Introduction
Diabetic nephropathy is a major microvascular
complication of diabetes mellitus and the leading cause of
end-stage renal disease (1). The
interventions in general clinical use are not capable of
efficiently slowing or reversing the progression of nephropathy.
Therefore, interventions which could optimally delay the
development of diabetic nephropathy are required.
The traditional Chinese medicine Fufang Xue Shuan
Tong (FXST) capsule, which contains SanQi, DanShen, XuanShen and
HuangQi, has been used to treat a series of fundus oculi diseases,
including diabetic retinopathy, for many years in China (2,3).
Since microvascular diseases share part of the same pathogenesis,
we hypothesized that FXST may also exhibit a nephropathy-protective
effect in diabetes.
Oxidative stress is increased in patients with
diabetes and in various tissue samples from experimental diabetes
(4–6). Accumulating evidence has shown that
oxidative stress markers, such as malondialdehyde (MDA) and
8-hydroxy-2′-deoxyguanosine (8-OHdG) are increased in diabetic
nephropathy states. Antioxidant enzymes, such as superoxide
dismutase (SOD), catalase and glutathione peroxidase, exhibit a
relatively low expression. Accordingly, oxidative stress is a
significant contributor to the pathogenesis of diabetic
nephropathy, and therefore those interventions with anti-oxidant
properties may attenuate the manifestations associated with
diabetic nephropathy (7,8). FXST was observed to attenuate the
up-regulation of oxidative stress in an experimental model of
diabetic retinopathy (3). Since
oxidative stress is a common cause of diabetic retinopathy and
nephropathy (9), we further
hypothesized that the nephropathy-protective effect of FXST was
also mediated by the down-regulation of oxidative stress. However,
the precise effects and mechanisms still need to be addressed using
cellular and molecular approaches.
To test these hypotheses, diabetes was induced in
rats by administration of a high-fat diet and low dose
streptozotocin (STZ) in the current study. FXST was initiated in
three different doses to assess the effects of FXST on diabetic
nephropathy and the potential causal mechanisms.
Materials and methods
Animal model
A total of 59 male Sprague-Dawley (SD) rats (150–180
g) were purchased from the Experimental Animal Center of Guangdong
Medical Sciences and were raised in the Department of Laboratory
animal center of Sun Yat-sen University. We were unable to induce
diabetes in 9 and 7 died during the study, therefore 43 rats
completed the study. The study followed the Guidelines for Animal
Care issued by the First Affiliated Hospital of Sun Yat-sen
University.
The rats were allocated a normal or high-fat diet
(HFD) (58.3% fat, 7.9% protein and 33.8% carbohydrate, as a
percentage of total kcal) ad libitum, respectively, for 5
weeks. Diabetes was induced in HFD rats by intraperitoneal
injection of STZ (Sigma, St. Louis, MI, USA), 40 mg/kg body weight.
The normal-diet rats were injected with an equal volume of vehicle
citrate buffer. Three days after STZ injection, the rats with a
non-fasting blood glucose of ≥16.7 mmol/l were considered diabetic
and selected for additional studies.
Experimental protocol
Diabetic rats were randomized into five groups:
Low-dose FXST group: LF group, n=7, FXST 450 mg/kg/day, oral
gavage; middle-dose FXST group: MF group, n=6, FXST 900 mg/kg/day,
oral gavage; high-dose FXST group: HF group, n=7, FXST 1800
mg/kg/per day, oral gavage; captopril group: CA group, n=7,
captopril 50 mg/kg/day, oral gavage; diabetic group: DM group, n=8,
no treatment. Normal-diet non-diabetic SD rats served as controls
(NC group, n=8).
The treatment was initiated 3 weeks after the
induction of diabetes. After 3 months of treatment, the rats were
fasted overnight and anesthetized by intraperitoneal injection of
10% chloral hydrate (0.3 ml/100 g body weight). The right kidneys
were removed for histological analysis. The left kidneys were
removed, decapsulated, weighed and then divided into cortical and
medullary sections. Kidney cortices were snap-frozen in liquid
nitrogen and stored at −80°C for further analysis.
