Introduction
Thymic carcinoma is a rare epithelial tumor of the
thymus. Its incidence appears to be higher in Asians than in
Caucasians (1). Completeness of
resection has been considered to be the most important determinant
of long-term survival in thymic carcinoma (2,3).
Systemic chemotherapy or radiation therapy is usually selected for
the patients with an unresectable or metastatic disease. However,
an optimal chemotherapeutic drug or regimen has not yet been
determined for advanced or recurrent thymic carcinoma. Previous
studies of chemotherapy for advanced thymic carcinoma were based on
the regimens for advanced thymoma or germ cell tumor, using
cisplatin-based chemotherapy (4–6).
Reports of secondary or salvage chemotherapy have been single case
reports or for thymic carcinomas of a small size (7–11).
Recently, Okuma et al (12) and Koizumi et al (13) reported the usefulness of oral S-1
monotherapy as second-line or later chemotherapy for advanced
thymic carcinoma. S-1 is an oral fluoropyrimidine agent composed of
tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP) and potassium
oxonate. Tegafur, which is a prodrug of 5-fluorouracil (5-FU), is
converted to 5-FU in vivo. One of the targets of 5-FU is
thymidylate synthase (TS). Tegafur is rapidly catabolized by
dihydropyrimidine dehydrogenase (DPD). CDHP, a component of S-1,
inhibits DPD and, thus, maintains high 5-FU activity. 5-FU activity
is accelerated by orotate phosphoribosyltransferase (OPRT) which
converts 5-FU to its active form. Thus, the anticancer activity of
S-1 is influenced by the expression levels of TS and OPRT.
In order to estimate the anticancer activity of 5-FU
drugs against thymic carcinoma, TS and OPRT protein expression
levels were investigated in thymic carcinomas using
immunohistochemistry (IHC).
Materials and methods
Patients and clinicopathological
characteristics
Thymic carcinoma tissue samples from 24 patients who
underwent surgery or core-needle biopsy between 1986 and 2009 at
Nagoya City University Hospital (Nagoya, Japan) were used in the
present study. All patients consented to the use of their tissues
for the analysis. The patients consisted of 12 males and 12 females
with a median age of 60 years, ranging from 33 to 84. Pathological
diagnosis revealed squamous cell carcinoma in 13, adenocarcinoma in
2, and other types of carcinoma (mucoepidermoid, neuroendocrine and
lympho epithelioma-like carcinomas) in 3 patients. Detailed
pathological findings were not determined for 6 patients. The
patients were staged according to the Masaoka clinical staging
system with the following results: 1 patient in stage I, 5 in stage
II, 8 in stage III, 2 in stage IVa and 8 in stage IVb. With regard
to treatment, 15 patients underwent surgery. For post-surgical
treatment, 3 of 15 patients had adjuvant radiotherapy, 3 had
chemotherapy and 1 had chemoradiotherapy. As induction therapy
prior to surgery, 4 patients underwent chemotherapy and radiation
therapy and 1 had chemotherapy. Nine patients were regarded as
inoperable and 6 of these 9 patients underwent chemoradiotherapy, 2
had radiation therapy and 1 had chemotherapy. Among the 24
patients, no patient received chemotherapy with 5-FU drugs.
To study the difference between thymic carcinoma and
carcinomas of other organs, 55 samples of lung carcinoma were
compared with the samples of thymic carcinoma. These 55 patients
included 33 adenocarcinomas and 22 squamous cell carcinomas. The 33
adenocarcinoma patients all underwent surgery and chemotherapy
between 2003 and 2009. The 22 squamous cell carcinoma patients
underwent surgery between 2003 and 2009. The clinicopathological
characteristics of the 55 lung carcinoma patients were as follows:
i) there were 33 males and 22 females, ii) pathological stage was
diagnosed as I, II and III in 34, 15 and 6 patients, respectively
and iii) the regimen of adjuvant chemotherapy was UFT in 33
patients, TS-1 in 5 patients and CBDCA+PTX in 4 patients.
