Introduction
Colorectal cancer is one of the most common
malignancies worldwide, particularly in Western populations, and is
thought to be correlated with colorectal adenoma, a type of
pre-neoplastic lesion. The mechanisms of colon adenoma genesis are
still unclear.
Evidence indicates that certain epidemiological
factors, including cigarette smoking, alcohol use and meat
consumption, may contribute to the risk of colorectal adenomas
(1,2). Moreover, exposure to certain toxins,
such as the heterocyclic aromatic amines formed during the cooking
of meat at high temperatures, may increase colorectal adenoma risk
(3). However, although numerous
individuals are exposed to environmental risk factors, colorectal
adenomas develop in only a small proportion of these individuals.
Additionally, previous studies suggested a marked association
between colonic adenoma risk and a family history of adenomas
(4,5), indicating that host genetic factors
may play a critical role in the genesis of colorectal adenomas.
Colon tissue is vulnerable to the effects of
external toxins by direct exposure. Xenobiotics can be
bio-activated into their ultimate carcinogen forms by phase I
enzymes and subsequently detoxified by phase II enzymes, such as
CYP1A1 and GSTM1, respectively (6). Genetic variation in the genes
encoding these enzymes may affect carcinogen
activation/detoxification and modulate DNA repair, possibly by
altering the genes’ expression and function. This may result in
oncogenesis.
Acetylation is an important biotransformation route
for these chemicals. In humans, the N-acetyltransferase 2
(NAT2) gene encodes a phase II enzyme that plays an
essential role in the metabolism of aromatic heterocyclic amines
and hydrazines via N- and O-acetylation (7). Alterations to the NAT2
acetylator status caused by variations in the NAT2 gene have
been reported to reduce enzymatic activity, resulting in
inefficient detoxification and thus leading to increased cancer
susceptibility (8). Several
NAT2 genetic variants have been identified in humans, of
which NAT2*4 is regarded as the most common allele linked to
rapid acetylation. NAT2*12A, NAT2*12C, NAT2*13
and NAT2*18 have also been classified as rapid alleles. The
remaining alleles are considered to be slow alleles (9–11).
Published studies have been conducted on the
association of NAT2 genetic variants with colorectal adenoma
risk and have yielded inconclusive results. Whether NAT2
polymorphisms are a risk factor for colorectal adenoma remains
uncertain. Therefore, in the present study, evidence-based
quantitative meta-analyses of the published studies were performed
to derive a more precise estimation of this association.
Materials and methods
Literature search strategy
A search of the Medline, EMBASE, OVID,
Sciencedirect, Google scholar and CNKI databases was performed
covering all studies published before March 2012 with no language
limitations. Combinations of the following keywords were used:
NAT2, Nacetyltransferase 2, colon,
colorectal, neoplasm, polyp, adenoma
and polymorphism. The bibliographies of all the retrieved
studies were searched for further relevant publications. Review
articles and the bibliographies of other identified relevant
studies were searched manually to identify additional eligible
studies.
Inclusion criteria
The following criteria were used for the literature
selection: i) studies should be concerned with the association of
NAT2 polymorphisms with colorectal adenoma risk; ii) studies
should be observational (case-control or cohort); iii) studies must
present the sample size, odds ratios (ORs) and the corresponding
95% confidence intervals (CIs) and genetic distributions or
information that may aid the interpretation of the results. After
extensive searching, all studies were reviewed in accordance with
the criteria defined above for further analysis.
Data extraction
Data were extracted and entered into a database by
two reviewers independently. In the case of conflicting
evaluations, agreement was reached following discussion. If a
consensus could not be reached, a third reviewer was consulted to
resolve the dispute and a final decision was then made according to
the majority decision. Carriers of at least one of the
high-activity alleles were identified as rapid acetylators and
individuals carrying two low-activity alleles were classified as
slow acetylators, as stated in the primary literature.