Biochemical analysis
Fasting blood samples were obtained from the tail
veins. Blood glucose was measured by glucometer (Roche, Basel,
Switzerland). Serum and urine levels of creatinine were detected
using an automatic biochemistry analyzer. Rats were kept in
metabolic cages to collect 24-h urine. Susequently, the urine
volume was measured and urine protein was tested by
chemiluminescence analysis. Urinary protein excretion (mg/24 h) was
assessed as: urine protein (mg/l) x urine volume (liters)/24 h.
Creatinine clearance rate (Ccr) was determined as: Ccr= urine
creatinine (μmol/l) x urine volume per min (ml/min)/serum
creatinine (μmol/l) and data were normalized for body weight.
Histological analysis
The right kidneys were fixed in 10% buffered
formalin, embedded in paraffin, sectioned at 4 μm and stained with
hematoxylin and eosin and periodic acid-silver metheramine (PASM).
In PASM-stained sections, the glomerular cross-sectional (Ag), tuft
(At) and mesangial matrix (Am) areas were measured in 30 glomerular
profiles per rat using Image Pro Express 6.0 software. The
mesangial matrix area was defined as the PASM-positive area.
Quantitative measurement of mesangial matrix expansion (mesangial
matrix index) was expressed as the PASM-positive area per total
glomerular tuft cross-sectional area (10). The glomerular volume (Vg) was
determined as: Vg = β/κ [Ag]3/2, where β is 1.38 as a shape factor
and κ is 1.1 as a distribution factor (11).
Measurement of lipid peroxidation
Kidney cortices (100 mg) were weighed and
homogenized. The protein concentration was determined by BCA
analysis (Kangchen, Shanghai, China). Lipid peroxidation, measured
as MDA, reflects the impact of oxidative stress in tissues. Tissue
MDA levels were measured according to the method described by
Ohkawa et al with a commercially available kit, following
the manufacturer’s instructions (Genmed, Shanghai, China) (12). The absorbance was measured with a
spectrophotometer at 535 nm. MDA levels are expressed as MDA
(μmol)/protein (μg).
SOD
SOD activity was assayed using the nitroblue
tetrazolium (NBT) method with a commercial assay kit according to
the manufacturer’s instructions (Genmed, Shanghai, China) (13). The absorbance was measured with a
spectrophotometer at 560 nm. One unit (U) of SOD is defined as the
amount of protein that inhibits the rate of NBT reduction by 50%.
The calculated SOD activity is expressed as SOD (U)/protein
(μg).
Statistical analysis
Results were shown as the means ± SD. Statistical
analysis was performed using the SPSS 11.0 statistical package.
One-way-analysis of variance (one-way-ANOVA) was used for
comparison of more than two groups followed by an LSD test for
multiple comparisons. The Kruskal-Wallis test was used when the
data departed substantially from a normal distribution.
Significance was defined as p<0.05.
Results
Metabolic data
The levels of fasting blood glucose were
significantly increased in the DM, LF, MF, HF and CA groups prior
to intervention and remained higher for the entire duration, as
compared with the NC group. Body weight showed the inverse result.
Three weeks after the induction of diabetes, the body weights were
significantly decreased and remained lower over the treatment
period in the diabetic rats with or without treatment, in
comparison to the NC group. The fasting blood glucose and body
weights in the DM and treatment groups did not reach statistical
significance (Table I).
 | Table IMetabolic data. |
Table I
Metabolic data.
