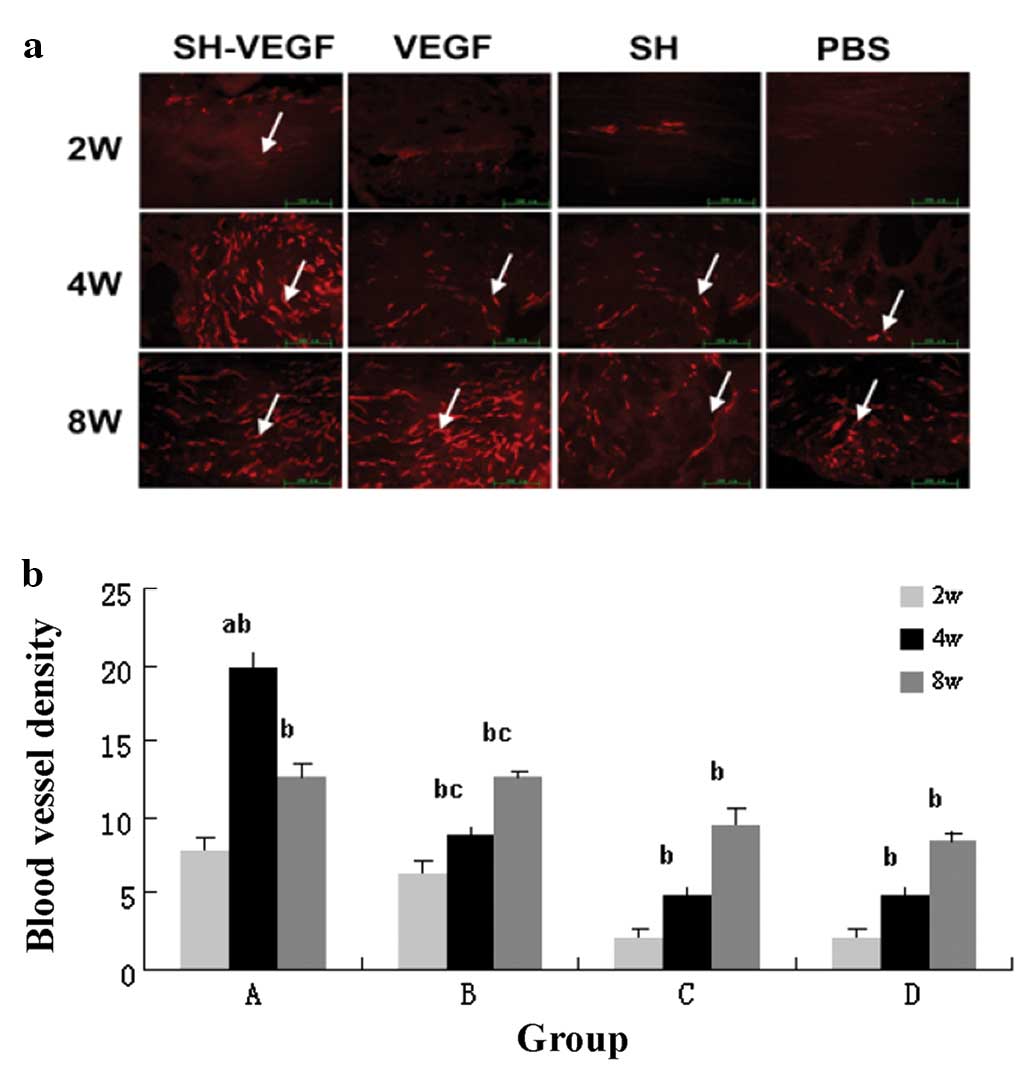
Fig. 4a shows the
immunofluorescent stained sections of the implanted site at 2, 4
and 8 weeks after implantation. At 2 weeks, the grafts in rabbits
from group A were filled by a few vessel lumina. No vascular
endothelial cells were observed in groups C and D, although in
group B there were few CD31-positive cells in the two ends of the
graft. In rabbits from groups C and D, a few CD31-positive cells
were found in the tibial and femoral ends. In group A,
CD31-positive cells were observed in the core of graft at 4 weeks;
however, vessel lumina were observed at the center of the graft in
groups C and D at 8 weeks while CD31-positive cells in the grafts
from group A were less than that at 4 weeks.
The data for microvessel density revealed that more
significant neovascularization occurred in group A than in the
other groups at every time point (Fig.
4b). Over time, the microvessel density increased gradually in
groups B–D with significant statistical significance. Also, the
microvessel density decreased in group A at 8 weeks, but there was
no significant statistical significance (Fig. 4b).
Discussion
In this study, we indicated that VEGF enhances
revascularization of allografts after ACL reconstruction, which was
similar with previous studies (11). Additionally, we found that
application of SH to VEGF was useful by acting as a drug-release
system of VEGF, leading to an acceleration in the process of graft
remodeling.
