Introduction
The development of periodontal inflammation is a
complex process, therefore animal models have been developed to
assist in its understanding. Experimental periodontitis induced by
the placement of nylon or cotton ligatures around molars (allowing
the retention of plaque), is one of the most widely used models
(1–4).
The main etiological factor of periodontal disease
is bacterial plaque, but the pathogenesis of the disease is
affected by environmental factors that modify or induce systemic
progression, such as stress (3,5,6).
Human studies suggest that negative life events and
psychological factors may contribute to an increased susceptibility
for periodontal disease (5,7–10).
It has been reported that stress produces
neuroendocrine changes and certain adverse effects on the immune
system, which affect the inflammatory response on periodontal
tissues (11,12).
The restriction movement technique has become a
standard procedure to study stress effects, particularly when using
rodents as study subjects (13,14).
This model has been previously used to associate chronic exposure
to stress and periodontal destruction (1–3,15,16).
Studies on animals indicated that chronic stress may
modulate pathophysiological states of inflammation, causing an
accelerated degradation of periodontal tissues (1).
Despite the accumulated evidence, a direct
association between periodontal disease and stress is not entirely
clear (17). In addition, there
are no previous studies or investigations that consider the effect
of habituation in study animals subjected to chronic stress. This
factor should be considered in the design and research methodology,
as the physiological response obtained may not represent the
reality (18,19).
Therefore, the purpose of this study was to clarify
the role of chronic stress on the severity of experimental
periodontitis in rats, taking into account previously unconsidered
issues.
Materials and methods
Experimental design
An experimental design of randomized blocks was
used. The independent variable was stress exposition and the
dependent variable was periodontal disease severity. All procedures
followed the guidelines of the Guide for the Care and Use of
Laboratory Animals, National Research Council (20) and were approved by the Bioethics
Committee of the University of Talca (Talca, Chile).
Animals
A total of 32 male Sprague Dawley (SD) rats of 12
weeks of age (330–430 g) were used. No blood relatives, with
appropriate health certificates, were obtained from the Institute