TS immunohistochemistry
TS protein expression was evaluated by IHC using
recombinant human TS-specific antibody (clone RTSSA; dilution,
1:1,500; Taiho Pharmaceutical, Co., Ltd., Saitama, Japan). A
standard protocol was used for immunostaining the 4-μm-thick
paraffin-embedded tissue sections of thymic carcinoma. The sections
were deparaffinized in xylene, dehydrated in ethanol, heated in a
microwave for antigen retrieval using pH 6.0 citrate buffer
solution, incubated with 0.3% hydrogen peroxidase in methanol to
block endogenous peroxidase activity and incubated with the
blocking solution (10% Block Ace) to block nonspecific binding.
RTSSA was applied as the primary antibody and the slides were
incubated overnight at 4°C. The slides were incubated with
EnVision™ as the second antibody for 45 min at room temperature and
visualized with 3,3′-diaminobenzidine and counterstained with
hematoxylin.
The slides were examined at low magnification and
the intensity of cytoplasmic staining was scored as follows: 0, no
staining or faint staining; 1+, moderate staining; 2+, strong
staining. We classified scores of 0 as negative and scores of 1+
and 2+ as positive for the TS antibody. We also evaluated cases
with <10% of tumor cells with moderate or strong staining as
negative.
OPRT immunohistochemisrty
OPRT protein expression was evaluated by IHC using
anti-OPRT polyclonal antibody (dilution, 1:1,200; Taiho
Pharmaceutical, Co., Ltd.). The staining procedure was the same as