Previous studies have shown that ligament healing is
a complex and multistage process, which is controlled by a variety
of factors. Growth factors are involved in the remodeling process
and play an important role (12,13).
VEGF is a potent direct angiogenic factor that stimulates
endothelial cell migration and activation in vitro and in
vivo. In addition, VEGF is a group of highly conservative
secreted glycoproteins. Thus, in this study, human VEGF
demonstrated beneficial biological effects in a rabbit model.
Combined with a VEGF receptor, VEGF played an important role in
inducing proliferation of endothelial cells in addition to
promoting endothelial cell migration and survival. The angiogenic
activation of endothelial cells probably plays a role in promoting
and regulating other biological events, such as inflammation,
fibroblast proliferation and extracellular matrix synthesis. We
found that exogenous VEGF improved the early revascularization
after ACL reconstruction using a B-PT-B allograft. Simultaneously,
the peak blood vessel formation in group A occurred at 4 weeks.
However, it declined at 8 weeks. The two reasons responsible for
the aforementioned decline may be the following: i) the biological
effect of one-time exogenous VEGF disappeared or ii) the body
underwent its own adjustment; a deficiency in blood vessels in the
normal ACL caused inevitable degradation.
However, the key for solving this problem is the
short half-life of VEGF in vivo. We chose SH in this
experiment for four reasons. Firstly, it has been widely used in
ophthalmic surgery; no serious side-effects have been noted, it is
well-tolerated, non-immunogenic and usually it does not cause any
inflammatory reactions. Secondly, SH is a glycosaminoglycan and
plays a protective, shock-absorbing and structure-stabilizing role
in connective tissue. Thirdly, SH is biodegradable. After a certain
period of time, the gel decomposes to biocompatible materials or is
absorbed into the surrounding tissues, disappearing from the site
of administration. The gel becomes progressively less viscous in
vitro and begins to dissolve during a period of 1–2 weeks.
Fourthly, SH could be used as a controlled and localized delivery
system in a variety of conditions including osteoarthritic pain,
basal cell carcinoma and actinic keratosis (8). It can be combined with certain
polymers by covalent bonding and wraps the drug in vivo to
form a slow-release system. Matsumoto et al (9) found that morphine intestinal
suppositories had slow-release characteristics after adding 3% of
SH. Surendrakumar et al (14) reported that recombinant human
insulin combined with SH could make average residence time and
half-life longer than pure insulin. In our study, we found that the
SH system displayed diffusion controlled limitation of VEGF
release. A sustained release was achieved over several hours. In
addition, VEGF with SH obviously promoted revascularization, more
effectively than pure VEGF (P<0.01). There was no significant
difference between groups C and D (P>0.05). This revealed that
SH itself could not promote revascularization, but it could prolong
the average residence time and enhance the effect of VEGF.
In addition, we found that the ultimate failure load
of the allograft soaked in VEGF solution was significantly lower
than that of the allograft soaked in SH or PBS solution 2 weeks
after ACL reconstruction. Meanwhile, the ultimate failure load of
groups A and B became significantly higher than that of the other
groups at 4 and 8 weeks. Therefore, we determined that the
biomechanical characteristics of the graft decreased at an early
phase and then increased apparently later by using exogenous VEGF.
We considered that there may be two reasons for this phenomenon.
Firstly, a number of newly formed vessels and infiltrative cells,
which were induced by VEGF administration, decreased the density of
the graft and enhanced the deterioration of the mechanical
properties of the grafted tendon. Secondly, it has been reported
that VEGF promotes matrix metalloproteinases (MMPs) which may
directly digest the matrix of the graft (15,16).
Moreover, it is important to reduce the effect of
the allograft itself in the process of revascularization. It is
known that B-PT-B allograft transplantation may cause inflammatory
rejection responses that may influence revascularization, and it
also increases the risks of disease transmission. Freezing at −80°C
can damage the fiber cells of the graft that are the main resource
of normal MHC antigens. Consequently, graft antigencity is reduced
(17). Pinkowski et al
(18,19), Arnoczky et al (1) and Shino et al (13) did not detect any immune rejection
response after transplantion of the allograft pretreated by deep
freezing at −80°C. As for the risks of disease transmission, γ
irradiation is now widely used as a safe and effective secondary
sterilization technique (20). In
this study, the B-PT-B allografts we used were pretreated by γ
irradiation and stored at −80°C for 3 months. We found that there
was no refection response phenomenon after reconstruction using
B-PT-B allograft in all 45 rabbits.
In conclusion, our study suggests that SH can be
used as an excellent carrier of VEGF by enhancing the effect of
VEGF on revascularization and the biomechanical properties of
grafts. This may be a useful method for improving the graft healing
quality after ACL reconstruction.
Acknowledgements
This research was supported by a grant
from the National Natural Science Foundation of China (nos.
30870639 and 30872619).