Statistical analysis
The OR of the NAT2 polymorphisms and
colorectal adenoma risk was estimated for each study. To detect
possible sample size biases, the OR and corresponding 95% CI of
each study were plotted against the number of participants. A
Chi-square-based Q statistic test was performed to assess
heterogeneity. If the result of the heterogeneity test was
P>0.1, ORs were pooled according to the fixed-effects model
(Mantel-Haenszel). Otherwise, the random-effects model (DerSimonian
and Laird) was used. The significance of the pooled ORs was
determined using a Z-test.
Publication bias was assessed by visual inspection
of funnel plots (12) in which the
standard error of the log(OR) of each study was plotted against the
corresponding log(OR). An asymmetric plot indicates a possible
publication bias. The symmetry of the funnel plot was evaluated
using Egger’s linear regression test (13). Statistical analyses were performed
using the STATA 11.0 software (StataCorp, College Station, TX,
USA).
Results
Literature search and meta-analysis
databases
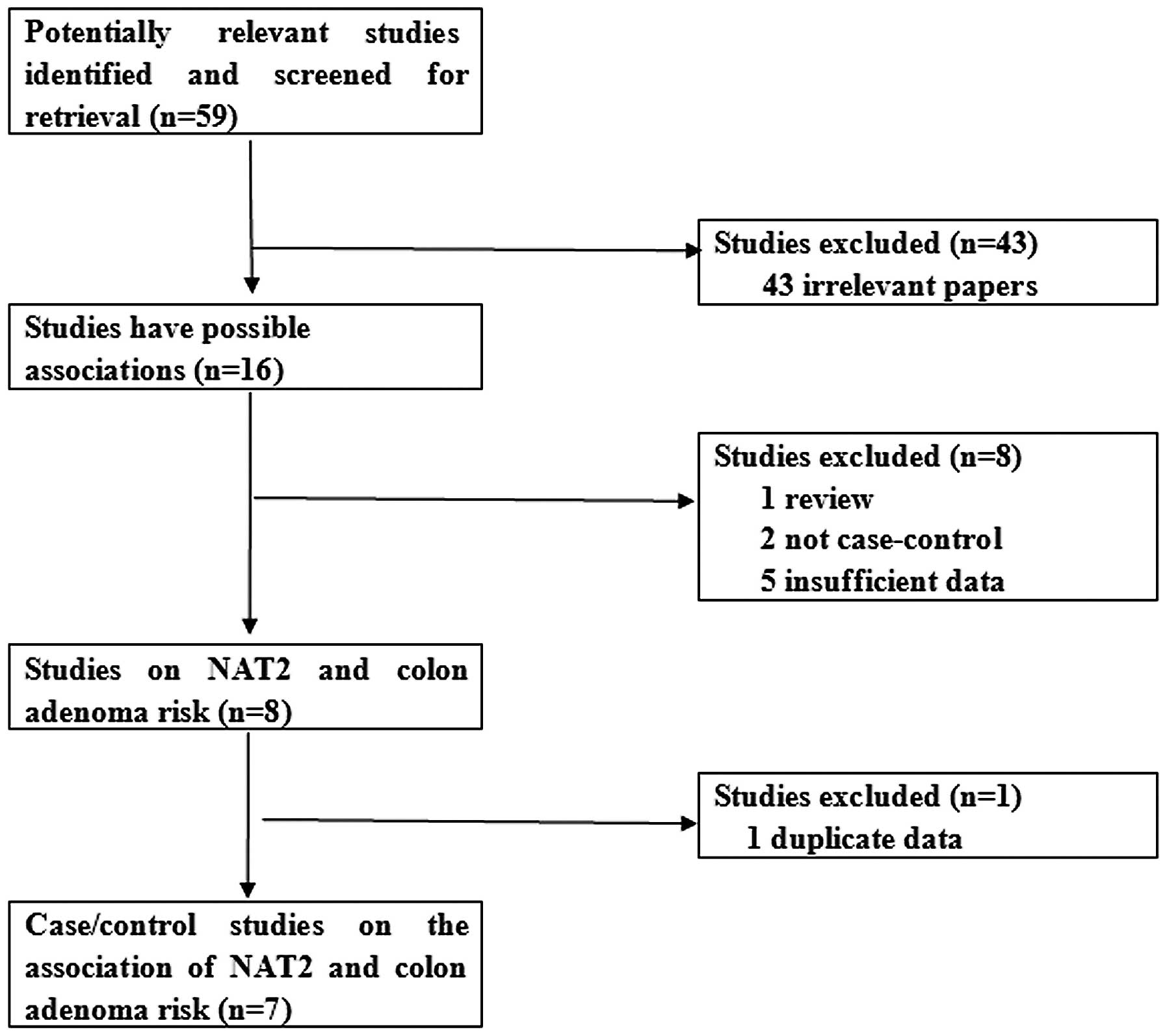
As shown in Fig. 1,
a total of 59 publications were searched and screened for
retrieval, of which 43 irrelevant studies were excluded. Thus, 16
studies were primarily identified, of which one review was excluded
(14). Subsequently, two articles
which were not case-control studies (15,16)
and five studies lacking sufficient information (17–21)
were also excluded. Eight publications were identified (22–29).