for TS, with the exception of the primary antibody.
The scores used for the intensity of cytoplasmic
staining were the same as for TS. Scores of 0 and 1+ were
classified as negative and scores of 2+ as positive for the OPRT
protein. We also evaluated cases with <10% of tumor cells with
moderate or strong staining as negative.
Statistical analysis
Survival curves were generated using the
Kaplan-Meier method and the log-rank test was used to determine
statistical significance of the difference between groups. A
log-rank test was used to compare the survival distributions of the
two groups. The Mann-Whitney U test was used to assess whether
there was a significant difference in the median values between the
two independent samples. The two-sided significance level was at
P<0.05. All of the analyses were performed using Ekuseru-Toukei
2010 of Excel software.
Results
Immunohistochemical analysis of TS
expression of thymic carcinoma
The immunostaining scores were as follows: 12
samples were scored as 0, 10 as 1+ and 2 as 2+. Those with a score
of 1+ or 2+ were considered TS-positive. In all of these samples,
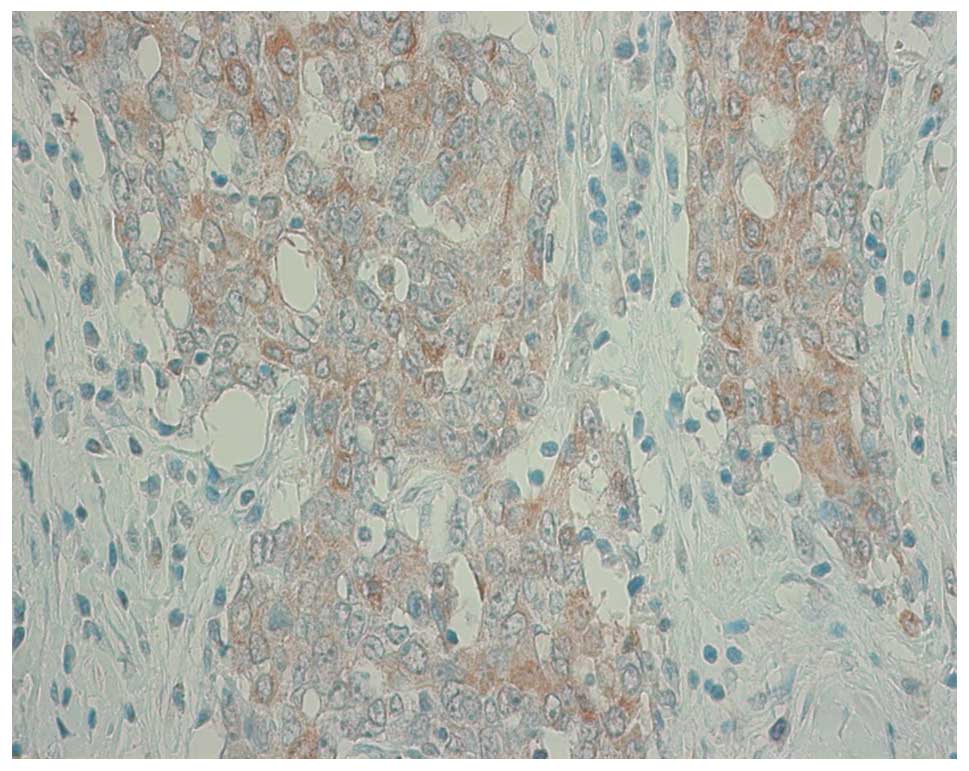
staining was counted over 10% of tumor cells. Fig. 1 shows a representative staining of
one sample which was scored as 2+. The association between TS
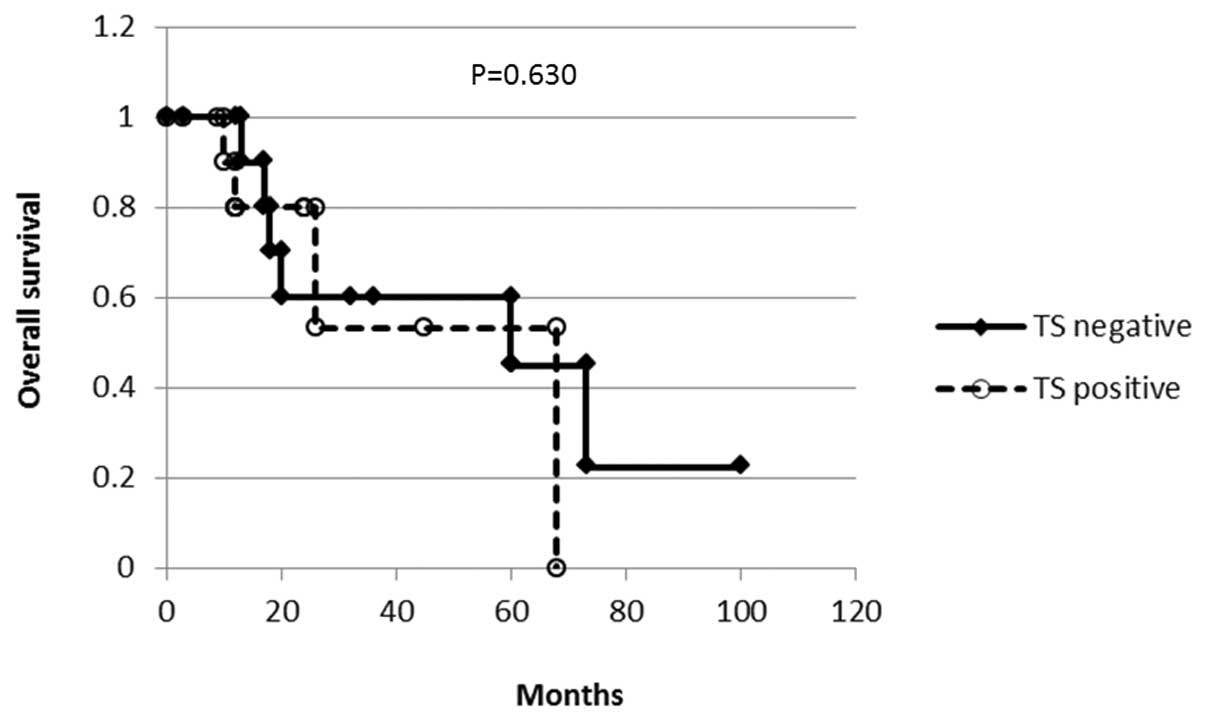
expression and overall survival was analyzed with the Kaplan-Meier
method (Fig. 2). No difference was
observed in survival according to the TS staining (P=0.630).
