Introduction
Esophageal cancer (EC) ranks as the eighth most
common malignancy and the sixth most common cancer-related cause of
mortality worldwide, which is characterized by a high incidence and
a striking worldwide geographic variation. In fact there exists a
so-called ‘Asian esophageal cancer belt’, an area that stretches
from the Caucasian mountains across Northern Iran, Afghanistan,
Kazakhstan, Uzbekistan and Turkmenistan, into northern China, and
China, Iran and Japan have the highest rate of EC in the world
(1–3). These high incidence areas are
associated with poverty or poverty-related diseases, and current
evidence also reveals that the incidence of EC tends to decrease
when wealth and accessibility to health care increases (4,5).
However, the cause and pathogenesis of EC remains
unclear. Histopathologically, EC is classified into two main types:
esophageal squamous cell carcinoma (ESCC) and esophageal
adenocarcinoma (EAC). A higher incidence rate of EAC occurs in
Western countries, while ESCC occurs more often in Oriental
countries, particularly in Mainland China (1,2,6). The
possible risk factors for developing EC have been reported to be
alcohol consumption, smoking, coffee consumption, low socioeconomic
status, poor oral health, hot drinks, high consumption of
nitrosamines, diet deficient in antioxidants and other
environmental factors (1–3,5,7,8).
Recent studies reveal that genetic alterations are also considered
as new risk factors for EC (9,10).
Numerous studies have shown that the cytochrome P450
superfamily are catalyzing enzymes for carcinogens. Cytochrome P450
2E1 (CYP2E1), a member of the CYP450 superfamily and an
ethanol-inducible enzyme, is involved in the metabolic activation
of numerous low molecular weight compounds, including
N-nitrosamines, aniline, vinyl chloride and urethane (11–14).
RsaI/PstI polymorphisms in the promoter gene region
are believed to affect the transcriptional activity of the gene,
and occur as a wild-type homozygous genotype (c1/c1), a
heterozygous genotype (c1/c2) and a variant homozygous rare
genotype (c2/c2), and the frequency of the variant c1 allele was
observed to be much higher in patients with esophageal diseases
than that of healthy individuals (15–17).
The first study on the association between EC and
the CYP2E1 RsaI/PstI polymorphism was conducted in
1996, but the result failed to reveal a significant association.
The second study in Japan also failed to identify a significant
difference between healthy controls and patients with EC (18). However, certain subsequent studies
on Chinese individuals indicated that a significant association
exists between the CYP2E1 RsaI/PstI polymorphism and
the risk of EC, yet certain studies revealed different or even
contradictory findings (16,17,19–33).
A previous meta-analysis was carried out concerning
EC and the CYP2E1 RsaI/PstI polymorphism, of which 11
published studies demonstrated that the CYP2E1
RsaI/PstI c2 allele may be a protective factor for EC
among Asian populations (34). In
the included 7 Chinese studies, the authors only searched PubMed
and did not categorize subgroups according to the source of
controls, which makes their studies unsuitable for reference for
mainland Chinese populations. Therefore, we conducted a
meta-analysis of case-control studies in order to review and
summarize the epidemiological evidence, and aimed to precisely
detect the correlation between the CYP2E1 RsaI/PstI
polymorphism and the risk of EC among Chinese individuals.
Materials and methods
Literature search
Strictly following the proposed Preferred Reporting
Items for Systematic Reviews and Meta-Analyses (PRISMA) (35) guidelines, our study aims to report
the present review and meta-analysis systematically. Initially, we
identified published and unpublished studies which tested the
association between the CYP2E1 RsaI/PstI polymorphism
and risk of EC by searching the following databases from their
creation until January 10th, 2012: PubMed, EMBASE, Web of Science,
the Chinese Biological Medicine Database (CBM), China National
Knowledge Infrastructure (CNKI) and the Chinese scientific
periodical database of VIP information (VIP). Terms used in the
search were as follows: i) esophag* and
oesophag*; ii) cancer, carcinoma, adenocarcinoma,
neoplasm, neoplasia and neoplastic; iii) cytochrome p450 2E1,
cytochrome p450II1, CYP2E1, CYPIIE1; iv) polymorphism; v) China and
Chinese, without restrictions. In addition, we also reviewed the
reference lists of retrieved manuscripts and recent reviews.
Study selection
We included any study that met with the following
criteria: i) case-control study design; ii) association between
CYP2E1 RsaI/PstI polymorphism and risk of EC was
investigated; iii) diagnosis of ESCC and EAC were either
histologically, pathologically or cytologically confirmed; and iv)
the odds ratios (ORs) and the corresponding 95% confidence
intervals (CIs), or the number of events required to calculate them
were reported. Two investigators independently evaluated the
eligibility of all studies retrieved from the databases on the
basis of the predetermined selection criteria. Disagreements were
resolved by discussion or in consultation with a third
investigator.
Data extraction
Two reviewers independently extracted data for the
study characteristics by using a standardized data-collection form.
Data were recorded as follows: first author’s last name, year of
publication, place of origin, study period and duration of
follow-up, characteristics of cancer cases, source of controls,
matching criteria, number of cases and controls, number of
different genotypes in cases and controls, Hardy-Weinberg
equilibrium (HWE) and minor allele frequency in controls. Any
disagreements were resolved by consensus.
Statistical analysis
We computed a pooled OR and 95% CI for the risk
allele using the Comprehensive Meta Analysis software (version 2.1)
to generate forest plots to determine whether there was a
statistical association between cases and controls and to assess
heterogeneity of the included studies. HWE was tested by a
Chi-square test at a significance level of P<0.05. Heterogeneity
was quantifiably evaluated using the Chi-square based Cochran’s Q
statistic (36) and the
I2 statistic, which yields results ranging from 0 to
100% (I2=0–25%, no heterogeneity; I2=25–50%,
moderate heterogeneity; I2=50–75%, large heterogeneity;
I2=75–100%, extreme heterogeneity) (37). If heterogeneity existed, the
random-effects model was used, otherwise, the fixed-effects model
was used. In addition, we investigated the influence of a single
study to the overall risk estimate by removing each study in turn
to test the robustness of the main results. Subgroup analysis was
also conducted if significant heterogeneity was identified (such as
Han individuals vs. ethnic minorities). If possible, potential
publication bias was assessed by visual inspection of the funnel
plots of the primary outcome (38).
Results
Identification of eligible studies
Of the 108 records retrieved initially, 17 studies
including 18 trails were identified for the association between the
CYP2E1 RsaI/PstI polymorphism and risk of EC,
including a total of 1,663 cases and 2,603 controls (16,17,19–33).
A flow chart for the study selection process is presented in
Fig. 1. Duplicates (the same study
searched from different databases) were excluded using the Endnote
X3 software.
Characteristics of the studies
The detailed characteristics of the included studies
are summarized in Table I. Of
these studies, 7 were published in English (16,17,19,22,28–30)
and 7 in Chinese (21,23–25,27,31,33),
2 were doctoral dissertations (20,32)
and 1 was a master’s thesis both in English and Chinese (26). The sample sizes ranged from 45 to
480. All of the cases were histologically, pathologically or
cytologically confirmed as EC. Of the cases, 6 studies clearly
confirmed the presence of ESCC (17,19,28,29,31,32),
while one had both ESCC and EAC (16). Controls were mainly healthy
populations and matched according to age, gender or were
cancer-free tissues. Of the controls 6 were hospital-based (HB)
(20,23–25,27,31),
10 were population-based (PB) (16,17,19,21,22,26,28,29,32,33)
and one was both (30). There were
two groups of Kazakhs (Xinjiang Province) (19,30)
and 16 groups of Han individuals (16,17,20–29,31–33).