| | Blood glucose
(mmol/l)
| Body weight (g)
|
|---|
| Group | n | Initial | Final | Initial | Final |
|---|
| NC | 8 | 4.99±0.58 | 4.99±0.84 | 412.50±22.96 | 547.75±34.50 |
| DM | 8 | 21.04±5.96a | 23.29±2.88a | 286.75±59.17a | 389.88±25.06a |
| LF | 7 | 19.56±4.94a | 21.70±5.97a | 255.85±20.88a | 386.71±28.04a |
| MF | 6 | 20.87±5.24a | 22.42±3.83a | 258.66±10.46a | 394.33±26.43a |
| HF | 7 | 19.09±2.82a | 22.27±3.65a | 257.14±18.08a | 383.71±31.54a |
| CA | 7 | 21.24±3.76a | 22.76±4.97a | 267.85±27.15a | 386.28±39.92a |
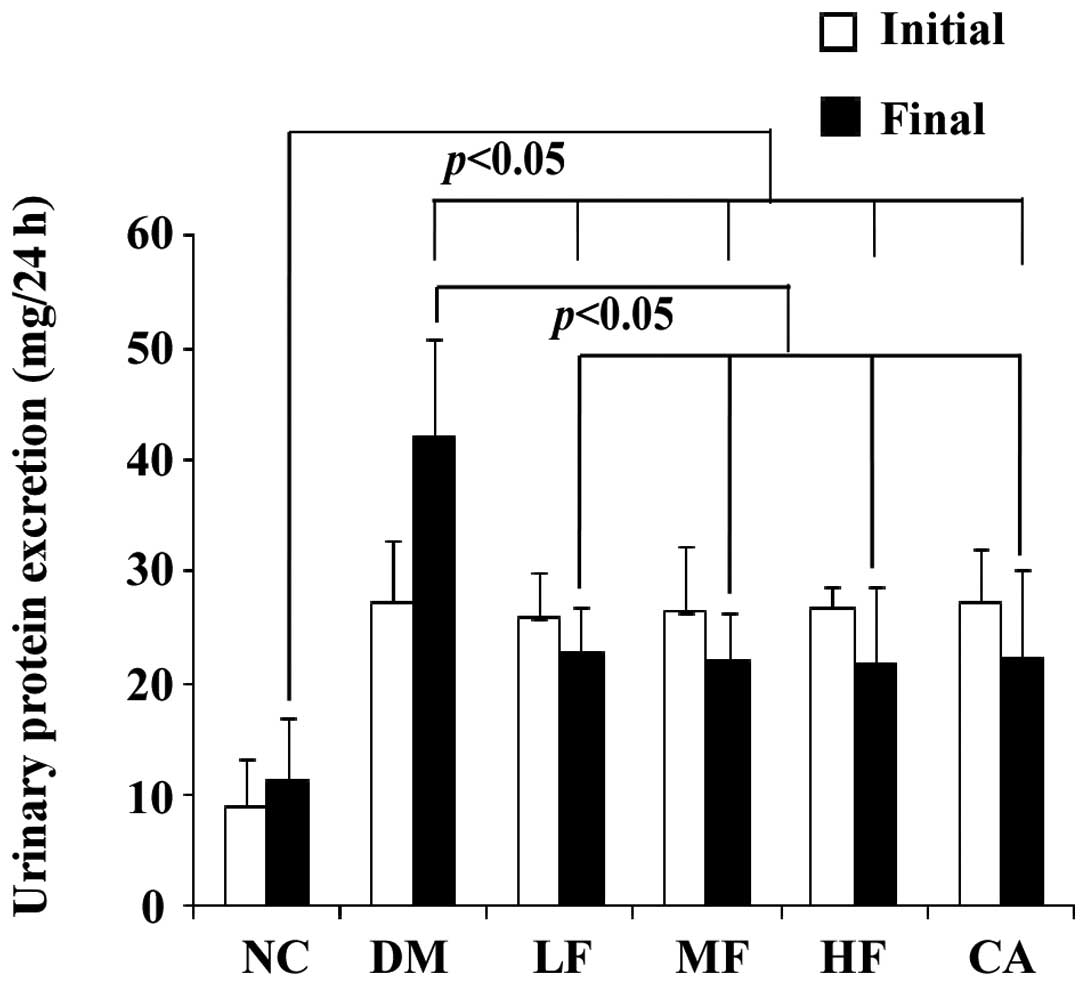
Urinary protein excretion
Urinary protein excretion was already significantly
elevated after 3 weeks of diabetes and markedly declined after 3
months of FXST and captopril treatments as compared to the
no-treatment DM group. However, urinary protein excretion in the
treatment groups remained higher than that in the NC group. The
differences of urinary protein excretion among the various doses of
FXST and captopril groups were not significant (Fig. 1).