of Biomedical Sciences (ICBM, University of Chile). The rats were
kept under controlled temperatures (22±1°C), under 12-h light/dark
(the light was turned on at 08:00 am) conditions, with freely
available food and water, in groups of four rats in polycarbonate
enclosures enriched with tissue paper and cardboard rolls (21), at the Animal Facility of the
University of Talca.
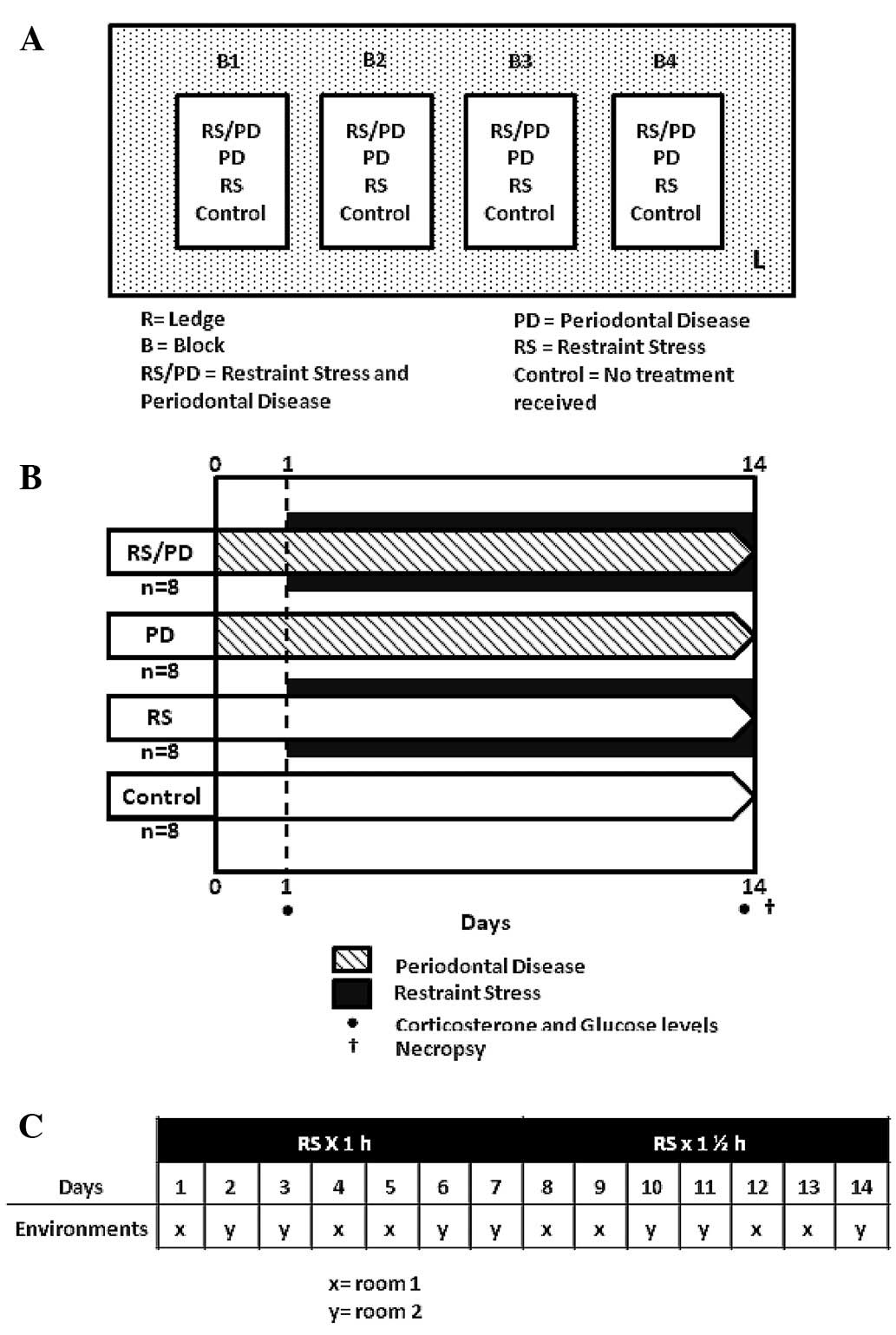
Rats were divided into four groups (Fig. 1A): the RS/PD group (n=8) received
stress by movement restriction and periodontitis; PD group (n=8)
received stress-induced periodontitis; RS group (n=8) received only
stress and the control group (n=8) received no treatment at
all.
Induction of experimental periodontal
disease
Significant events of the pilot phase are shown in
Fig. 1B. Prior to the intraoral
procedures, the rats were anesthetized with 10% ketamine/2%
xylazine/1% acepromazine (Drag Pharma Chile Invetec S.A., Santiago,
Chile) at a ratio of 50/5/1 mg/kg intramuscularly (IM). Having
established anesthesia, we applied a ligature (4-0, Ethicon,
Johnson & Johnson Company) around the neck of the left
mandibular first molars (M1s) from each rat (Fig. 2A and B). The rats remained with
ligatures throughout the experimental period of 15 days (to allow
retention of plaque). Each day the correct position of the bands
was confirmed.
Restraint stress (RS) model
From day 1 of the pilot phase, animals were stressed
1 h/day for the first 7 days, and then 1.5 h in the following 7
days, in two different environments. The animals were alternated to
avoid habituation to the stressor stimulus and environment
(Fig. 1C) in properly ventilated
acrylic cylinders (60 mm in diameter). Each session was undertaken
for 1–1.5 h between 08:00 and 12:00 am, during which rats were
fasted from food or water.
Laboratory assays
After the first and last cycle of restriction of
movement, plasma samples were obtained from the leg vein of each
subject and stored at −20°C, until determination of plasma levels
of corticosterone and glucose in the Laboratory of Animal
Physiology and Endocrinology (University of Concepción, Chillán,
Chile). Corticosterone was quantified by means of a commercial
ELISA kit (DRG International, Austin, TX, USA), validated for rat
corticosterone, with an intrassay coefficient of 3%. Glucose was
determined with a kit (Roche, Mannheim, Germany) based on the
GOD-POD method (glucose oxidase and peroxidase) and was measured at
505 nm using a spectrophotometer (Thermo Electron Co., Vantaa,
Finland).