The genotypes were analyzed by polymerase chain
reaction-restriction fragment length polymorphism (PCR-RFLP);
genotype distributions in the controls of all studies were in
accordance with HWE, with the exception of 4 studies (17,19,23,29).
 | Table ICharacteristics of the studies
included in this meta-analysis. |
Table I
Characteristics of the studies
included in this meta-analysis.
| Author (Refs.) | Site | Group | Source | Sample | Genotype
| Genotyping
method | P-value (HWE) for
controls | Adjustment |
|---|
| c1/c1 | c1/c2 | c2/c2 |
|---|
| Lin et al
(16) | Linxian County,
Henan Province | T | ESCC and EAC | 45 | 36 | 6 | 3 | PCR-RFLP | 0.345 | Age, gender |
| C | PB | 45 | 20 | 22 | 3 |
| Tan et al
(17) | Linxian County,
Henan Province | T | ESCC | 150 | 107 | 31 | 12 | PCR-RFLP | 0.009 | Age, gender,
smoking |
| C | PB | 150 | 66 | 77 | 7 |
| Shi (20) | Linzhou City, Henan
Province | T | EC | 116 | 74 | 37 | 5 | PCR-RFLP | 0.922 | Age, gender,
smoking, fermented vegetable |
| C | HB | 106 | 46 | 48 | 12 |
| Xi’an City, Shaanxi
Province | T | EC | 71 | 39 | 28 | 4 | PCR-RFLP | 0.368 | |
| C | HB | 62 | 30 | 24 | 8 |
| Gao et al
(21) | Huai’an City,
Jiangsu Province | T | EC | 144 | 83 | 44 | 13 | PCR-RFLP | 0.525 | Age, gender, tea,
smoking, alcohol |
| C | PB | 233 | 140 | 75 | 14 |
| Shi et al
(23) | Wuhan City, Hubei
Province | T | EC | 98 | 72 | 19 | 7 | PCR-RFLP | 0.039 | Age, gender |
| C | HB | 120 | 54 | 45 | 21 |
| Gao et al
(22) | Huai’an City,
Jiangsu Province | T | EC | 93 | 55 | 31 | 7 | PCR-RFLP | 0.20 | Age, gender,
smoking, drinking, dietary habits |
| C | PB | 196 | 121 | 62 | 13 |
| Shi et al
(24) | Nanjing City,
Jiangsu Province | T | EC | 78 | 48 | 24 | 6 | PCR-RFLP | 0.141 | Age, gender,
smoking, alcohol |
| C | HB | 118 | 60 | 42 | 16 |
| Yin et al
(25) | Huai’an City,
Jiangsu Province | T | EC | 106 | 52 | 49 | 5 | PCR-RFLP | 0.411 | Age, gender |
| C | HB | 106 | 55 | 45 | 6 |
| Lu et al
(19) | Kazakh, Xinjiang
Autonomous Region | T | ESCC | 104 | 81 | 20 | 3 | PCR-RFLP | <0.001 | Age, gender |
| C | PB | 104 | 25 | 74 | 5 |
| Li (26) | Taiyuan City,
Shanxi Province | T | EC | 60 | 32 | 18 | 10 | PCR-RFLP | 0.781 | Age, gender,
smoking, drinking habits |
| C | PB | 199 | 101 | 69 | 29 |
| Liu et al
(28) | Huai’an City,
Jiangsu Province | T | ESCC | 77 | 34 | 33 | 10 | PCR-RFLP | 0.91 | Age, gender |
| C | PB | 79 | 45 | 29 | 5 |
| Dong et al
(27) | Gansu Province | T | EC | 120 | 84 | 26 | 10 | PCR-RFLP | 0.428 | Age, gender |
| C | HB | 120 | 57 | 44 | 19 |
| Xia et al
(31) | Jingjiang City,
Jiangsu Province | T | ESCC | 45 | 30 | 12 | 3 | PCR-RFLP | 0.708 | Not reported |
| C | HB | 45 | 26 | 17 | 2 |
| Qin et al
(30) | Kazakh, Xinjiang
Autonomous Region | T | EC | 120 | 94 | 23 | 3 | PCR-RFLP | 0.29 | Age, gender,
dietary habits |
| C | PB and HB | 240 | 128 | 90 | 22 |
| Guo et al
(29) | Lanzhou City, Gansu
Province | T | ESCC | 80 | 57 | 16 | 7 | PCR-RFLP | <0.001 | Age, gender |
| C | PB | 480 | 225 | 180 | 75 |
| Yang (32) | Feicheng City,
Shandong Province | T | ESCC | 27 | 19 | 8 | 0 | PCR-RFLP | 0.442 | Age, gender,
alcohol, education, smoking |
| C | PB | 44 | 31 | 11 | 2 |
| Zhang and Wu
(33) | Gansu Province | T | EC | 129 | 75 | 40 | 14 | PCR-RFLP | 0.443 | Age, gender |
| C | PB | 156 | 70 | 56 | 21 |
Meta-analyses
The main results of the heterogeneity test and
meta-analysis are listed in Table
II.
 | Table IIMain results of the heterogeneity
test and subgroups meta-analyses. |
Table II
Main results of the heterogeneity
test and subgroups meta-analyses.
| | Heterogeneity
| Meta-analyses
|
|---|
| Genetic model | Study or
subgroup | P-value | I2
(%) | OR (95% CI) | P-value |
|---|
| c2 vs. c1 | Total | | <0.001 | 80 | 0.64
(0.50–0.81) | 0.0003 |
| Ethnicity | Han | <0.001 | 72 | 0.71
(0.57–0.89) | 0.002 |
| Kazakh | 0.12 | 58 | 0.28
(0.17–0.46) | <0.001 |
| Source of
control | PB | <0.001 | 85 | 0.65
(0.45–0.53) | 0.02 |
| HB | 0.02 | 59 | 0.62
(0.46–0.82) | 0.0007 |
| Both | Single study | 0.35
(0.23–0.55) | <0.001 |
| Tumor type | EC | <0.001 | 76 | 0.69
(0.53–0.89) | 0.005 |
| ESCC | <0.001 | 84 | 0.56
(0.33–0.93) | 0.03 |
| c2/c2 vs.
c1/c1 | Total | | 0.03 | 42 | 0.70
(0.56–0.89) | 0.003 |
| Ethnicity | Han | 0.04 | 42 | 0.75
(0.59–0.95) | 0.02 |
| Kazakh | 0.35 | 0 | 0.32
(0.13–0.80) | 0.01 |
| Source of
control | PB | 0.30 | 16 | 1.02
(0.76–1.38) | 0.89 |
| HB | 0.69 | 0 | 0.44
(0.30–0.66) | <0.001 |
| Both | Single study | 0.23
(0.07–0.80) | 0.02 |
| Tumor type | EC | 0.05 | 46 | 0.63
(0.48–0.82) | 0.0008 |
| ESCC | 0.19 | 31 | 0.94
(0.61–1.46) | 0.80 |
| c1/c2 vs.
c1/c1 | Total | | <0.001 | 81 | 0.54
(0.38–0.75) | 0.0003 |
| Ethnicity | Han | <0.001 | 72 | 0.62
(0.46–0.84) | 0.002 |
| Kazakh | 0.002 | 90 | 0.18
(0.05–0.68) | 0.01 |
| Source of
control | PB | <0.001 | 88 | 0.52
(0.30–0.92) | 0.02 |
| HB | 0.05 | 53 | 0.59
(0.42–0.85) | 0.004 |
| Both | Single study | 0.35
(0.20–0.59) | <0.001 |
| Tumor type | EC | 0.004 | 62 | 0.66
(0.50–0.88) | 0.004 |
| ESCC | <0.001 | 87 | 0.38
(0.18–0.82) | 0.01 |
| c2/c2 vs.