Creatinine clearance
Creatinine clearance remained higher 3 weeks after
induction of diabetes and averaged at lower levels after 3 months
of FXST and captopril therapy, albeit it remained largely unchanged
in the DM group. Creatinine clearances in the LF, MF, HF and CA
groups were higher than those in the NC group, although they did
not reach statistical significance. The amelioration of creatinine
clearance in the intervention groups remained significant when
normalized for body weight. Moreover, when normalized for body
weight, creatinine clearance in the DM group was reduced, with the
most likely reason being that rats gained weight over the study
period (Table II). Following
3-month FXST treatment, urinary protein excretion and creatinine
clearance markedly decreased, but remained slightly higher than the
normal levels, indicating that FXST was capable of delaying but not
completely reversing disease progression.
 | Table IICreatinine clearance. |
Table II
Creatinine clearance.
| | Creatinine clearance
(ml/min)
| Relative creatinine
clearance (ml/min/100 g body weight)
|
|---|
| Group | n | Initial | Final | Initial | Final |
|---|
| NC | 8 | 1.28±0.42 | 1.43±0.92 | 0.31±0.10 | 0.26±0.17 |
| DM | 8 | 3.89±1.51a | 3.87±0.74a | 1.37±0.48a | 0.99±0.18a |
| LF | 7 | 3.64±1.65a | 2.04±1.13b | 1.43±0.67a | 0.54±0.32b |
| MF | 6 | 3.49±0.84a | 1.93±1.48b | 1.35±0.30a | 0.50±0.39b |
| HF | 7 | 3.51±0.55a | 1.82±1.19b | 1.38±0.28a | 0.49±0.36b |
| CA | 7 | 3.47±1.00a | 1.81±0.74b | 1.32±0.42a | 0.48±0.23b |
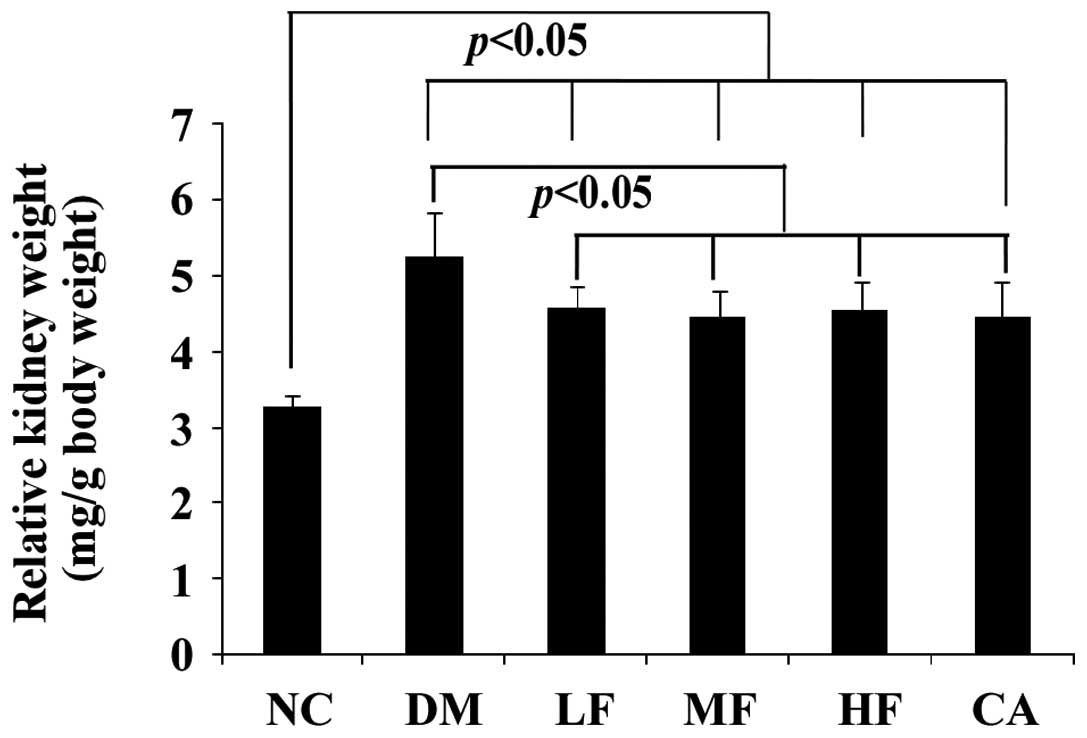
Kidney weight and relative kidney
weight
Induction of diabetes significantly increased the
kidney weight and relative kidney weight. The changes were highly
suppressed in the LF, MF, HF and CA groups and did not reach
statistical significance. However, the relative kidney weight in
the NC group remained lower than that in the treatment groups. The
potential reason was that the diabetic rats did not gain weight as
significantly as the normal rats during the study (Table III, Fig. 2).