Tissue preparation
After the experimental phase, the animals were
sacrificed with an overdose of anesthetic. Immediately thereafter,
the mandible was hemisected (two halves by a cut between the lower
incisors) and fixed in 10% neutral-buffered formalin. The
decalcified tissue blocks were maintained in 5% nitric acid for 7
days after conventional histopathology (H&E staining).
Incidence
Determination of periodontal disease by
histopathological examination was established with a conventional
technique of 112 plates of H&E-stained tissues, which were
obtained from the mandible processing preparations. The plates were
analyzed by an academic (CR) from the University of Talca. The
observer was unaware of the group to which the study samples
belonged (single-blind model). The incidence of periodontal disease
was established by the presence of inflammation or destruction of
periodontal tissues.
Severity
To establish the severity of the disease in the
periodontal tissues of the M1s, we examined the presence or absence
of pathological histology, according to the parameters of Garcia
(22) and Liu et al
(23) (Fig. 3A). The degree of inflammation in
the gingival tissue (24,25) (Fig.
3B) and bone (26) (Fig. 3C) was also determined.
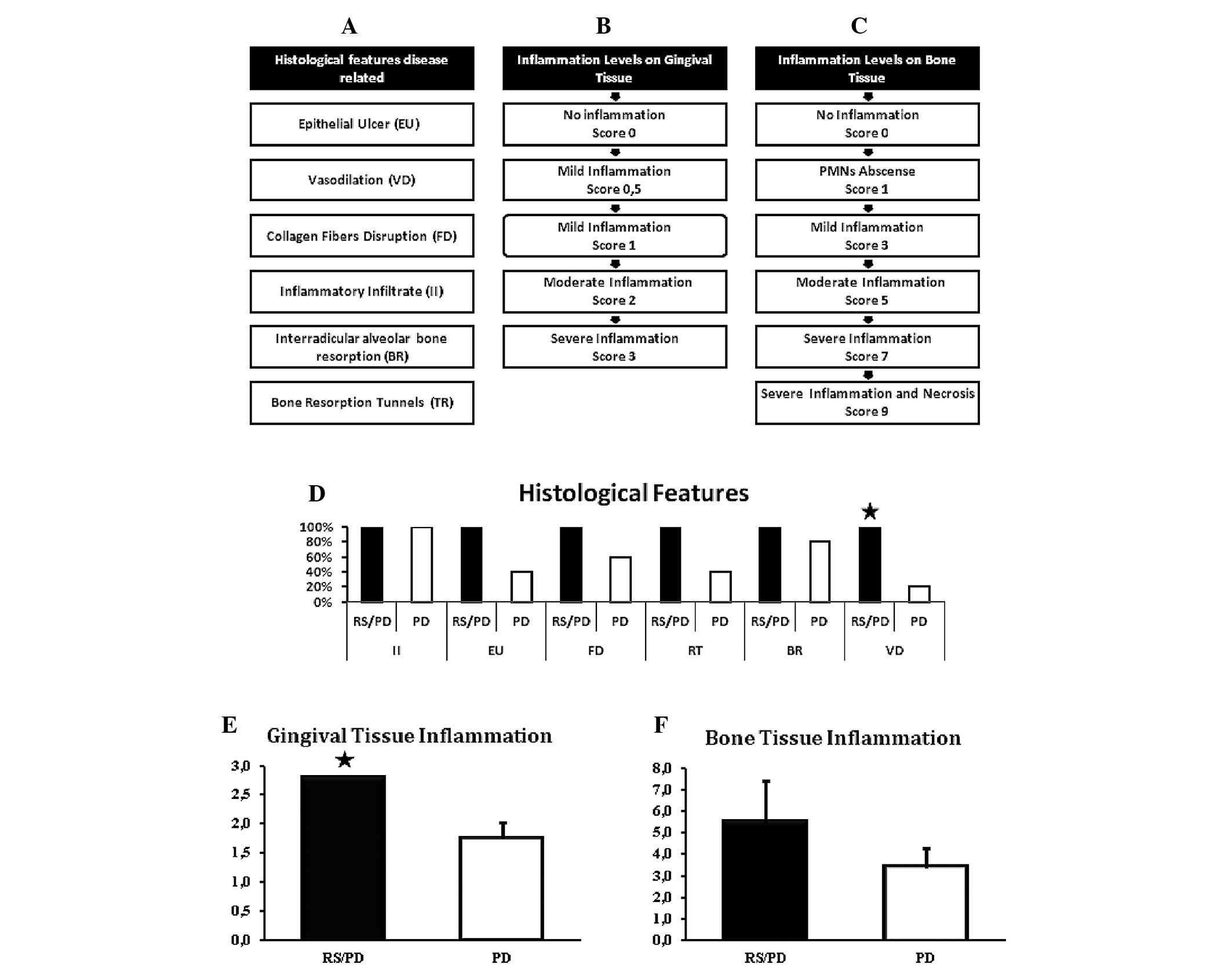 | Figure 3(A) The severity of experimental
periodontal disease (PD) was determined. Associated histological
features with periodontal disease [modified from Liu et al
(23) and Garcia (22)] are shown: epithelial ulceration,
lack of continuity of the gingival epithelium, vasodilation,
increased luminal diameter blood vessels, disrupted collagen
fibers, continuity of loss of collagen fibers in connective tissue,
presence of inflammatory infiltrate, groups of lymphocytes observed
in field; interradicular alveolar bone resorption, continuity loss
of interradicular bone, replaced by connective tissue or other;
tunnels in bone resorption, osteoclastic resorption in depth areas.
(B) Degree of inflammation in the gingival tissues [modified from
Liu et al (24) and Luan
