Introduction
The aging of human skin includes intrinsic aging and
photoaging, characterized by a thining epidermis, decrease in
collagens and deposition of abnormal elastic fibers (1). It is well-documented that human
fibroblasts generate matrix metalloproteinases (MMPs), which
specifically degrade the majority of the extracellular matrix (ECM)
components and play an important role in skin natural aging and
photoaging (2). Vascular
endothelial growth factor (VEGF) is the only growth factor with a
specific effect on angiogenesis. After stimulation, vascular
endothelial cells and other cells produce VEGF, which may be
involved in the process of inflammation and carcinogenesis
(3).
The photorejuvenation technique is a type of
non-ablative treatment using continuous wavelength intense pulsed
light (IPL) at low energy density (4). Photorejuvenation is mostly used to
treat photoaging and telangiectasias, among others (5). The underlying mechanism is associated
with the photothermal and photochemical effects generated by IPL
(6).
To date, no data are clear on the effects of IPL on
human skin cells and the associated mechanism. Our research aimed
to investigate the effects of IPL on cell proliferation and protein
secretion of VEGF and/or MMP-1 and MMP-2 in human fibroblasts and
vascular endothelial cells, and to study the effects of IPL on mRNA
expression levels of procollagen type I and III in cultured human
fibroblasts.
Materials and methods
Cell culture and IPL irradiation
Fibroblasts were isolated from circumcised foreskin
(using 0.5% dispase, 0.25% trypsinase and 0.1% EDTA). Fibroblasts
and a vascular endothelial cell line (ECV034) were cultured in
medium and plated in 35-mm dishes and/or 96-well plates with the
same amounts of cells. Subconfluent primary fibroblasts and the
vascular endothelial cell line were irradiated with IPL with a
certain wavelength and dosage (wavelength 520–1200 nm, 5 pulses,
pulse width 15 msec, pulse delay 5 msec, fluence 15, 29 and 35
mJ/cm2). After 24-h culture, cells were collected for
morphological observation by light microscopy and the following
procedures.
Cell viability assay
Cellular viability was measured by MTT [3-(4,
5-dimethyl-thiazol-2-yl)-2, 5-diphenyldiphenyltetrazoliumbromide]
assay. A 5 mg/ml MTT solution (20 μl) was added per 100 μl medium.
After 4 h, 100 μl of dimethylsulfoxide (DMSO) was added to each
well and the absorbance (A) values at 490 nm were recorded on the
microplate reader.
Detection of certain cytokines
After IPL irradiation, the conditioned medium was
collected at various time intervals for ELISA. VEGF, MMP-1 and
MMP-2 were detected with the Human Cytokine Sandwich ELISA kit
(JingMei Bioengineer Company, Shenzhen, China). According to the
instructions, 50 μl samples, specifically diluted antibody, enzyme
reagent and color reagent were added and washed step by step in the
pre-coated 96-well immunoplates. The standard curve demonstrated a
direct association between OD values and secreted cytokine
levels.
Detection of mRNA expression of type I
and III procollagens
The total mRNA of fibroblasts was harvested by
Tri-reagent (Gibco-BRL, Gaithersburg, MD, USA) after IPL
irradiation. The ratio of OD260/OD280 was between 1.8–2.0,
suggesting no degradation and no protein contamination. DNA was
denatured at 94°C for 6 sec and PCR was performed immediately for
30 cycles, with denaturation at 94°C for 50 sec, annealing at 55°C
for 50 sec, extension at 72°C for 70 sec and the final extension at
72°C for 7 min. Table I lists the
primer sequences and the expected length of type I and III
procollagens in RT-PCR amplification. The RT-PCR products were run
through gel electrophoresis using 2% agarose gel. The quantitative
analysis of the optical density of the cDNA bands was performed by
Bio-Rad Quantity One® Image software. The density value
of each band of collagen type I and III was divided by the density
value of its corresponding GAPDH band for normalization. The
resultant values were expressed as relative intensities.
 | Table IPrimers used in the RT-PCR
amplification of the human procollagen type I and III and GAPDH
mRNAs. |
Table I
Primers used in the RT-PCR
amplification of the human procollagen type I and III and GAPDH
mRNAs.