c1/c1+c1/c2 | Total | | 0.12 | 29 | 0.73
(0.58–0.92) | 0.008 |
| Ethnicity | Han | 0.15 | 27 | 0.78
(0.61–0.99) | 0.04 |
| Kazakh | 0.38 | 0 | 0.34
(0.13–0.85) | 0.02 |
| Source of
control | PB | 0.47 | 0 | 1.03
(0.76–1.38) | 0.54 |
| HB | 0.8 | 0 | 0.49
(0.33–0.72) | 0.0003 |
| Both | Single study | 0.25
(0.07–0.87) | 0.03 |
| Tumor type | EC | 0.14 | 33 | 0.66
(0.50–0.87) | 0.003 |
| ESCC | 0.31 | 16 | 0.96
(0.62–1.49) | 0.87 |
| c1/c2+c2/c2 vs.
c1/c1 | Total | | <0.001 | 85 | 0.48
(0.34–0.70) | 0.0001 |
| Ethnicity | Han | <0.001 | 81 | 0.56
(0.40–0.79) | 0.001 |
| Kazakh | 0.003 | 85 | 0.17
(0.05–0.59) | 0.009 |
| Source of
control | PB | <0.001 | 88 | 0.56
(0.33–0.95) | 0.03 |
| HB | <0.001 | 82 | 0.42