et al (25)] is shown: no
inflammation, no presence of inflammatory cells (score 0), mild
inflammation (score 0.5), limited inflammation of the epithelium,
mild inflammation (score 1), inflammation of the connective tissue
near the epithelium, with 2–4 inflammatory cells/field; moderate
inflammation (score 2), inflammation of the tissue with 5–10
inflammatory cells/field; severe inflammation (score 3),
inflammation in the connective tissue consistent with an abscess.
(C) Degree of inflammation in bone tissue [taken from Graves et
al (26)] is shown. The scale
was used according to the number of PMNs at the center of the
inflammatory infiltrate (1, no PMNs; 3, slight infiltrate; 5,
moderate infiltrate; 7, severe infiltrate; and 9, severe infiltrate
with cell necrosis). (D) Presence of histological features
associated with periodontal disease (percentage) are shown. It is
noted that all features are present in 100% of animals in the RS/PD
group, which is significantly different to PD in vasodilation
(significant difference for Chi-square test, P≤0.05). (E)
Restriction of movement increases inflammation of gingival tissue
in rats with periodontal disease. Rats in the RS/PD group have an
average value of 2.8 (moderate to severe inflammation). Rats in the
PD group presented a mean value of 1.8 (mild to moderate
inflammation). The difference among the groups was statistically
significant (P=0.001 for Student’s t-test). (F) Inflammation of
bone tissue. Rats in RS/PD group had an average value of 5.5
(moderate to severe inflammation). The rats in the PD group
presented a mean value of 3.4 (inflammation of mild to moderate
type). The difference among the groups was not statistically
significant (P= 0.064 for Student’s t-test). PMNs,
polymorphonuclear leukocytes. |
Statistical analysis
Qualitative data were analyzed by the Chi-square
test with Pearson’s correlation. Quantitative data were assessed
using the Mann-Whitney U test and Student’s t-test. P≤0.05 was
considered to indicate a statistically significant result.