 | Table IIIKidney weight and relative kidney
weight. |
Table III
Kidney weight and relative kidney
weight.
| Group | n | Body weight (g) | Kidney weight
(g) | Relative kidney
weight (mg/g body weight) |
|---|
| NC | 8 | 547.75±34.50 | 1.79±0.10 | 3.28±0.15 |
| DM | 8 |
389.88±25.06a | 2.05±0.15a | 5.27±0.56a |
| LF | 7 |
386.71±28.04a | 1.76±0.03b |
4.58±0.29a,b |
| MF | 6 |
394.33±26.43a | 1.75±0.03b |
4.46±0.34a,b |
| HF | 7 |
383.71±31.54a | 1.73±0.03b |
4.55±0.38a,b |
| CA | 7 |
386.28±39.92a | 1.71±0.02b |
4.47±0.45a,b |
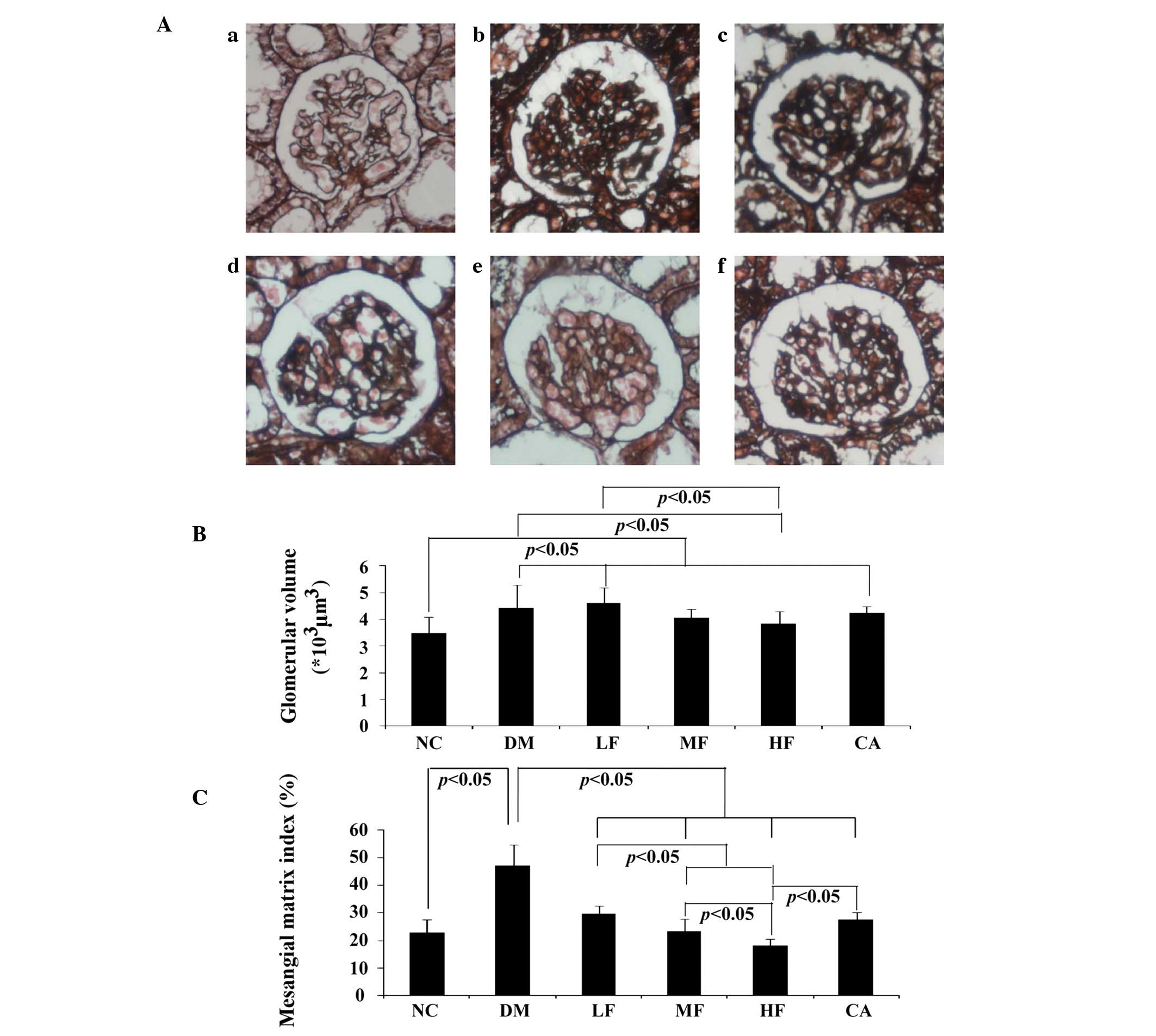
Histological analysis
PASM-stained glomeruli are representatively shown in
Fig. 3Aa–f. Glomerular hypertrophy
and mesangial matrix expansion, measured as glomerular volume and
mesangial matrix index respectively, were markedly elevated in the
DM group. Mesangial matrix expansion was attenuated in the
treatment groups after 3-month FXST or captopril therapy, and the
HF group showed the most prominent effect in antagonizing mesangial
matrix expansion. By contrast, glomerular hypertrophy was
ameliorated only in the MF and HF groups, but not in the LF or CA
groups. Significant diabetic glomerulosclerosis was not observed in
any rat kidney (Fig. 3B and
C).
MDA
A marked decrease was detected in the levels of
renal cortical MDA in the LF, MF, HF and CA groups as compared with
the DM group, showing that FXST and captopril reduced the oxidative
status. However, in comparison to the NC group, the levels in these
groups remained higher. Furthermore, the finding that the decrease
in the LF group was less prominent than that in the MF and HF group
indicated that the antioxidative effect of FXST was dose-dependent
(Fig. 4).
SOD
The levels of renal cortical SOD were significantly
increased in the LF, MF, HF and CA groups as compared with the DM
group, but remained lower than those in the NC group. Captopril