| Primer | Sequence | Length of sequence
(bp) |
|---|
| Collagen I | | |
| Upstream |
5′-CTGGTCCCAAGGGTAACAG-3′ | 285 |
| Downstream |
5′-GCCAGGAGAACCACGTTC-3′ | |
| Collagen III | | |
| Upstream |
5′-CTGCCATCCTGAACTCAAGAGTGG-3′ | 447 |
| Downstream |
5′-CCATCCTCCAGAACTGTGTAGG-3′ | |
| GAPDH (control) | | |
| Upstream |
5′-AACCATGAGAAGTATGACAACAGC-3′ | 580 |
| Downstream |
5′-CATGTGGGGCCATGAGGTCCACCAC-3′ | |
Statistical analysis
The experimental data are expressed as mean ± SD and
analyzed by SPSS 10.0 software. P<0.05 was considered to
indicate a statistically significant result.
Results
Effect of IPL irradiation on the
morphology of skin fibroblasts
Subconfluent primary fibroblasts were treated with
doses of IPL irradiation (18, 29 and 35 J/cm2,
wavelength 590–1,200 nm) and cultured for 24 h. There was no clear
morphological change observed compared with the non-irradiated
fibroblasts under light microscope by the end of the experiment
(Fig. 1).
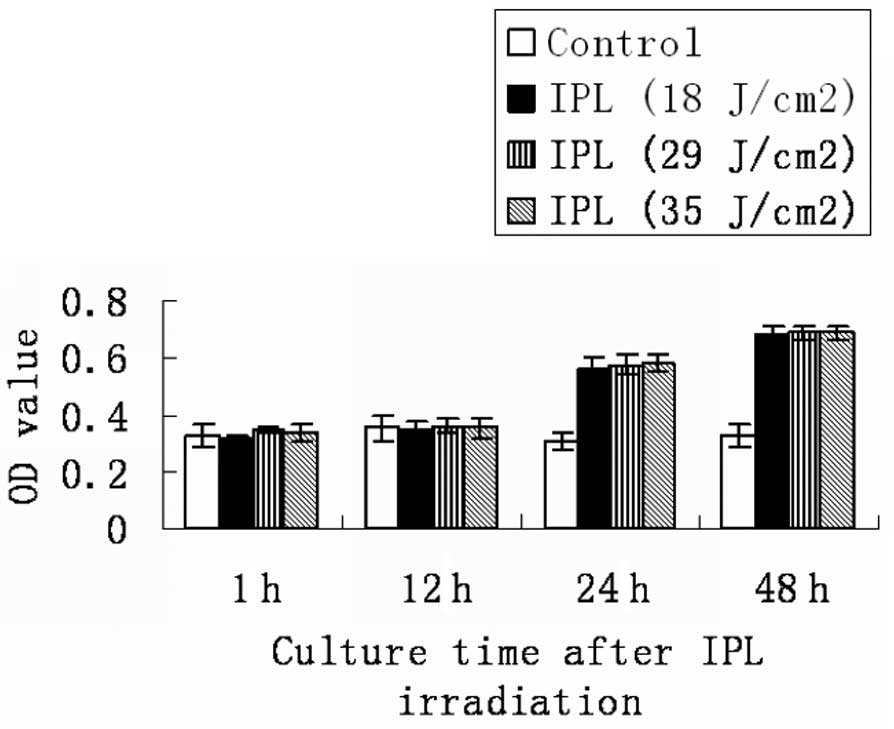
Effect of IPL on the cell proliferation
of skin fibroblasts
Fibroblasts were treated with doses of IPL
irradiation (18, 29 and 35 J/cm2). Fig. 2 and Table II show that there is no significant
difference in fibroblast viability or proliferation at the
time-points of 1 or 12 h after IPL irradiation (P>0.05).