Results
SD rats treated with RS had higher levels
of plasma corticosterone
Table I shows
plasma corticosterone (ng/ml) and glucose (mmol/l) levels. Results
of the Mann-Whitney U test established significant differences
between the groups treated with RS (RS/PD and RS), compared with
final measurements of corticosterone in the groups not treated with
RS (PD and control; P≤0,05). There were no statistical differences
in the measured glucose levels.
 | Table ICorticosterone and glucose plasma
levels (mean ± SD) before and after restraint cycles. |
Table I
Corticosterone and glucose plasma
levels (mean ± SD) before and after restraint cycles.
| Corticosterone levels
(ng/ml)
| Glucose levels
(mmol/l)
|
|---|
| Groups | Initial | Final | Initial | Final |
|---|
| RS/PD | 195.1±71.5 | 348.2±135.4a | 4.4±2.0 | 18.8±6.0 |
| PD | 186.2±55.4 | 232.0±69.2 | 2.8±2.3 | 13.5±7.3 |
| RS | 205.0±141.0 | 329.5±120.1a | 4.1±2.2 | 13.5±2.4 |
| Control | 200.3±74.2 | 169.7±6.3 | 3.9±3,0 | 11.2±7.0 |
Histopathological findings
Fig. 2C–K shows the
main histological aspects observed in this investigation.
Incidence of experimental periodontal
disease
All the rats treated with molar ligation (RS/PD and
PD groups) had inflammation in periodontal tissues (gingival or
bone). There was no periodontal inflammation in the untreated
animals.
Severity according to the presence of
histological features associated with periodontal disease
RS increased the presence of features associated
with periodontal disease (Fig.
3D). Rats in the RS/PD group tended to exhibit greater
disruption of collagen fibers of connective tissue, epithelial
ulcers, resorption tunnels, interradicular alveolar bone resorption
and vasodilation, compared with the PD group, with a statistically
significant difference only for vasodilation (P<0.05 for the
Chi-square test).
Severity of periodontal disease according
to the degree of gingival inflammation
RS increased the severity of gingival inflammation
(Fig. 3E). Rats in the RS/PD group
showed an average value of 2.8 inflammatory infiltrate,
corresponding to moderate to severe inflammatory process. The PD
group demonstrated a value of 1.8, indicating mild to moderate
inflammation. To evaluate the difference we used the Student’s
t-test, which found a statistically significant difference
(P=0.001).
Severity of periodontal disease according
to the degree of bone inflammation
RS did not increase the severity of inflammation in
bone marrow (Fig. 3F). Rats in the
RS/PD group tended to have a score value of 5–7 points (average
5.5), corresponding to moderate to severe inflammatory process. PD
group rats presented with score values of 3–5 points (mean 3.4),
indicating a mild to moderate inflammatory process. To evaluate the
values we used the Student’s t-test, which found no statistically
significant differences (P=0.064), however, there was a tendency to
increased inflammation in the RS/PD group.
Discussion
Results of this study have shown that RS was an
effective method for causing chronic stress in rats, measured with
plasma corticosterone levels (ng/ml). Animals treated with RS had
higher levels of circulating corticosterone, with significant
differences between groups receiving environmental enrichment vs.
those that did not. This parameter is widely used as a marker of
physiological changes associated with the presence and intensity of
stress (14). Glucose showed only
increased values in the RS/PD group, with no significant trend,
which is in contrast to studies in which this parameter is
increased in groups receiving chronic stress (2). This discrepancy may be due to some
extent to the methodology used.
With regard to the various histopathological
features analyzed, there was an increased presence of 100%
vasodilation in rats in the RS/PD group (treated with stress and
periodontal disease) vs. 20% of the PD group (treated only with
periodontal disease). The increase of vasodilation observed in this
investigation is associated with clinical features evidenced by
Lindhe and Karring (27), who
explained that early inflammatory changes associated with
periodontal disease are likely to be expressed in the dentogingival
plexus with increased blood supply to the affected area. If the
inflammation it perpetuates, local factors, risk factors and host
susceptibility may be considered in periodontal tissue
destruction.
Significant differences expected in other
characteristics of the analysis were not observed; it is likely
that trends become apparent with monitoring and observation over an
extended time period.
One of the parameters used to evaluate the role of
RS in the severity of periodontal disease was the observation of
the inflammatory infiltrate using the scale used by Liu et
al (24), which according to
the severity of inflammation, was: 0 for no inflammation; 1, mild;
2, moderate and 3, for severe. Rats in the RS/PD group had an
average value of 2.8 inflammatory infiltrate corresponding to a
moderate to severe inflammatory process, compared with the PD group
where a value of 1.8 shows a mild to moderate inflammation.