(0.23–0.75) | 0.004 |
| Both | Single study | 0.32
(0.19–0.52) | <0.001 |
| Tumor type | EC | <0.001 | 82 | 0.55
(0.37–0.82) | 0.003 |
| ESCC | <0.001 | 87 | 0.41
(0.21–0.85) | 0.02 |
Our meta-analyses gave a significant association of
the CYP2E1 RsaI/PstI polymorphism with EC risk [for
the allele contrast c2 vs. c1: OR=0.64; 95% CI, 0.50–0.81;
P<0.001 (Fig. 2); for c2/c2 vs.
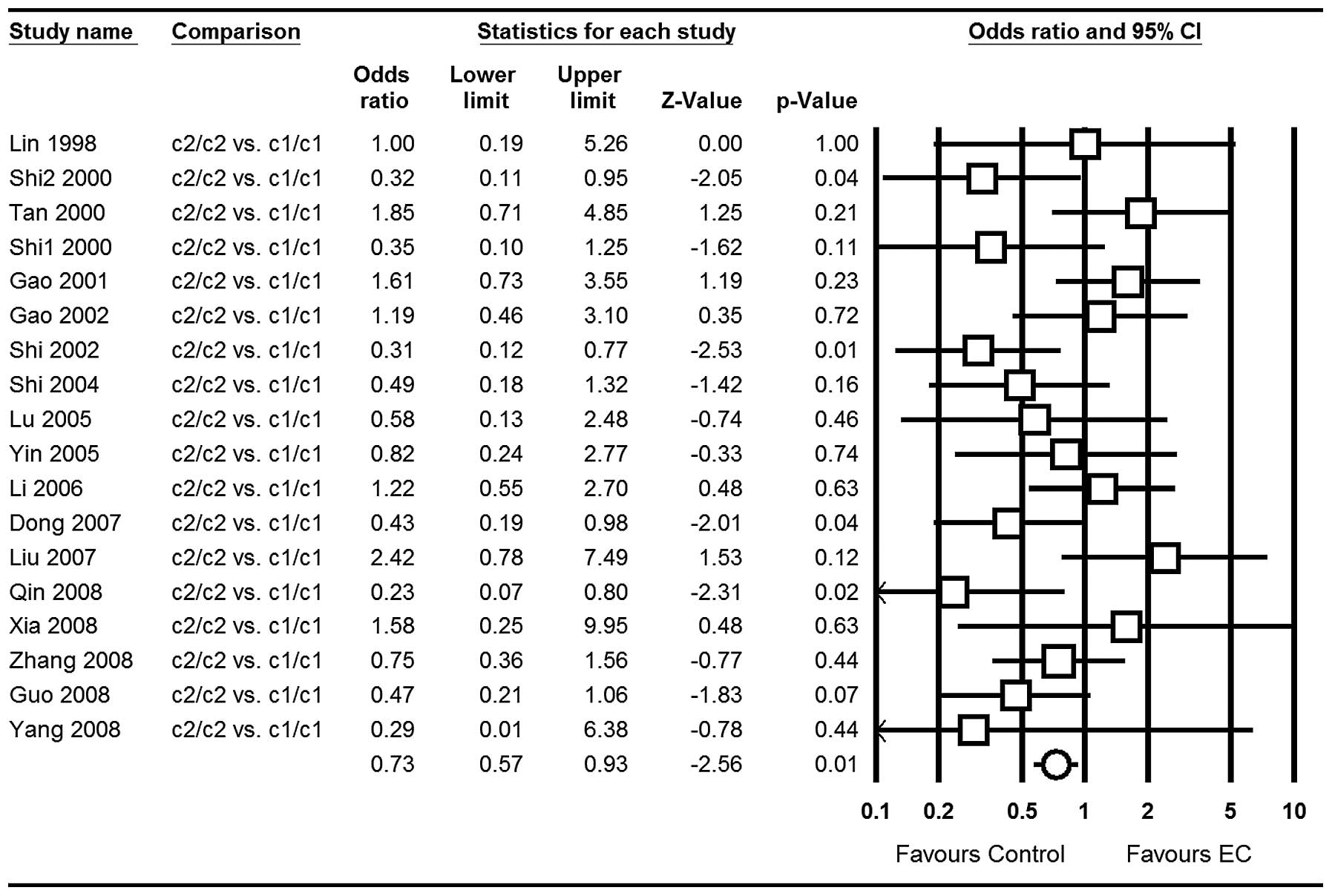
c1/c1: OR=0.73; 95% CI, 0.57–0.93; P=0.01 (Fig. 3); for c2/c2 vs. c1/c1+c1/c2:
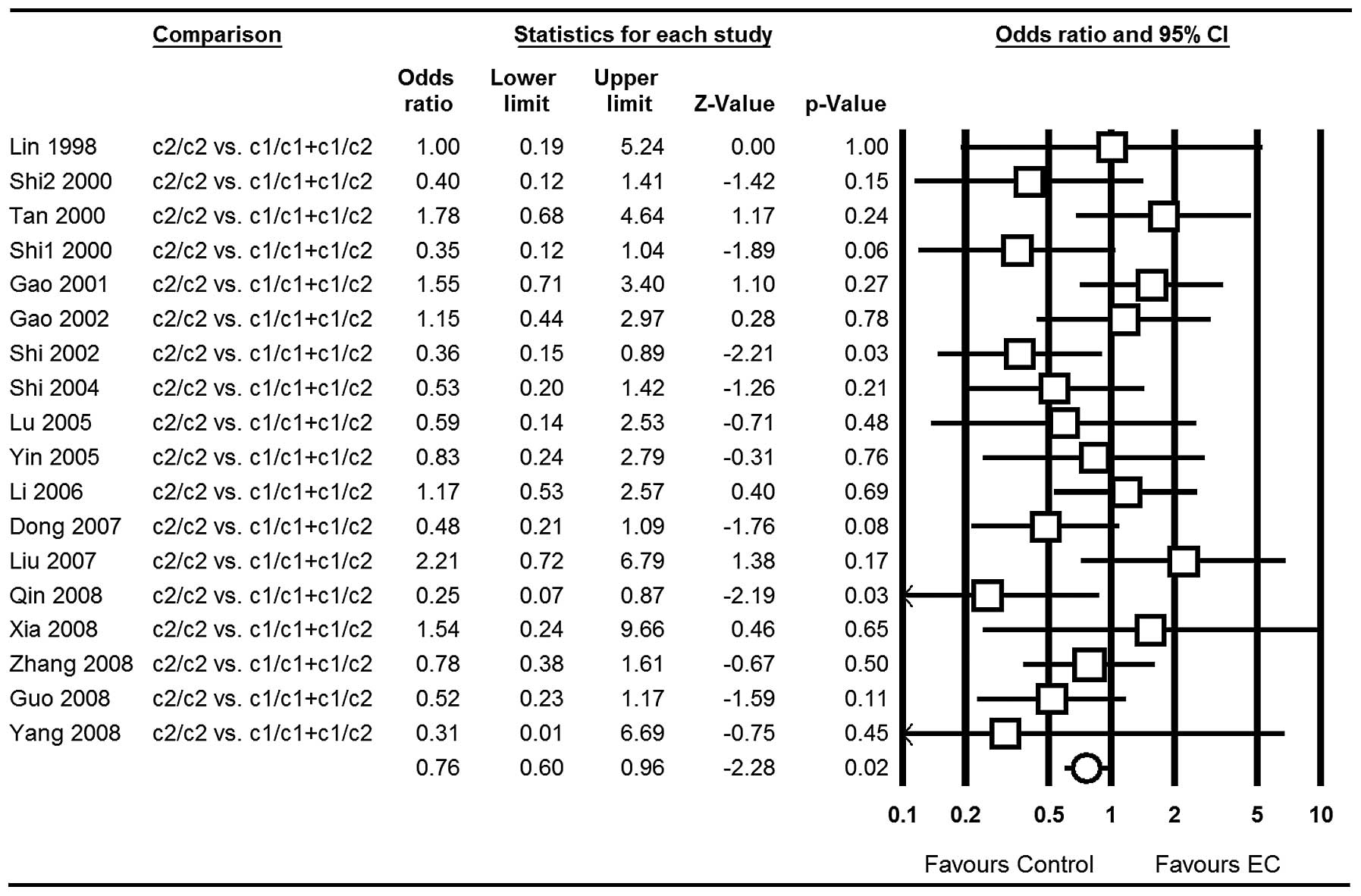
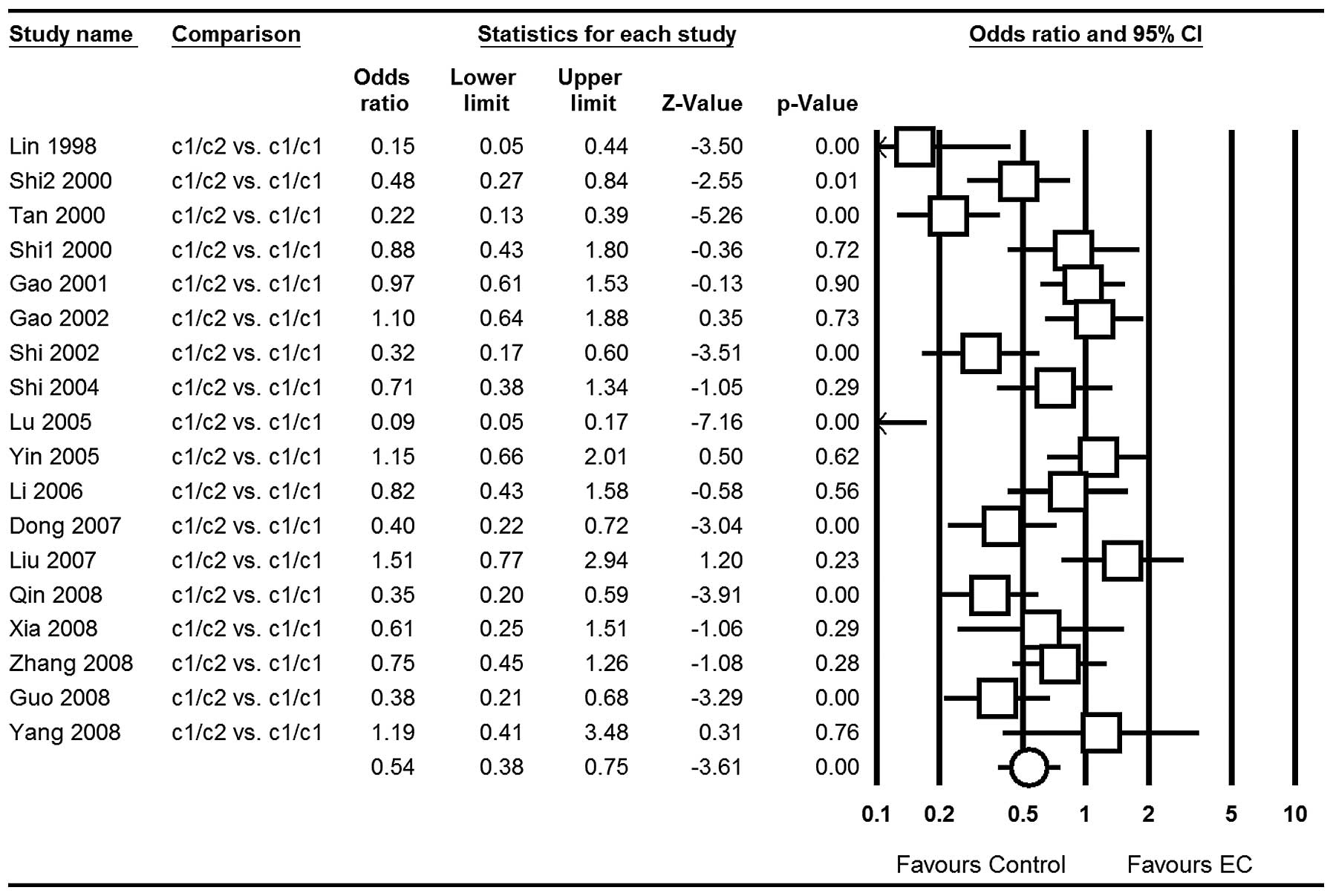
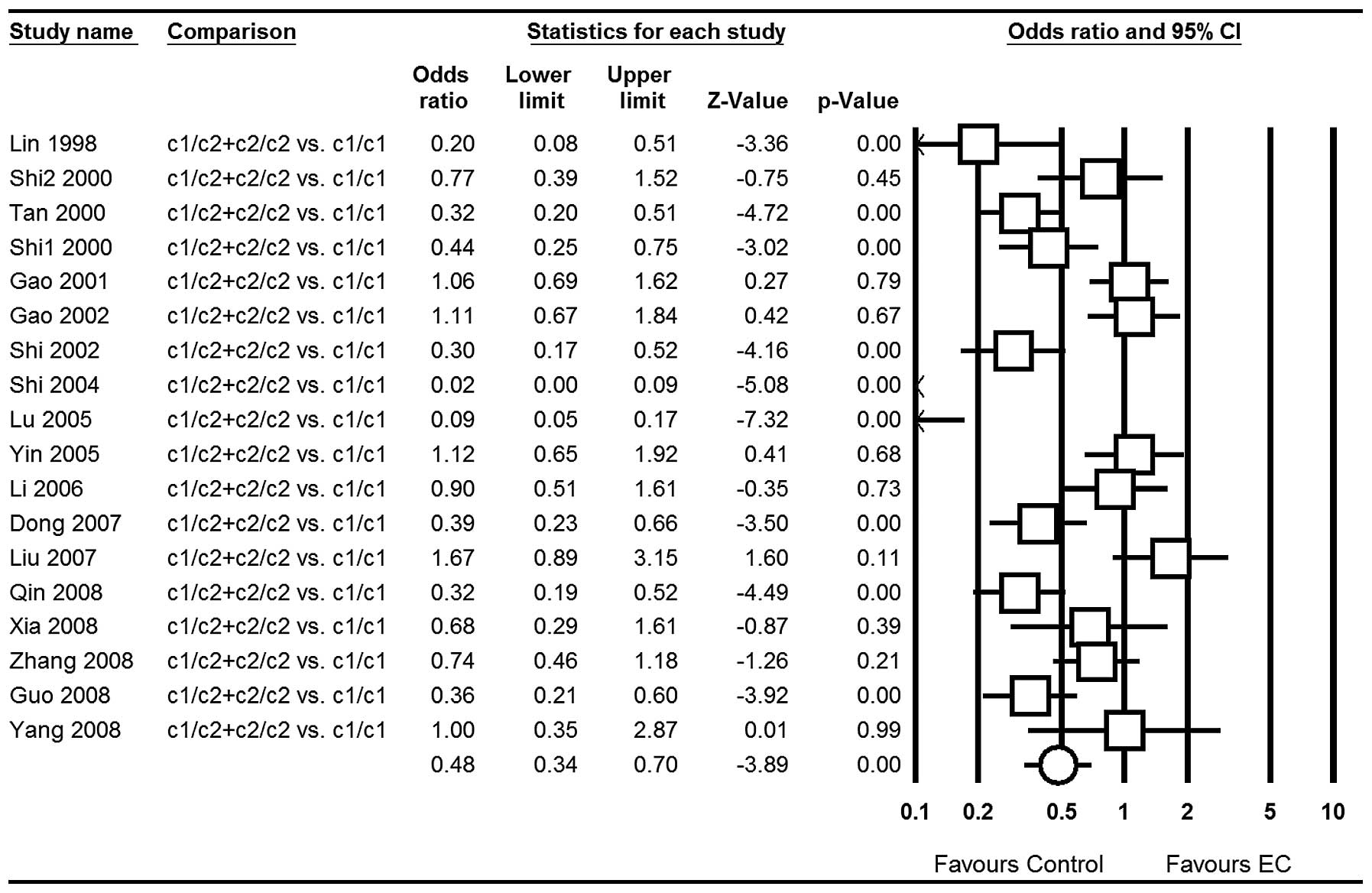
OR=0.76; 95% CI, 0.60–0.96; P=0.02 (Fig. 4); for c1/c2 vs. c1/c1: OR=0.54; 95%
CI, 0.38–0.75; P<0.001 (Fig.