However, the cellular viability and proliferation of fibroblasts
increased clearly after 24- and 48-h IPL treatment compared with
the controls (P<0.05).
 | Table IITime effects of IPL on the cell
proliferation of primary fibroblasts. |
Table II
Time effects of IPL on the cell
proliferation of primary fibroblasts.
| Culture time after
IPL radiation (h)
|
|---|
| Treatment | 1 | 12 | 24 | 48 |
|---|
| Sham | 0.329±0.038 | 0.352±0.042 | 0.307±0.028 | 0.329±0.038 |
| IPL (18
J/cm2) | 0.313±0.012 | 0.344±0.036 | 0.565±0.040a | 0.678±0.032b |
| IPL (29
J/cm2) | 0.344±0.012 | 0.358±0.023 | 0.577±0.038a | 0.688±0.027b |
| IPL (35
J/cm2) | 0.336±0.028 | 0.351±0.031 | 0.582±0.029a | 0.688±0.027b |
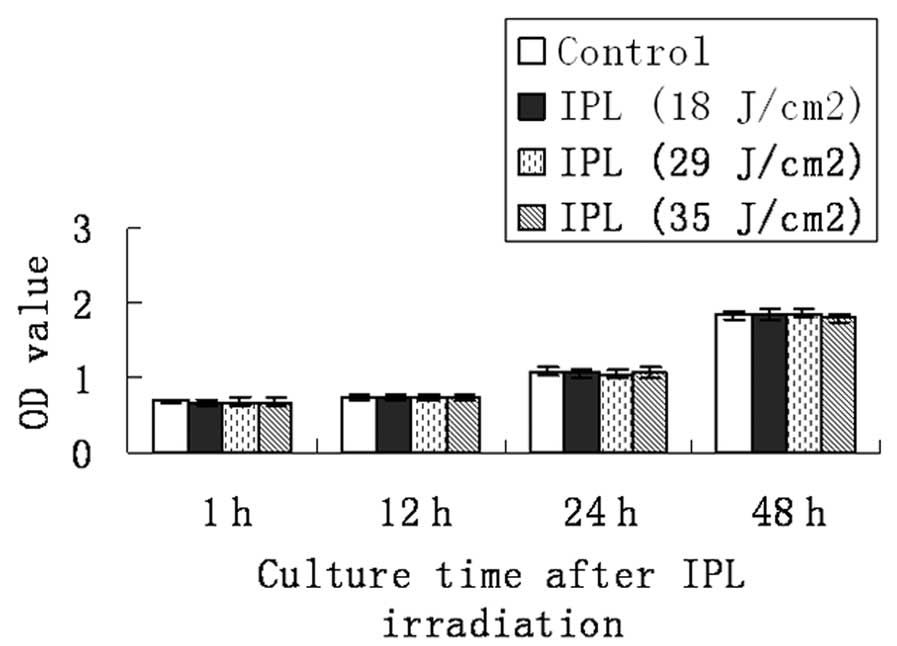
Effect of IPL irradiation on cell
proliferation of vascular endothelial cell line
Subconfluent vascular endothelial cell line (ECV034)
was treated with the same doses of IPL irradiation and culture time
as mentioned above. No obvious changes in cellular viability and
proliferation in the vascular endothelial cell line were observed
by the end of our experiment (P>0.05; Fig. 3 and Table III).
 | Table IIITime effects of IPL irradiation on
cell proliferation of vascular endothelial cell line. |
Table III
Time effects of IPL irradiation on
cell proliferation of vascular endothelial cell line.