showed the most pronounced effect in activating the antioxidant
system. However, the difference did not reach statistical
significance (Fig. 5).
Discussion
The major findings of the current study are that
FXST decreases urinary protein excretion, reduces creatinine
clearance and ameliorates the diabetic nephropathy-related
histopathological changes. FXST retards the progression of diabetic
nephropathy through modulations of oxidative stress. The beneficial
effects of FXST have been shown to be similar to those of
captopril.
In its normal state, the kidney generates a
substantial amount of oxidative stress due to its high metabolic
activity, which is balanced by an extensive antioxidant system.
However, under pathological conditions such as diabetes, oxidative
stress balance shifts towards a pro-oxidant state that accelerates
tissue injury, and the kidney has been shown to be a target of
oxidative stress-mediated tissue damage (14). As in our study, induction of
diabetes resulted in a marked increase in the SOD levels in the
renal cortex of diabetic rats. The MDA levels were also found to be
significantly reduced. Therefore, oxidative stress is a potential
mechanism for diabetic nephropathy, since it promotes the formation
of lipid peroxidation products and decreases the antioxidant
defense by decreasing the level of antioxidant enzymes.
Accordingly, therapeutic strategies with anti-oxidant properties
may eliminate the manifestations associated with diabetic
nephropathy. In our study, FXST down-regulated the level of
oxidative stress in the renal cortices of diabetic rats. Therefore,
FXST may be a novel strategy with which to slow the progression of
renal disease.
One of the components of FXST, SanQi, attenuated the
high level of oxidative stress in the rat liver in a model of
alcoholic fatty liver disease and showed an anti-oxidative effect
in the serum of diabetic rats (15,16).