The association between TS protein expression and
Masaoka stages (stage I, II and III vs. stage IV) was analyzed for
the difference in TS expression between the stages. The tumors of
stage IV showed significantly higher TS expression than stages I,
II and III (Table I).
 | Table IAssociation between TS protein
expression and Masaoka stage. |
Table I
Association between TS protein
expression and Masaoka stage.
| Masaoka stage
|
|---|
| TS score | I, II, III | IV |
|---|
| 0 | 10/14 | 2/10 |
| 1 | 4/14 | 6/10 |
| 2 | 0/14 | 2/10 |
| Median score | 0 | 1 |
Immunohistochemical analysis of OPRT
expression of thymic carcinoma
The immunostaining scores were as follows: 4 samples
were scored as 0, 10 as 1+ and 10 as 2+. Samples with a score of 0
or 1+ were considered as OPRT-negative and those with 2+ as
OPRT-positive. In all of these samples, staining was counted over
10% of tumor cells. Fig. 3 shows a
representative staining of score 2+. The association between OPRT
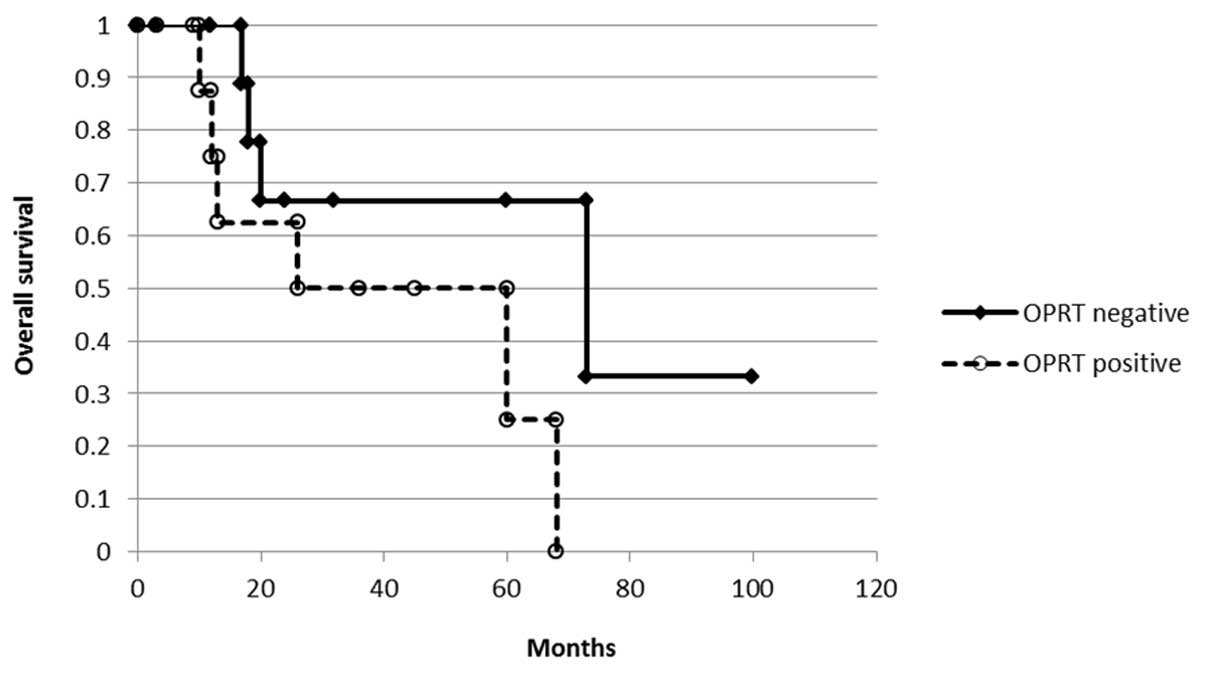
expression and overall survival was analyzed with the Kaplan-Meier
method (Fig. 4). There was no
difference according to the OPRT staining (P= 0.101) but there was
a tendency for OPRT-negative cases to demonstrated a longer
survival than OPRT-positive cases.