| Culture time after
IPL radiation (h)
|
|---|
| Treatment | 1 | 12 | 24 | 48 |
|---|
| Sham | 0.689±0.030 | 0.739±0.052 | 1.086±0.059 | 1.847±0.056 |
| IPL (18
J/cm2) | 0.671±0.040 | 0.725±0.035 | 1.066±0.062 | 1.856±0.072 |
| IPL (29
J/cm2) | 0.674±0.050 | 0.723±0.037 | 1.051±0.059 | 1.868±0.061 |
| IPL (35
J/cm2) | 0.674±0.050 | 0.738±0.040 | 1.079±0.061 | 1.802±0.063 |
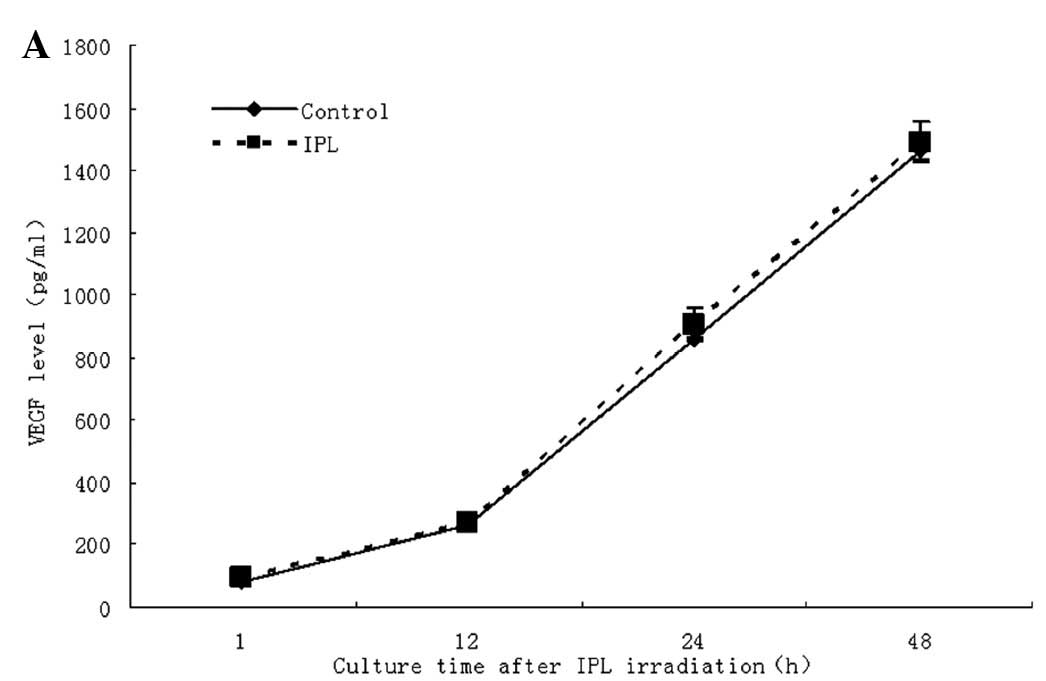
Effect of IPL on VEGF secretion in
primary fibroblasts and vascular endothelial cells
To investigate the effects of IPL, fibroblasts and
the vascular endothelial cell line (ECV034) were treated with
various wavelengths and doses of IPL irradiation (590–1200 nm and
18, 29 and 35 J/cm2). After culturing for 1, 12, 24 and
48 h after irradiation, the levels of VEGF in cell supernatants
were determined by ELISA. No clear regulation of VEGF secretion was
observed in fibroblasts and the ECV034 cell line (Fig. 4A and B).
Effect of IPL on mRNA expression of
procollagen type I and III in fibroblasts
The cultured fibroblasts were treated with IPL
irradiation (570–590 nm and 18 J/cm2) and the mRNA
expression was measured after culturing irradiated fibroblasts for
1, 12, 24 and 48 h. Compared with sham irradiation, the mRNA
expression levels of procollagen types I and III did not increase
markedly after short-term culture. The procollagen levels were
clearly increased (P<0.05) when the irradiated fibroblasts were
cultured from 24 to 48 h after IPL irradiation and the upregulation
effect of IPL was also time-dependent (Fig. 5A and B).
Effect of IPL irradiation on the
secretion of MMP-1 and MMP-2 in fibroblasts
The fibroblasts were irradiated by IPL (590–1200 nm,
29 J/cm2) and cultured for 24 h. The conditioned
supernatant was collected and measured by ELISA for levels of MMP-1
and MMP-2. The results showed that IPL irradiation had no
significant effect on the secretion levels of MMP-1 and MMP-2
proteins (P>0.05; Table
IV).
 | Table IVEffects of IPL irradiation on MMP-1
and MMP-2 secretion from fibroblasts. |
Table IV
Effects of IPL irradiation on MMP-1
and MMP-2 secretion from fibroblasts.
| MMP (ng/ml) | Controls | IPL (29
J/cm2) |
|---|
| MMP-1 | 3.89±0.16 | 3.75±0.18 |
| MMP-2 | 1.50±0.14 | 1.41±0.11 |
Discussion
The development of non-ablative laser and light
treatment provide an alternative to the traditional ablative
modalities and improve overall skin texture and tone. Non-ablative
laser and light therapy (also termed photorejuvenation) is a
relatively new concept for the impovement of the visual appearance
of photodamaged skin and acne scars. The remarkable advantage of
non-ablative therapy is the limited downtime after treatment.