Therefore, RS modulates the inflammatory process in gingival tissue
in rats treated with periodontal disease. These findings are
consistent with those obtained by Takada et al (2), where rats subjected to restriction of
movement with periodontal disease had a higher presence of
inflammatory infiltrate, vasodilation and disorganization of
connective tissue fibers than untreated subjects. Furthermore,
Peruzzo et al (3) noted
that restricting movement increased the expression of inflammatory
factors and resorption in periodontal tissues in rats. Therefore,
rats subjected to restriction of movement would have produced a
greater inflammatory process vs. the untreated animals.
With regard to the degree of inflammation in the
inter-radicular bone tissue, the rats in the RS/PD group had an
average value of inflammatory infiltrate in the bone tissue of 5.5,
corresponding to a moderate to severe inflammation vs. the PD
group, which presented a value of 3.4, corresponding to a mild to
moderate inflammation. Thus, RS also modulates the progress of the
inflammatory process in bone tissue in rats treated with
periodontal disease, although this difference was not statistically
significant.
Consequently, RS would influence inflammatory
processes in the gingival tissue and bone, but only in samples with
periodontal disease (molars using a nylon ligature). However, RS by
itself is unable to produce a more severe inflammatory process.
This is consistent with Gaspersic et al (1), who suggested that stress by itself
does not cause periodontal disease (no cause-effect). However, only
when periodontal disease is present, stress may play a role,
causing accelerated degradation of periodontal tissues, thus,
creating a correlation between increased severity parameters and
the presence of elevated levels of corticosterone.
The methodology used in this study shows that
chronic RS increases the severity of inflammation, in the gingival
tissue and bone. This finding is based on evidence obtained by
conventional histopathological analysis only, which represents a
limitation. For future studies, it would be necessary to evaluate
the role of chronic stress on the severity of destructive processes
in bone tissue, increasing the duration of the pilot phase and
using other advanced histological techniques, since in this model
it was not possible to measure bone destruction. Another
alternative for these limitations is that repeated stimuli may
generate a reduction in physiological responses elicited by
exposure to a repeated homotypic (same) stressor, a phenomenon
known as habituation (18). This
possibility was considered in the study design, and to prevent it,
we used a method of inducing high-intensity stress, accompanied by
an interval between cycles and different environments. The
influence of habituation cannot be ruled out, which may represent a
limitation to our study. Nevertheless, the results of the present
study showed that RS modulates periodontal inflammation and that
the rat model described is suitable for investigating the
association between stress and periodontal disease.
Acknowledgements
We would like to thank Cristian
Fernández for his support and the Dirección de Investigación (DI)
of the University of Talca (Talca, Chile) for its cooperation. A
preliminary report was presented at the IADR/LAR General Session,
Iguaçu Falls, Brazil, in 2012.
References
|
1
|
Gaspersic R, Stiblar-Martincic D and
Skaleric U: Influence of restraint stress on ligature-induced
periodontitis in rat. Eur J Oral Sci. 110:125–129. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takada T, Yoshinari N, Sugiishi S, Kawase
H, Yamane T and Noguchi T: Effect of restraint stress on the
progression of experimental periodontitis in rats. J Periodontol.
75:306–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peruzzo DC, Benatti BB, Antunes IB,
Andersen ML, Sallum EA, Casati MZ, Nociti FH Jr and Nogueira-Filho
GR: Chronic stress may modulate periodontal disease: a study in
rats. J Periodontol. 79:697–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oz H and Puleo D: Animal models for
periodontal disease. J Biomed Biotechnol. 7548572011.PubMed/NCBI
|
|
5
|
Genco RJ, Ho AW, Grossi SG, Dunford RG and
Tedesco LA: Relationship of stress, distress, and inadequate coping
behaviors to periodontal disease. J Periodontol. 70:711–723. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hildebrand HC, Epstein J and Larjava H:
The influence of psychological stress on periodontal disease. J
West Soc Periodontol Periodontal Abstr. 48:69–77. 2000.PubMed/NCBI
|
|
7
|
Green LW, Tryon WW, Marks B and Huryn J:
Periodontal disease as a function of life events stress. J Hum
Stress. 12:32–36. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freeman R and Gross S: Stress measures as
predictors of periodontal disease-a preliminary communication.