References
|
1.
|
Arnoczky SP, Warren RF and Ashlock MA:
Replacement of the anterior cruciate ligament using a patellar
tendon allograft. An experimental study. J Bone Joint Surg Am.
68:376–385. 1986.PubMed/NCBI
|
|
2.
|
Bernatchez PN, Soker S and Sirois MG:
Vascular endothelial growth factor effect on endothelial cell
proliferation, migration, and platelet-activating factor synthesis
is Flk-1-dependent. J Biol Chem. 274:31047–31054. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chandrashekar L: Reply: hyaluronic acid: a
unique topical vehicle for the localized delivery of drugs to the
skin. J Eur Acad Dermatol Venereol. 20:1348–1349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Delay BS, McGrath BE and Mindell ER:
Observations on a retrieved patellar tendon autograft used to
reconstruct the anterior cruciate ligament. A case report J Bone
Joint Surg Am. 84:1433–1438. 2002.PubMed/NCBI
|
|
5.
|
Dodge-Khatami A, Backer CL, Holinger LD,
Mavroudis C, Cook KE and Crawford SE: Healing of a free tracheal
autograft is enhanced by topical vascular endothelial growth factor
in an experimental rabbit model. J Thorac Cardiovasc Surg.
122:554–561. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Guo L, Yang L, Duan XJ, et al: Second-look
arthroscopy study after anterior cruciate ligament reconstruction:
effect of residual anterior cruciate ligament tissue upon
accelerating revascularization of allologous bone-patellar
tendon-bone grafting. Zhonghua Yi Xue Za Zhi. 89:2030–2033.
2009.(In Chinese).
|
|
7.
|
Kelly RM, Meyer JD, Matsuura JE, et al: In
vitro release kinetics of gentamycin from a sodium hyaluronate gel
delivery system suitable for the treatment of peripheral vestibular
disease. Drug Dev Ind Pharm. 25:15–20. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Spitzer MS, Yoeruek E, Kaczmarek RT, et
al: Sodium hyaluronate gels as a drug-release system for
corticosteroids: release kinetics and antiproliferative potential
for glaucoma surgery. Acta Ophthalmol. 86:842–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Matsumoto Y, Yamamoto I, Watanabe Y and
Matsumoto M: Enhancing effect of viscous sodium hyaluronate
solution on the rectal absorption of morphine. Biol Pharm Bull.
18:1744–1749. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Tan H, Yang B, Duan X, et al: The
promotion of the vascularization of decalcified bone matrix in vivo
by rabbit bone marrow mononuclear cell-derived endothelial cells.
Biomaterials. 30:3560–3566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Munaut C, Noel A, Hougrand O, Foidart JM,
Boniver J and Deprez M: Vascular endothelial growth factor
expression correlates with matrix metalloproteinases MT1-MMP, MMP-2
and MMP-9 in human glioblastomas. Int J Cancer. 106:848–855. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Scheffler SU, Unterhauser FN and Weiler A:
Graft remodeling and ligamentization after cruciate ligament
reconstruction. Knee Surg Sports Traumatol Arthrosc. 16:834–842.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shino K, Kawasaki T, Hirose H, Gotoh I,
Inoue M and Ono K: Replacement of the anterior cruciate ligament by
an allogeneic tendon graft. An experimental study in the dog. J
Bone Joint Surg Br. 66:672–681. 1984.PubMed/NCBI
|
|
14.
|
Surendrakumar K, Martyn GP, Hodgers EC,
Jansen M and Blair JA: Sustained release of insulin from sodium
hyaluronate based dry powder formulations after pulmonary delivery
to beagle dogs. J Control Release. 91:385–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wei X, Mao Z, Hou Y, et al: Local
administration of TGFbeta-1/VEGF165 gene-transduced bone
mesenchymal stem cells for Achilles allograft replacement of the
anterior cruciate ligament in rabbits. Biochem Biophys Res Commun.
406:204–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yoshikawa T, Tohyama H, Katsura T, et al:
Effects of local administration of vascular endothelial growth
factor on mechanical characteristics of the semitendinosus tendon
graft after anterior cruciate ligament reconstruction in sheep. Am
J Sports Med. 34:1918–1925. 2006. View Article : Google Scholar
|
|
17.
|
Pattison JE, Hugtenburg RP and Green S:
Enhancement of natural background gamma-radiation dose around
uranium microparticles in the human body. J R Soc Interface.
7:603–611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Pinkowski JL, Reiman PR and Chen SL: Human
lymphocyte reaction to freeze-dried allograft and xenograft
ligamentous tissue. Am J Sports Med. 17:595–600. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Pinkowski JL, Rodrigo JJ, Sharkey NA and
Vasseur PB: Immune response to nonspecific and altered tissue
antigens in soft tissue allografts. Clin Orthop Relat Res.
326:80–85. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Reikeras O, Sigurdsen UW and Shegarfi H:
Impact of freezing on immunology and incorporation of bone
allograft. J Orthop Res. 28:1215–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|