Another component of FXST, Danshen, improved antioxidation of the
patients with acute coronary syndromes following percutaneous
transluminal coronary intervention (17). Danshen reduced the level of MDA and
enhanced the level of SOD in cerebral tissues in a focal cerebral
ischemia rat model (18). The
third component of FXST, Huangqi, elevated the activity of SOD and
reduced MDA in patients with primary nephrotic syndrome (19). In an in vivo study, Huangqi
antagonized hydrogen peroxide (H2O2)-induced
oxidative injury in cardiomyocytes (20). Polyphenols in Xuanshen, the fourth
ingredient of FXST, also possess high antioxidant activity
(21). Therefore, the anti-oxidant
effect of FXST may be due to the anti-oxidant properties of all of
its active ingredients. These ingredients, when combined together,
exhibit optimal activity in anti-oxidative stress (3).
Under normal circumstances, oxidative stress is
counteracted by antioxidant enzymes such as SOD, which normally
scavenges superoxide. The role of SOD is crucial to the regulation
of oxidative stress in diabetes. Enhanced activity of antioxidant
enzymes has been reported as an adaptive mechanism to protect cells
against the toxicity of free radicals. The decreased SOD in the
untreated diabetic rats may indicate that the protective ability in
response to elevated levels of oxidative stress was impaired.
Increased SOD activity induced by FXST may be a response to
increased generation of superoxide anions in diabetes and may
therefore result in the amelioration of oxidative stress. MDA, an
end-product of lipid peroxidation and a measure of free radical
generation, reflects the impact of oxidative stress in cells and
tissues. In the present study, renal cortical MDA concentrations in
diabetic rats were significantly elevated. This is consistent with
previously studies. The increased MDA levels indicate the
occurrence of lipid oxidative damage, which is suggested in the
development of diabetic nephropathy. Treatment with FXST
significantly reduced the levels of MDA. These results indicate
that FXST exhibits an anti-peroxidative effect. Taken together, our
results have shown that SOD activity increased, whereas MDA
activity was reduced in the renal cortex of FXST-treatment diabetic
rats.
Oxidative stress is well known as an important
factor in the progression of diabetic complications, is involved in
molecular changes associated with exacerbation of renal injury, and
results in mesangial expansion and increased extracellular matrix
deposition (22). Therefore, as
shown in our study, the anti-oxidant activity of FXST eliminated
the pathological abnormalities of diabetic nephropathy, thereby
reducing urinary protein excretion, which was a marker for the
development of nephropathy in diabetes. Therefore, FXST may arrest
the progression of renal disease. Moreover, the renoprotective
activity of FXST was mediated by modulation of oxidative stress in
diabetic nephropathy.
In conclusion, Fufang Xue Shuan Tong capsule, a
traditional Chinese medicine, delayed the development of
proteinuria, reduced creatinine clearance, attenuated the
pathological abnormalities of diabetic nephropathy, thereby showing
predominant kidney-protective action. This finding may be
attributable to the fact that the increased oxidative stress in the
kidney cortex of diabetic rats was antagonized by FXST. The
protective role of FXST is not inferior to that of captopril, one
of most commonly used drugs for the treatment of diabetic
nephropathy.
Acknowledgements
This study was supported by the
Natural Science Foundation of China (Nos. 81070659 and 81001190);
the Research Fund for the Doctoral Program of Higher Education of
China (No. 2009171110054); the Natural Science Foundation of
Guangdong Province of China (No. 1251008901000030); the Science and
Technique Research Project of Guangzhou Municipality, Guangdong
Province, China (No. 2010J-E521); the Foundation for the Author of
Excellent Doctoral Dissertation of Guangdong Province, China (No.
80000-3226201); and the Yat-Sen outstanding innovative
postgraduates and supervisors training program of Sun Yat-Sen
University (No. 80000-3126200-211).
References
|
1
|
American Diabetes Assocation: Standards of
medical care in diabetes – 2011. Diabetes Care. 34(Suppl 1): 11–61.