The association between the OPRT protein expression
and Masaoka stage was analyzed. The tumors at stage IV showed a
significantly higher OPRT expression than those at stage I, II or
III (Table II).
 | Table IIAssociation between OPRT protein
expression and Masaoka stage. |
Table II
Association between OPRT protein
expression and Masaoka stage.
| Masaoka stage
|
|---|
| OPRT score | I, II, III | IV |
|---|
| 0 | 4/14 | 0/10 |
| 1 | 7/14 | 3/10 |
| 2 | 3/14 | 7/10 |
| Median score | 1 | 2 |
Comparison of TS expression between
thymic carcinoma and non-small cell lung cancer (NSCLC)
To examine the differences in TS expression between
thymic carcinoma and NSCLC, TS protein expression was analyzed for
the 33 lung adenocarcinomas and 22 lung squamous cell carcinomas.
The TS expression scores of the 33 lung adenocarcinomas were as
follows: 16 samples were scored as 0 and 17 as 1+. With regard to
the 22 lung squamous cell carcinomas, 7 samples were 0, 6 as 1+ and
9 as 2+. The TS protein expression of lung squamous cell carcinoma
was significantly higher than that in thymic carcinoma (P= 0.0407),
whereas there was no difference between thymic carcinoma and lung
adenocarcinoma (Fig. 5A).
The differences in TS expression between thymic
squamous cell carcinoma and NSCLC were also assessed. TS protein
expression of lung squamous cell carcinoma was significantly higher
than that in thymic squamous cell carcinoma (P=0.0358), whereas
there was no difference between the thymic squamous cell carcinoma
and lung adenocarcinoma (data not shown).
Comparison of OPRT expression between
thymic carcinoma and NSCLC
To examine the differences of OPRT expression
between thymic carcinoma and NSCLC, OPRT protein expression of the
33 lung adenocarcinoma and 22 lung squamous cell carcinomas was
analyzed. OPRT expression in the lung adenocarcinomas was as
follows: 11 samples were scored as 0, 18 as 1+ and 4 as 2+. With
regard to the lung squamous cell carcinoma samples, 2 were scored
as 0, 11 as 1+ and 9 as 2+. OPRT protein expression in thymic
carcinoma was significantly higher than that in lung adenocarcinoma
(P=0,0170), whereas there was no difference between the thymic
carcinoma and the lung squamous cell carcinoma (Fig. 5B).
The differences in OPRT expression between thymic
squamous cell carcinoma and NSCLC were also assessed. There was no
difference between the thymic squamous cell carcinoma and NSCLC,
lung squamous cell carcinoma or lung adenocarcinoma (data not
shown).
Discussion
The TS and OPRT protein expression of the 24 thymic
carcinomas was evaluated with the aim of predicting the effect of
5-FU drugs for thymic carcinomas.
As thymic carcinoma is a rare thymic epithelial
tumor, a clinical trial of chemotherapy for advanced or recurrent
cases is difficult to plan. In addition, no clinical trial on a
large scale has been conducted due to the difficulties in
recruiting patients. There have been case reports of effective S-1
treatments for thymic carcinoma. S-1 has been used for patients
with advanced lung cancer (14).
In lung cancer, TS and OPRT expression has been reported to be
related to the effect of 5-FU drugs (15–17).
In thymic carcinoma, however, TS and OPRT expression has not yet
been reported.
TS is the enzyme that generates deoxythymidine
monophosphate (dTMP), which is subsequently phosphorylated to
thymidine triphosphate for use in DNA synthesis and repair. High
levels of TS expression have been reported in association with
aggressiveness (18), metastasis
and poor prognosis in various cancers (15–17,19,20).