IPL is a laser-like device that uses a flash lamp to
produce a non-coherent pulsed light from 515 to 1,200 nm with
variable pulse durations and intervals. Photorejuvenation is mainly
used in the treatment of certain skin diseases, including
photoaging (7) and telangiectasias
(8). IPL also works on dermal
cells, fibers and vessels (9) by
the principle of selective photothermolysis. The major advantage of
this device is its versatility in providing the wide range of
wavelengths, pulse durations and pulse delay that allow the
treatment of numerous types of lesions. This versatility makes IPL
able to adjust to the type, depth and the size of the lesion as
well as the skin type of the patient to achieve maximum clearance
(10). The advantages of the new
IPL technique are manifold, with optimal treatment parameters,
including pulse and wave-OPT system, and almost without epidermal
loss, side effects or recovery periods.
It is well-known that collagen in the dermis is
mainly composed of type I (80%) and III (10%) collagens, which are
responsible for the elasticity and integrity of the skin.
Fibroblasts produce and secrete procollagen which consists of
collagens. For non-ablative therapy, an epidermal surface
temperature of 40–48°C, is ideal since this correlates with a
dermal temperature of 55–65°C, which is required for collagen
denaturation. It was reported by Talwar et al (11) that procollagen type I and III
levels decreased in photoaging and/or aged skin. After IPL
treatment, fibroblasts promote the production of procollagen, which
may have some association with IPL by direct stimulation and/or
photothermolysis. In this way IPL increases the substantial
production and rearrangement of collagens in the dermis (4,6) and
makes the skin more elastic and rejuvenated.
Our research focused on primary fibroblasts and the
vascular endothelial cell line (ECV034) to confirm whether IPL
irradiation could increase the production of procollagen from
cultured fibroblasts and/or increase endothelial cell proliferation
and VEGF production. There was no morphological change observed
under light microscopy, which suggests that IPL has no heat-injury
effect on cultured fibroblasts (Fig.
1). In addition, the cell proliferation of fibroblasts
increased dramatically 24 and 48 h after administration of a series
of doses (18, 29 and 35 J/cm2) of IPL irradiation
compared with sham irradiation but IPL showed no effect on the
ECV034 cell line (Tables II and
III, Figs. 2 and 3). The RT-PCR assay also revealed mRNA
expression levels of the procollagen type I and III from
fibroblasts were increased at 12, 24 and 48 h after IPL irradiation
(Fig. 5). ELISA results revealed
that IPL could stimulate fibroblasts to secret type I and III
procollagens and consequently increase the level of collagens. Our
study demonstrated that IPL irradiation on Chinese Han fibroblasts
resulted in increased cell proliferation and procollagen mRNA
levels.
VEGF is the most important cytokine of the vascular
endothelial growth factor family, which plays an important role in
the growth and differentiation of vascular, as well as lymphatic,
endothelial cells. VEGF is the pivotal angiogenic growth factor
activating endothelial cells to migrate, proliferate and form
capillary tubes (12). VEGF
induces increased vascular permeability, angiogenesis and then the
skin repair process. In this study, IPL showed no up- or
downregulatory effects on VEGF levels in fibroblasts or vascular
endothelial cells at any time-point after IPL irradiation (Fig. 4). The data showed that IPL has no
direct effects on the cellular proliferation or cytokine secretion
from the vascular endothelial cell line, which supports that the
mechanism of IPL on telangiectasias has no association with VEGF.
It may have an association with the destruction and deposition of
vascular endothelial cells after IPL photothermolysis.