5); for the dominant model c1/c2+c2/c2 vs. c1/c1: OR=0.48; 95%
CI, 0.34–0.70; P<0.001 (Fig.
6)] in total populations.
When stratified by ethnicity, all genetic models
also produced statistically significant results. When studies were
stratified for control source, an association was detected for all
genetic models, with the exception of PB in c2/c2 vs. c1/c1 and
c2/c2 vs. c1/c1+c1/c2 (both P>0.05). In the stratified analysis
by tumor type, the results were similar to the control source; the
ESCC type showed no association in c2/c2 vs. c1/c1 and c2/c2 vs.
c1/c1+c1/c2 (both P>0.05).
Sensitivity analysis
The majority of studies indicated that the frequency
distributions of genotypes in the controls were in accordance with
HWE, whereas deviations from HWE were observed in 4 studies of the
PstI/RsaI polymorphism (all P<0.05) (17,19,23,29).
However, the corresponding pooled ORs were not substantially
altered whether or not these studies were included (Table III). In addition, sensitivity
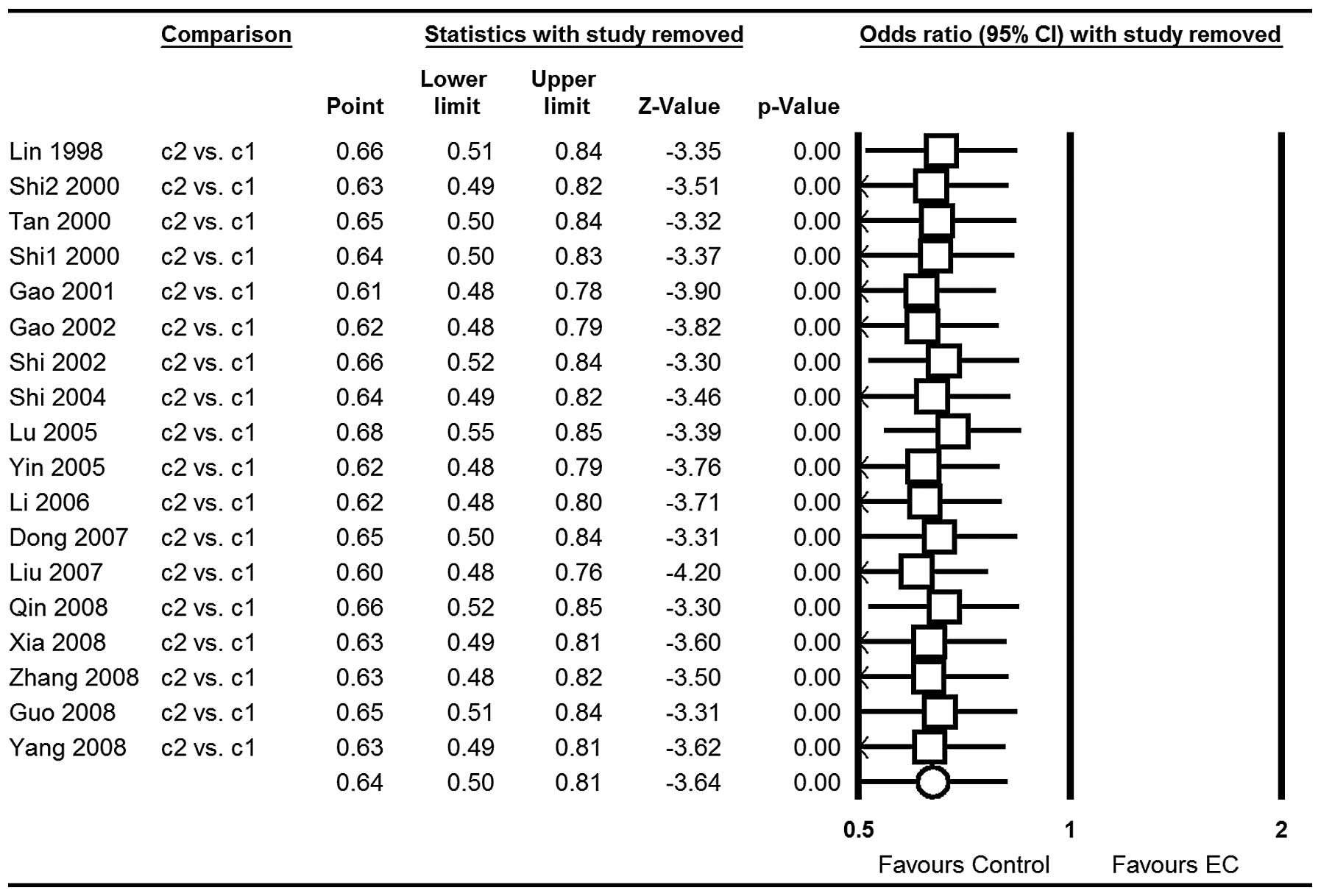
analysis indicated that no single study influenced the pooled OR
qualitatively and this suggests the stability of the result (c2 vs.
c1 for example; Fig. 7).
 | Table IIISensitivity analysis after
elimination of the studies where frequency distributions of
genotypes in the controls were inconsistent with HWE. |
Table III
Sensitivity analysis after
elimination of the studies where frequency distributions of
genotypes in the controls were inconsistent with HWE.
| | Heterogeneity
| Meta-analyses
|
|---|
| Genetic model | Subgroup | P-value | I2
(%) | OR (95% CI) | P-value | Model |
|---|
| c2 vs. c1 | Total | <0.001 | 80 | 0.64 (0.50,
0.81) | 0.0003 | Random |
| AE | <0.001 | 71 | 0.76 (0.60,
0.96) | 0.02 | Random |
| c2/c2 vs.
c1/c1 | Total | 0.03 | 42 | 0.70 (0.56,
0.89) | 0.003 | Fixed |
| AE | 0.08 | 37 | 0.75 (0.57,
0.98) | 0.03 | Fixed |
| c1/c2 vs.
c1/c1 | Total | <0.001 | 81 | 0.54 (0.38,
0.75) | 0.0003 | Random |
| AE | 0.001 | 62 | 0.71 (0.54,
0.93) | 0.01 | Random |
| c2/c2 vs.