A tumor with high TS expression tends to be resistant to a TS
inhibitor compared with a tumor showing low TS expression. TS
expression in squamous cell carcinoma has been shown to be higher
than in adenocarcinoma in general (21,22),
suggesting its relative resistance to 5-FU.
In the present study, TS and OPRT expression was
demonstrated in 24 thymic carcinomas using immunohistochemistry.
The TS expression in advanced-stage thymic carcinoma was
significantly higher than those in early stages, suggesting
resistance to 5-FU. Similar results have been reported with other
types of cancer (15–17,19).
We compared TS expression of thymic carcinoma with lung
adenocarcinoma and squamous cell carcinoma. The level of TS
expression in thymic carcinoma was similar to lung adenocarcinoma
but significantly lower than in squamous cell carcinoma. These
results indicate the possibility of using S-1 as a therapeutic drug
for thymic carcinoma. These data may be relevant to the antitumor
effect of another anticancer drug, pemetrexed, which is also
approved for NSCLC. Pemetrexed is chemically similar to folic acid
and is classified as a folate antimetabolite. Pemetrexed inhibits
three enzymes including TS which are used in purine and pyrimidine
synthesis (23). It is approved
for lung adenocarcinoma, with a lower level of TS expression, but
is not approved for lung squamous cell carcinoma having a higher
level of TS expression.
OPRT is an enzyme involved in pyrimidine
biosynthesis and contributes to the conversion of 5-FU into fdUMP,
an active form of 5-FU. In the present study, OPRT expression of
thymic carcinoma was significantly higher in patients of advanced
stage than in those of earlier stages. OPRT expression in thymic
carcinoma was significantly higher than in lung adenocarcinoma, and
was similar to that of squamous cell carcinoma. Thymic carcinoma
was shown to have a combination of relatively low expression of TS
and high expression of OPRT and is likely to be sensitive to
antitumor drugs such as 5-FU.
TS expression in thymic carcinoma was higher in more
advanced stages. This appears to be useful in predicting that
thymic carcinomas at an advanced stage are more resistant to S-1
than those in early stages. However, as OPRT expression was also
higher in more advanced stages, 5-FU is more highly activated and
subsequently may be effective in advanced stages.
While the results of this study are encouraging, it
is acknowledged that any conclusions should be tempered with
certain reservations. The small number of samples limited the
statistical power of the study. Thymic carcinoma is a rare disease
and it is difficult to undertake a large scale study. Our study was
confined to the protein expression of the enzymes TS and OPRT using
immunohistochemical analysis. Since these may be regulated at
various levels, a further study to examine the expression of mRNA,
by using in situ hybridization (ISH), may enhance the
results of the present study.
In conclusion, we evaluated TS and OPRT protein
expression in thymic carcinoma. The combination of relatively low
expression of TS and high expression of OPRT suggests a sensitivity
of thymic carcinoma to 5-FU drugs.
References
|
1
|
Shimosato Y, Kameya T, Nagai K and Suemasu
K: Squamous cell carcinoma of the thymus. An analysis of eight
cases. Am J Surg Pathol. 1:109–121. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yano M, Sasaki H, Yokoyama T, et al:
Thymic carcinoma: 30 cases at a single institution. J Thorac Oncol.
3:265–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yano M, Sasaki H, Yokoyama T, et al:
Thymic carcinoma with dissemination: a retrospective analysis of
ten patients. Gen Thorac Cardiovasc Surg. 56:335–339. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loehrer PJ Sr, Jiroutek M, Aisner S, et
al: Combined etoposide, ifosfamide, and cisplatin in the treatment
of patients with advanced thymoma and thymic carcinoma: an
intergroup trial. Cancer. 91:2010–2015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koisumi T, Takabayashi Y, Yamagashi S, et
al: Chemotherapy for advanced thymic carcinoma: clinical response
to cisplatin, doxorubicin, vincristine, and cyclophosphamide (ADOC
chemotherapy). Am J Clin Oncol. 25:266–268. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoh K, Goto K, Ishii G, et al: Weekly
chemotherapy with ciaplatin, vincristine, doxorubicin, and
etoposide is an effective treatment for advanced thymic carcinoma.