With regard to human skin aging, it is not only
associated with the decrease in new collagen production, but also
with the increase of collagen degradation. MMPs are a group of ECM
enzymes that degrade all known protein components of the ECM
(13). The upregulation of MMPs,
particularly collagenase-1 (MMP-1), stromelysin-1 (MMP-3) and
gelatinase A (MMP-2), is responsible for the lysis of dermal
collagen and elastic fibers during skin aging (14). MMPs originating from keratinocytes
and fibroblasts are considered to play a primary role in this
process. In response to UV irradiation, mitogen-activated protein
kinase signaling pathways are activated mediating the upregulation
of MMP expression. However, in response to IPL irradiation, there
was no upregulation of the secretion levels of MMP-1 and MMP-2
proteins on fibroblasts (Table
IV), which suggests that IPL irradiation could not degrade the
protein components of ECM as does UV irradiation.
IPL irradiation induced cell proliferation in
cultured primary fibroblasts but had no such effect on the vascular
endothelial cell line. IPL had no regulatory effect on VEGF
secretion for either cultured fibroblasts or ECV304. In addition,
IPL had no upregulatory effect on MMP-1 and MMP-2 secretion from
fibroblasts. However, IPL irradiation promoted the transcription of
type I and III procollagen mRNA in fibroblasts directly, which
suggests at a part of the mechanism of photorejuvenation.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (81000700 and
81171518).
References
|
1
|
Berneburg M, Plettenberg H and Krutmann J:
Photoaging of human skin. Photodermatol Photoimmunol Photomed.
16:239–244. 2000. View Article : Google Scholar
|
|
2
|
Ohnishi Y, Tajima S, Akiyama M, Ishibashi
A, Kobayashi R and Horii I: Expression of elastin-related proteins
and matrix metalloproteinases in actinic elastosis of sun-damaged
skin. Arch Dermatol Res. 292:27–31. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ouchi N, Shibata R and Walsh K:
AMP-activated protein kinase signaling stimulates VEGF expression
and angiogenesis in skeletal muscle. Circ Res. 96:838–846. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bitter PH: Noninvasive rejuvenation of
photodamaged skin using serial, full-face intense pulsed light
treatments. Dermatol Surg. 26:835–843. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holck DE and Ng JD: Facial skin
rejuvenation. Curr Opin Ophthalmol. 14:246–252. 2003. View Article : Google Scholar
|
|
6
|
Negishi K, Tezuka Y, Kushikata N and
Wakamatsu S: Photorejuvenation for Asian skin by intense pulsed
light. Dermatol Surg. 27:627–632. 2001.PubMed/NCBI
|
|
7
|
Negishi K, Wakamatsu S, Kushikata N,
Tezuka Y, Kotani Y and Shiba K: Full-face photorejuvenation of
photodamaged skin by intense pulsed light with integrated contact
cooling: initial experiences in Asian patients. Lasers Surg Med.
30:298–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Angermeier MC: Treatment of facial
vascular lesions with intense pulsed light. J Cutan Laser Ther.
1:95–100. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Campolmi P, Bonan P, Cannarozzo G,
Bruscino N, Troiano M, Prignano F and Lotti T: Intense pulsed light
in the treatment of non-aesthetic facial and neck vascular lesions:
report of 85 cases. J Eur Acad Dermatol Venereol. 25:68–73. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fodor L, Peled IJ, Rissin Y, Ramon Y,
Shoshani O, Eldor L, Gaiman A and Ullmann Y: Using intense pulsed
light for cosmetic purposes: our experience. Plast Reconstr Surg.
113:1789–1795. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Talwar HS, Griffiths CE, Fisher GJ,
Hamilton TA and Voorhees JJ: Reduced type I and type III
procollagens in photodamaged adult human skin. J Invest Dermatol.
105:285–290. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gale NW and Yancopoulos GD: Growth factors
acting via endothelial cell-specific receptor tyrosine kinases:
VEGFs, angiopoietins, and ephrins in vascular development. Genes
Dev. 13:1055–1066. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bramono DS, Richmond JC, Weitzel PP,
Kaplan DL and Altman GH: Matrix metalloproteinases and their
clinical applications in orthopaedics. Clin Orthop Relat Res.
428:272–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hornebeck W: Down-regulation of tissue
inhibitor of matrix metalloprotease-1 (TIMP-1) in aged human skin
contributes to matrix degradation and impaired cell growth and
survival. Pathol Biol (Paris). 51:569–573. 2003. View Article : Google Scholar : PubMed/NCBI
|