c1/c1+c1/c2 | Total | 0.12 | 29 | 0.73 (0.58,
0.92) | 0.008 | Fixed |
| AE | 0.19 | 24 | 0.77 (0.59,
1.00) | 0.05 | Fixed |
| c1/c2+c2/c2 vs.
c1/c1 | Total | <0.001 | 85 | 0.48 (0.34,
0.70) | 0.0001 | Random |
| AE | <0.001 | 80 | 0.62 (0.43,
0.89) | 0.01 | Random |
Publication bias
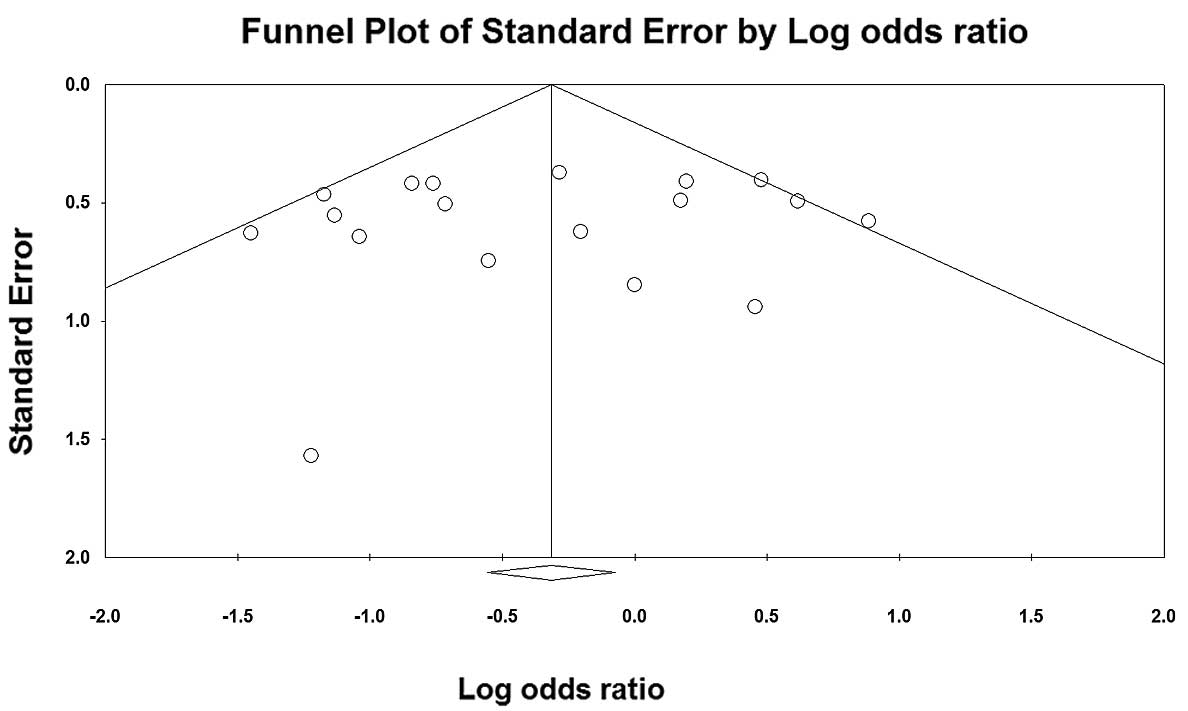
Funnel plot based on c2/c2 vs. c1/c1 (the genetic
model was pooled using a fixed-effects model) was chosen to assess
publication bias. The symmetrical shape of the funnel plots
(Fig. 8) implied that slight bias
of the studies occurred.
Discussion
EC is an increasingly common cancer with a poor
prognosis, which is likely to be caused by multi-factors, including
environmental risk and genetic factors (3,7–9). In
recent years, environmental and genetic susceptivity, and their
interactions, were used to evaluate the risks of EC, but the
results were inconsistent and this difference may be due to
geographical distribution. Environmental risk factors were once
regarded as the major cause of EC, but an epidemiological study on
immigrants moving to Changzhi City in Shanxi Province after a
century of residing in Linzhou City in Henan Province (>200 km
apart and the environment is different), observed that the
detection rate of ESCC in the immigrant population was similar to
that of the residents in Linzhou City. It indicated that changes in
environment and time do not affect the incidence rate of ESCC,
since genetic factors play an important role (39).
Unlike other factors, the positive association
between family history of EC risk is consistent with previous
studies, which suggests a genetic susceptibility in EC pathogenesis
(40–50). A meta-analysis in 2003 involved 13
studies to evaluate risk factors of EC in China and revealed that
family history was a significant factor (OR=4.0; 95% CI, 2.29–6.99)
(51). The majority of studies
concluded that this is caused by various hereditary
susceptibilities to gene-related tumors. As for genetic
susceptibility, it has been reported that gene polymorphisms of
metholenetetrahydrofolate reductase (MTHFR) (52), cytochrome P450 1A1 (CYP1A1)
(52), glutathione S-transferase
M1 (GSTM1) (52), GSTT1 (53), CYP2A6 (54), CYP2E1 (34), human 8-oxoguanine glycosylase 1
(hOGG1) (55), X-ray repair
cross-complementing group 1 (XRCC1) (56), xeroderma pigmentosum group D (XPD)
(56), p53 (57) and others, are correlated with
EC.
The CYP2E1 gene that encodes the CYP2E1 enzyme has
been mapped to chromosome 10q24.3-qter, and is an important member
of the cytochrome P450 superfamily. CYP2E1 is a naturally
ethanol-inducible enzyme mainly involved in the metabolic
activation of low molecular weight compounds, such as
N-nitrosamines and in alcohol metabolism (11–14).
The RsaI/PstI polymorphisms in the promoter gene
region are reported to affect the transcriptional activity of the
gene. Numerous large-sample and unbiased epidemiological studies of
CYP2E1 RsaI/PstI polymorphisms could confirm it as a
predisposition gene for EC risk, particularly in China (15–33).
Molecular biological studies have also demonstrated that the rare
allele of the RsaI/PstI polymorphism in the CYP2E1
gene is associated with increased transcriptional activity
(58), which may play an important
role in EC development.
Our meta-analysis summarized all the available data
on the association between the CYP2E1 RsaI/PstI
polymorphism and EC risk, including a total of 4,266 subjects. The
results clearly suggested that there was a significant association
between the CYP2E1 RsaI/PstI polymorphism and EC
susceptibility. The RsaI/PstI c2 allele is a factor
which lowers the possibility of EC, which may change and increase
the ability to activate mutagens and carcinogens (OR=0.64; 95% CI,
0.50–0.81).
When subgroups were analyzed by ethnicity, the c2
allele was considered as a decreased risk factor in both Han and
Kazakh subgroups, suggesting the ethnic differences in genetic
backgrounds and the environment they lived in was non-related
factor, which was in accordance with Wang et al (39).
In mainland China, ESCC is one of the most common
malignancies, and has a great geographic variation of occurrence;
the Northwest of China shows an exceptionally high occurrence
(2). However, the meta-analysis of
ESCC subgroup failed to identify any significant association in
c2/c2 vs. c1/c1 (OR=0.94; 95% CI, 0.61–1.46; P=0.80) and c2/c2 vs.
c1/c1+c1/c2 (OR=0.96; 95% CI, 0.62–1.49, P=0.87) genetic models.
This phenomenon could have resulted since the included studies did
not indicated the tumor type were all ESCC, with the exception of
Lin et al (16) (both ESCC
and EAC). Thus, future studies should clearly report the cancer
type included.
Similar results also appeared in the PB controls in
the c2/c2 vs. c1/c1 (OR=1.02; 95% CI, 0.76–1.38; P=0.89) and c2/c2
vs. c1/c1+c1/c2 (OR=1.03; 95% CI, 0.76–1.38; P=0.54) genetic
models, and this could be since the HB studies have certain biases
for such controls and may only represent a sample of an ill-defined
reference population, and may not be representative of the general
population; or it may be that numerous subjects in the PB controls
were susceptible individuals. Therefore, the use of proper and
representative PB control subjects is important to reduce biases in
such genetic studies.