Community Dent Oral Epidemiol. 21:176–177. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wimmer G, Janda M, Wieselmann-Penkner K,
Jakse N, Polansky R and Pertl C: Coping with stress: its influence
on periodontal disease. J Periodontol. 73:1343–1351. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hilgert JB, Hugo FN, Bandeira DR and
Bozzetti MC: Stress, cortisol, and periodontitis in a population
aged 50 years and over. J Dent Res. 85:324–328. 2006.PubMed/NCBI
|
|
11
|
Pistorius A, Krahwinkel T, Willershausen B
and Boekstegen C: Relationship between stress factors and
periodontal disease. Eur J Med Res. 7:393–398. 2002.PubMed/NCBI
|
|
12
|
Breivik T, Thrane PS, Murison R and Gjermo
P: Emotional stress effects on immunity, gingivitis and
periodontitis. Eur J Oral Sci. 104:327–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paré WP and Glavin GB: Restraint stress in
biomedical research: a review. Neurosci Biobehav Rev. 10:339–370.
1986.
|
|
14
|
Buynitsky T and Mostofsky D: Restraint
stress in biobehavioral research: recent developments. Neurosci
Biobehav Rev. 33:1089–1098. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakajima K, Hamada N, Takahashi Y,
Sasaguri K, Tsukinoki K, Umemoto T and Sato S: Restraint stress
enhances alveolar bone loss in an experimental rat model. J
Periodontol Res. 41:527–534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenoff Segundo A, Semenoff TA, Borges
AH, Pedro FL and Sakai V: Methodological model of chronic stress
associated with ligature-induced periodontitis in rats: a
radiographic study. Braz Oral Res. 24:455–459. 2010.PubMed/NCBI
|
|
17
|
Saini R, Saini S and Saini SR:
Periodontitis and psychological stress: a dental view. Ind
Psychiatry J. 19:66–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grissom N and Bhatnagar S: Habituation to
repeated stress: get used to it. Neurobiol Learn Mem. 92:215–224.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rivera CA, Droguett DA, Kemmerling U and
Venegas BA: Chronic restraint stress in oral squamous cell
carcinoma. J Dent Res. 90:799–803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council, Institute for
Laboratory Animal Resources: Guide for the Care and Use of
Laboratory Animals. National Academy Press; Washington, DC:
1996
|
|
21
|
Olsson IA and Dahlborn K: Improving
housing conditions for laboratory mice: a review of ‘environmental
enrichment’. Lab Anim. 36:243–270. 2002.
|
|
22
|
Garcia MF: Estudio de la dinámica ósea
mandibular y de los procesos reabsortivos de la cresta alveolar en
ratas diabéticas y controles. Revista Académica Electrónica de la
UNR 1: 1852-0707, 2008 (Available at: http://hdl.handle.net/2133/1509uri).
|
|
23
|
Liu L, Li C, Cai C, Xiang J and Cao Z:
Cyclophilin A (CypA) is associated with the inflammatory
infiltration and alveolar bone destruction in an experimental
periodontitis. Biochem Biophys Res Commun. 391:1000–1006. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu R, Bal HS, Desta T, Krothapalli N,
Alyassi M, Luan Q and Graves DT: Diabetes enhances periodontal bone
loss through enhanced resorption and diminished bone formation. J
Dent Res. 85:510–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luan Q, Desta T, Chehab L, Sanders VJ,
Plattner J and Graves DT: Inhibition of experimental periodontitis
by a topical boron-based antimicrobial. J Dent Res. 87:148–152.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Graves DT, Naguib G, Lu H, Leone C, Hsue H
and Krall E: Inflammation is more persistent in type 1 diabetic
mice. J Dent Res. 84:324–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lindhe J and Karring NT: Periodontología
Clínica e Implantología Odontológica. 1. 5th edition. Editorial
Medica Panamericana; Mexico: pp. 289–290. 2009
|