2011.
|
|
2
|
Zhang J, Huang Q, Liu Y, et al: Effect of
complex dribbing-pill of xue shuan tong on thrombus formation and
microcirculation in rat. Zhong Yao Cai. 26:881–882. 2003.PubMed/NCBI
|
|
3
|
Ye XF and Xu GZ: Effect of Fufang Xue
Shuan Tong on retinal oxidative stress in diabetic rats. Chin J
Oeul Fundus Dis. 26:176–178. 2010.
|
|
4
|
Skrha J, Hodinar A, Kvasnicka J and
Hilgertova J: Relationship of oxidative stress and fibrinolysis in
diabetes mellitus. Diabet Med. 13:800–805. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Mattia G, Bravi MC, Laurenti O, et al:
Reduction of oxidative stress by oral N-acetyl-L-cysteine treatment
decreases plasma soluble vascular cell adhesion molecule-1
concentrations in non-obese, non-dyslipidaemic, normotensive,
patients with non-insulin-dependent diabetes. Diabetologia.
41:1392–1396. 1998.
|
|
6
|
Ihara Y, Toyokuni S, Uchida K, et al:
Hyperglycemia causes oxidative stress in pancreatic beta-cells of
GK rats, a model of type 2 diabetes. Diabetes. 48:927–932. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan HZ, Zhang L, Guo MY, et al: The
oxidative stress status in diabetes mellitus and diabetic
nephropathy. Acta Diabetol. 47:71–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nam SM, Lee MY, Koh JH, et al: Effects of
NADPH oxidase inhibitor on diabetic nephropathy in OLETF rats: the
role of reducing oxidative stress in its protective property.
Diabetes Res Clin Pract. 83:176–182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baynes JW: Role of oxidative stress in
development of complications in diabetes. Diabetes. 40:405–412.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okada S, Shikata K, Matsuda M, et al:
Intercellular adhesion molecule-1-deficient mice are resistant
against renal injury after induction of diabetes. Diabetes.
52:2586–2593. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ota T, Takamura T, Ando H, Nohara E,
Yamashita H and Kobayashi K: Preventive effect of cerivastatin on
diabetic nephropathy through suppression of glomerular macrophage
recruitment in a rat model. Diabetologia. 46:843–851. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paoletti F, Mocali A and Aldinucci D:
Superoxide-driven NAD(P)H oxidation induced by EDTA-manganese
complex and mercaptoethanol. Chem Biol Interact. 76:3–18. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agardh CD, Stenram U, Torffvit O and
Agardh E: Effects of inhibition of glycation and oxidative stress
on the development of diabetic nephropathy in rats. J Diabetes
Complications. 16:395–400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen ZY, Yan MX, Cai DL, He BH, Chen H and
Liu QS: Effect of SanQi on alcoholic fatty liver disease. Chin J
Trad Chin Med Pharm. 21:614–616. 2006.
|
|
16
|
Sun W, Feng LY, Zhao ZJ, Liu TH and Yang
MJ: Study on antioxidant effects and inhibition of podocyte
apoptosis of PNS on DN rat. Chin J Trad Chin Med Pharm.
26:1062–1067. 2011.
|
|
17
|
Chen JX, Li AY, Wang ZY and Liu HX: Impact
of Danshen powder injection on oxidative stress after the
intervention in the patients with acute coronary syndromes of blood
stasis type. World J Integr Trad West Med. 6:122–124. 2011.
|
|
18
|
Liu C, Min LQ, Ji ZS, Wang Q, Jia YJ and
Li SY: Protective effects of salvia miltiorrhizae on
oxidative stress in rats with focal cerebral ischemia. Chin J Clin
Rehabil. 10:37–39. 2006.
|
|
19
|
Mo ZY, Liang D, Shitu Y, Huang PP and Chen
XW: Effect of astragalus on oxidative stress status in primary
nephrotic syndrome. Chin J Integr Trad Western Nephrol. 5:209–211.
2004.
|
|
20
|
Guan FY, Li H, Yu XX and Yang XJ: Effects
of astragalus injection on myocardial cell damages due to oxidative
stress. Chin J Rehabil Theory Pract. 16:830–832. 2010.
|
|
21
|
Liu ZJ, Li L, Wang J, Yan J and Liu CM:
Antioxidant activities of polyphenol from Scrophularia
ningpoensis Hemsl. Lishizhen Med Materia Medica Res.
21:796–798. 2010.
|
|
22
|
Shah IM, Mackay SP and McKay GA:
Therapeutic strategies in the treatment of diabetic nephropathy – a
translational medicine approach. Curr Med Chem. 16:997–1016.
2009.
|