The sensitivity of the frequency distributions of
genotypes in the controls were inconsistent with HWE, and the
stability of results by deleting one study each time suggested that
they were not substantially altered and the results were stable.
Hence, the heterogeneity of the studies did not substantially lower
the statistical validity of the study and the CYP2E1
RsaI/PstI polymorphism is clearly associated with EC
risk in Chinese individuals.
Compared with the previous meta-analysis (34), this meta-analysis grouped subgroups
with more accuracy than before, and contained more studies and a
more accurate association estimation. Certain limitations of our
meta-analysis must be acknowledged. Firstly, heterogeneity among
the studies, resulting from different defined controls or other
factors, may influence the results of this analysis. In certain
studies, the controls were selected randomly from a healthy or
normal population, but in other studies controls were selected from
HB cancer-free patients. In addition, the matching criteria of the
control group differed in age and gender. The variant risks (eg.
gender, living habits) of EC in these different populations may
affect the results. Secondly, it is well-known that a single gene
has only a moderate effect on EC development. The combinations of
certain genotypes may be the more discriminating factor than a
single locus genotype. In our meta-analysis, just as the previous
one, the connection between disequilibrium and haplotype analysis
was not performed. Thirdly, although primary studies had adjusted
for covariates, the ORs of this meta-analysis were obtained without
correction, while a more precise analysis should be performed if
individual data were available, which would allow for the
adjustment by the covariates, including age, gender, ethnicity,
smoking and other factors. Fourthly, the sample size was still
relatively small; thus, we could not fully assess the effects.
Finally, a potential limitation of any meta-analysis is the
‘file-drawer’ effect, in which studies with negative results may
remain unpublished, and this may bias the literature toward
positive findings. Although we endeavored to search for unpublished
studies and the funnel plots also did not detect obvious
publication bias, we still cannot confirm all the relevant studies
were included.
In conclusion, evidence from the included studies
suggests that the CYP2E1 RsaI/PstI polymorphism plays
an important role in EC development for mainland Chinese
individuals. It also suggests that the c2 allele significantly
decreases the susceptibility to EC. For future study, studies with
larger sample sizes, stricter selection of patients, well-matched
PB controls and clearly reported cancer types are required. In
addition, the potential gene-gene and gene-environment interactions
of the CYP2E1 RsaI/PstI polymorphism and EC should be
further investigated.
Acknowledgements
This study was partly supported by
grants from the Foundation of Hubei University of Medcine (2011
CZX01) and Taihe Hospital (2010 D22), without commercial or
for-profit organizations.
References
|
1
|
Szumiło J: Epidemiology and risk factors
of the esophageal squamous cell carcinoma. Pol Merkur Lekarski.
26:82–85. 2009.PubMed/NCBI
|
|
2
|
Zheng S, Vuitton L, Sheyhidin I, Vuitton
DA, Zhang Y and Lu X: Northwestern China: a place to learn more on
oesophageal cancer. Part one: behavioural and environmental risk
factors. Eur J Gastroenterol Hepatol. 22:917–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Umar SB and Fleischer DE: Esophageal
cancer: epidemiology, pathogenesis and prevention. Nat Clin Pract
Gastroenterol Hepatol. 5:517–526. 2008. View Article : Google Scholar
|
|
4
|
Guo P and Li K: Trends in esophageal
cancer mortality in China during 1987–2009: age, period and birth
cohort analyzes. Cancer Epidemiol. 36:99–105. 2012.
|
|
5
|
Ibiebele TI, Hughes MC, Whiteman DC and
Webb PM; Australian Cancer Study: Dietary patterns and risk of
oesophageal cancers: a population-based case-control study. Br J
Nutr. 107:1207–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown LM, Devesa SS and Chow WH: Incidence
of adenocarcinoma of the esophagus among white Americans by sex,
stage, and age. J Natl Cancer Inst. 100:1184–1187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamangar F, Chow WH, Abnet CC and Dawsey
SM: Environmental causes of esophageal cancer. Gastroenterol Clin
North Am. 38:27–57. 2009. View Article : Google Scholar
|
|
9
|
Kuwano H, Kato H, Miyazaki T, et al:
Genetic alterations in esophageal cancer. Surg Today. 35:7–18.
2005. View Article : Google Scholar
|
|
10
|
Hagymási K and Tulassay Z: Risk factors
for esophageal cancer, and possible genetic background. Orv Hetil.
150:407–413. 2009.(In Hungarian).
|
|
11
|
Yang CS and Ishizaki H: Deuterium isotope
effect on the metabolism of N-nitrosodimethylamine and related
compounds by cytochrome P4502E1. Xenobiotica. 22:1165–1173. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raucy JL, Kraner JC and Lasker JM:
Bioactivation of halogenated hydrocarbons by cytochrome P4502E1.
Crit Rev Toxicol. 23:1–20. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ingelman-Sundberg M, Ronis MJ, Lindros KO,
Eliasson E and Zhukov A: Ethanol-inducible cytochrome P4502E1:
regulation, enzymology and molecular biology. Alcohol Alcohol
Suppl. 2:131–139. 1994.PubMed/NCBI
|
|
14
|
Pentiuk OO, Kachula SO and Herych OKh:
Cytochrome P4502E1. Polymorphism, physiological function,
regulation, and role in pathology. Ukr Biokhim Zh. 76:16–28.
2004.(In Ukrainian).
|
|
15
|
Lucas D, Ménez C, Floch F, et al:
Cytochromes P4502E1 and P4501A1 genotypes and susceptibility to
cirrhosis or upper aerodigestive tract cancer in alcoholic
caucasians. Alcohol Clin Exp Res. 20:1033–1037. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin DX, Tang YM, Peng Q, Lu SX, Ambrosone
CB and Kadlubar FF: Susceptibility to esophageal cancer and genetic
polymorphisms in glutathione S-transferases T1, P1, and M1 and
cytochrome P450 2E1. Cancer Epidemiol Biomarkers Prev. 7:1013–1018.
1998.PubMed/NCBI
|
|
17
|
Tan W, Song N, Wang GQ, et al: Impact of
genetic polymorphisms in cytochrome P450 2E1 and glutathione
S-transferases M1, T1, and P1 on susceptibility to esophageal
cancer among high-risk individuals in China. Cancer Epidemiol
Biomarkers Prev. 9:551–556. 2000.PubMed/NCBI
|
|
18
|
Hori H, Kawano T, Endo M and Yuasa Y:
Genetic polymorphisms of tobacco- and alcohol-related metabolizing
enzymes and human esophageal squamous cell carcinoma
susceptibility. J Clin Gastroenterol. 25:568–575. 1997. View Article : Google Scholar
|
|
19
|
Lu XM, Zhang YM, Lin RY, et al:
Relationship between genetic polymorphisms of metabolizing enzymes
CYP2E1, GSTM1 and Kazakh’s esophageal squamous cell cancer in
Xinjiang, China. World J Gastroenterol. 11:3651–3654.
2005.PubMed/NCBI
|
|
20
|
Shi QL: Genetic susceptibility,
environment risk factors and their interaction in esophageal
cancer. PhD dissertation,. 2000.(Available at: http://www.globethesis.com/?t=1104360185996737uri).
|
|
21
|
Gao CM, Takezaki T, Sugimura H, et al: The
Impact of CYP2E1 RsaI, GSTT1 and GSTM1 polymorphisms on the
risk of esophageal cancer. China Cancer. 10:346–349. 2001.(In
Chinese).
|
|
22
|
Gao C, Takezaki T, Wu J, et al:
Interaction between cytochrome P-450 2E1 polymorphisms and
environmental factors with risk of esophageal and stomach cancers
in Chinese. Cancer Epidemiol Biomarkers Prev. 11:29–34.