Cancer. 98:926–931. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oguri T, Achiwa H, Kato D, et al: Efficacy
of docetaxel as a second-line chemotherapy for thymic carcinoma.
Chemotherapy. 50:279–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komatsu Y, Koizumi T, Tanabe T, et al:
Salvage chemotherapy with carboplatin and paclitaxel for
cisplatin-resistant thymic carcinoma – three cases. Anticancer Res.
26:4851–4855. 2006.PubMed/NCBI
|
|
9
|
Loehrer P, Yiannoutsos C and Dropcho S: A
phase II trial of pemetrexed in patients with recurrent thymoma or
thymic carcinoma. Am Soc Clin Oncol. 24:70792006.
|
|
10
|
Magois E, Guigay J, Blancard PS, et al:
Multimodal treatment of thymic carcinoma: report of nine cases.
Lung Cancer. 59:126–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tagawa T, Ohta M, Kuwata T, et al: S-1
plus cisplatin chemotherapy with concurrent radiation for
thymicbasaloid carcinoma. J Thorac Oncol. 5:572–573. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okuma Y, Shimokawa T, Takagi Y, et al: S-1
is an active anticancer agent for advanced thymic carcinoma. Lung
Cancer. 70:357–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koizumi T, Agatsuma T, Komatsu Y and Kubo
K: Successful S-1 monotherapy for chemorefractory thymic carcinoma.
Anticancer Res. 31:299–301. 2011.PubMed/NCBI
|
|
14
|
Totani Y, Saito Y, Hayashi M, et al: A
phase II study of S-1 monotherapy as second-line treatment for
advanced non-small cell lung cancer. Cancer Chemother Pharmacol.
64:1181–1185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ceppi P, Volante M, Saviozzi S, et al:
Squamous cell carcinoma of the lung compared with other histotypes
shows higher messenger RNA and protein levels of for thymidylate
synthase. Cancer. 107:1589–1596. 2006. View Article : Google Scholar
|
|
16
|
Ishihama H, Chida M, Araki O, et al:
Comparison of 5-fluorouracil-related gene expression levels between
adenocarcinomas and squamous cell carcinomas of the lung. Jpn J
Clin Oncol. 39:33–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng Z, Li X, Schell MJ, et al:
Thymidylate synthase in situ protein expression and survival in
stage I nonsmall-cell lung cancer. Cancer. 112:2765–2773. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sasaki H, Fukai I, Kiriyama M, et al:
Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA
levels in thymoma. Surg Today. 33:83–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnston PG, Lenz HJ and Leichman CG:
Thymidylate synthase gene and protein expression correlate and are
associated with response to 5-fluorouracil in human colorectal and
gastric tumors. Cancer Res. 55:1407–1412. 1995.PubMed/NCBI
|
|
20
|
Lenz H, Leichman CG, Danenberg KD, et al:
Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a
predictor for primary tumor response and overall survival. J Clin
Oncol. 14:176–182. 1995.PubMed/NCBI
|
|
21
|
Obara S, Yamamoto K, Hosogai N and
Yoshimura Y: Evaluation of TS-1 based treatment and expression of
thymidylate synthase and dihydropyrimidine dehydrogenase on oral
squamous cell carcinoma. Oral Oncol. 41:276–282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogiuchi Y, Maruoka Y, Ando T, et al:
Thymidylate synthase, thymidine phosphorylase and orotate
phosphoribosyl transferase levels as predictive factors of
chemotherapy in oral squamous cell carcinoma. Acta Histochem
Cytochem. 41:39–46. 2008. View Article : Google Scholar
|
|
23
|
Chattopadhyay S, Moran RG and Goldman ID:
Pemetrexed: biochemical and cellular pharmacology, mechanisms, and
clinical applications. Mol Cancer Ther. 6:404–417. 2007. View Article : Google Scholar : PubMed/NCBI
|