2002.PubMed/NCBI
|
|
23
|
Shi Y, Zhou XW, Zhou YK and Ren S:
Analysis of CYP2E1, GSTMl genetic polymorphisms in relation to
human lung Cancer and esophageal carcinoma. J Huazhong Univ Sci
Tech. 31:14–17. 2002.(In Chinese).
|
|
24
|
Shi RH, Wang W, Yu LZ, Huang XY, Chen ZQ
and Zhao ZQ: The association between cytochrome P-450 2E1 and
Glutathione enzyme gene P1 gene polymorphisms and esophageal cancer
susceptibility. Chin J Digestive Endoscopy. 21:392–394. 2004.(In
Chinese).
|
|
25
|
Yin LH, Pu YP, Song YH, Hu X, Liu YZ and
Kai HT: Polymorphisms of susceptible genes for esophageal cancer
risk in Huaian population in Jiangsu province. Tumor. 25:357–361.
2005.(In Chinese).
|
|
26
|
Li H: Case-control study of the risk
factors to esophageal carcinoma. MSc thesis. 2006, (Available at:
http://globethesis.com/?t=2144360185452638uri).
|
|
27
|
Dong CX, Wu J, Jin Y and Zhang J:
Correlation between genetic polymorphism of CYP2E1, GSTM1 and
esophageal cancer in Gansu. Chin J Gastroenterol Hepatol.
16:115–118. 2007.(In Chinese).
|
|
28
|
Liu R, Yin LH and Pu YP: Association of
combined CYP2E1 gene polymorphism with the risk for esophageal
squamous cell carcinoma in Huai’an population, China. Chin Med J
(Engl). 120:1797–1802. 2007.PubMed/NCBI
|
|
29
|
Guo YM, Wang Q, Liu YZ, Chen HM, Qi Z and
Guo QH: Genetic polymorphisms in cytochrome P4502E1, alcohol and
aldehyde dehydrogenases and the risk of esophageal squamous cell
carcinoma in Gansu Chinese males. World J Gastroenterol.
14:1444–1449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin JM, Yang L, Chen B, et al: Interaction
of methylenetetrahydrofolate reductase C677T, cytochrome P4502E1
polymorphism and environment factors in esophageal cancer in Kazakh
population. World J Gastroenterol. 14:6986–6992. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia H, Zhou J and Chen HJ: The research on
the relationship between CD44, CYP2E1 genetic polymorphisms and
esophageal cancer. Chin Oncol. 18:195–198. 2008.(In Chinese).
|
|
32
|
Yang YF: A study on risk factors and
biomarkers associated with the different stages of esophageal
squamous cell carcinogenesis in Feicheng county. PhD dissertation.
2008, (Available at: http://www.globethesis.com/?t=1114360245996153uri).
|
|
33
|
Zhang B and Wu J: Correlation between
genetic polymorphism of CYP2E1 and susceptibility of esophageal
cancer in Gansu Province. J Chengde Med Coll. 25:245–248. 2008.(In
Chinese).
|
|
34
|
Niu Y, Yuan H, Leng W, Pang Y, Gu N and
Chen N: CYP2E1 RsaI/PstI polymorphism and esophageal cancer risk: a
meta-analysis based on 1,088 cases and 2,238 controls. Med Oncol.
28:182–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: the PRISMA statement. BMJ. 339:b25352009.
View Article : Google Scholar
|
|
36
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang LD, Liu B, Feng CW, et al:
Histopathological comparison of the lesions at esophagus, gastric
cardia and antrum on the subjects from Linzhou, Henan and the
Linzhou migrated from Changzhi, Shanxi. J Zhengzhou Univ (Medical
Sciences). 41:5–9. 2006.
|
|
40
|
Tran GD, Sun XD, Abnet CC, et al:
Prospective study of risk factors for esophageal and gastric
cancers in the Linxian general population trial cohort in China.
Int J Cancer. 113:456–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morita M, Saeki H, Mori M, Kuwano H and
Sugimachi K: Risk factors for esophageal cancer and the multiple
occurrence of carcinoma in the upper aerodigestive tract. Surgery.
131(Suppl 1): S1–S6. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akbari MR, Malekzadeh R, Nasrollahzadeh D,
et al: Familial risks of esophageal cancer among the Turkmen
population of the Caspian littoral of Iran. Int J Cancer.
119:1047–1051. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dhillon PK, Farrow DC, Vaughan TL, et al:
Family history of cancer and risk of esophageal and gastric cancers
in the United States. Int J Cancer. 93:148–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garavello W, Negri E, Talamini R, et al:
Family history of cancer, its combination with smoking and
drinking, and risk of squamous cell carcinoma of the esophagus.
Cancer Epidemiol Biomarkers Prev. 14:1390–1393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tavani A, Negri E, Franceschi S and La
Vecchia C: Risk factors for esophageal cancer in lifelong
nonsmokers. Cancer Epidemiol Biomarkers Prev. 3:387–392.
1994.PubMed/NCBI
|
|
46
|
Chak A, Lee T, Kinnard MF, et al: Familial
aggregation of Barrett’s oesophagus, oesophageal adenocarcinoma,
and oesophagogastric junctional adenocarcinoma in Caucasian adults.
Gut. 51:323–328. 2002.
|
|
47
|
Li JY, Ershow AG, Chen ZJ, et al: A
case-control study of cancer of the esophagus and gastric cardia in
Linxian. Int J Cancer. 43:755–761. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morita M, Kuwano H, Nakashima T, et al:
Family aggregation of carcinoma of the hypopharynx and cervical
esophagus: special reference to multiplicity of cancer in upper
aerodigestive tract. Int J Cancer. 76:468–471. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Z, Tang L, Sun G, et al: Etiological
study of esophageal squamous cell carcinoma in an endemic region: a
population-based case control study in Huaian, China. BMC Cancer.
6:2872006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yu Y, Taylor PR, Li JY, et al:
Retrospective cohort study of risk-factors for esophageal cancer in
Linxian, People’s Republic of China. Cancer Causes Control.
4:195–202. 1993.
|
|
51
|
Tian F, Cheng QS and Yu LL: Meta-analysis
of risk factors of esophageal cancer in China. Journal of the
Fourth Military Medical University. 24:2196–2198. 2003.(In
Chinese).
|
|
52
|
Liu YX, Wang B, Wan MH, Tang WF, Huang FK
and Li C: Meta-analysis of the relationship between the
metholenetetrahydrofolate reductase C677T genetic polymorphism,
folate intake and esophageal cancer. Asian Pac J Cancer Prev.
12:247–252. 2011.PubMed/NCBI
|
|
53
|
Moaven O, Raziee HR, Sima HR, et al:
Interactions between glutathione-S-transferase M1, T1 and P1
polymorphisms and smoking, and increased susceptibility to
esophageal squamous cell carcinoma. Cancer Epidemiol. 34:285–290.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tong Z, ZhuGe J and Yu Y: Researches on
the polymorphism of cytochrome P450 2A6. Zhonghua Yi Xue Yi Chuan
Xue Za Zhi. 19:424–427. 2002.(In Chinese).
|
|
55
|
Xing DY, Tan W, Song N and Lin DX:
Ser326Cys polymorphism in hOGG1 gene and risk of esophageal cancer
in a Chinese population. Int J Cancer. 95:140–143. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang J, Zhang J, Zhao Y, et al: The
Arg194Trp polymorphism in the XRCC1 gene and cancer risk in Chinese
mainland population: a meta-analysis. Mol Biol Rep. 38:4565–4573.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhao Y, Wang F, Shan S, et al: Genetic
polymorphism of p53, but not GSTP1, is association with
susceptibility to esophageal cancer risk - a meta-analysis. Int J
Med Sci. 7:300–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hayashi S, Watanabe J and Kawajiri K:
Genetic polymorphisms in the 5’-flanking region change
transcriptional regulation of the human cytochrome P450IIE1 gene. J
Biochem. 110:559–565. 1991.
|