Two studies conducted by Tiemersma et al (22,28)
concerned the same research subjects. Thus, the study concerning
cigarette smoking was selected (28). Finally, seven case-control studies
were selected (23–29).
The included studies were all written in English, of
which one involved a Caucasian population (28) and the remaining six involved
multi-ethnic populations. We were able to extract information about
smoking status from two of the studies (25,28).
We established a database of the extracted
information from each study. The relevant information is shown in
Table I. The first author and the
number and characteristics of cases and controls for each study are
presented, as well as the other necessary information. The genetic
distributions of the control groups were in Hardy-Weinberg
equilibrium. The distributions of the NAT2 acetylator
variants (classified as rapid or slow) are presented in Table II.
 | Table ICharacteristics of the studies
included in the meta-analysis. |
Table I
Characteristics of the studies
included in the meta-analysis.
| First author
(ref.) | Publication
year | Cases
(male/female) | Controls
(male/female) | Type of
controls | Age range (mean),
years
| Ethnicity | Country |
|---|
| Cases | Controls |
|---|
| Lang (24) | 1994 | 41 (28/13) | 205 (129/76) | 205 volunteers
(population-based) | 36–84 (60.6) | 20–80 (46.9) | Mixed | USA |
| Probst-Hensch
(26) | 1996 | 441 (280/161) | 484 (326/158) | 484 controls (age-
and gender-matched; population-based) | 50–74 (61.7) | 50–74 (61.6) | Mixed | USA |
| Ishibe (23) | 2002 | 146 (111/35) | 228 (144/84) | 228 controls (age-
and gender-matched; hospital-based) | 18–74 (58) | 18–74 (59) | Mixed | USA |
| Tiemersma (28) | 2004 | 431 (236/195) | 433 (160/273) | 433 controls (age-
and gender-matched; hospital-based) | 18–75 (58.8) | 18–75 (50.4) | Caucasian | Netherlands |
| Moslehi (25) | 2006 | 772 (535/237) | 777 (536/241) | 777 controls
(gender- and ethnicity-matched; population-based) | 55–74 (NA) | 55–74 (NA) | Mixed | USA |
| Shin (27) | 2008 | 557 (418/139) | 1493
(1250/243) | 1493 controls
(hospital-based) | 40–75 (59.6) | 40–75 (57.2) | Mixed | USA |
| Wang (29) | 2011 | 914 (550/364) | 1185 (745/440) | 1185 controls
(age-, gender- and ethnicity-matched; hospital-based) | NA (61) | NA (62) | Mixed | USA |
 | Table IIDistribution of NAT2
acetylator variants among colorectal adenoma cases and controls
included in the meta-analysis. |
Table II
Distribution of NAT2
acetylator variants among colorectal adenoma cases and controls
included in the meta-analysis.
| First author | Year | Genotyping
method | Cases
| Controls
|
|---|
| Rapid | Slow | Rapid | Slow |
|---|
| Lang | 1994 | Use of
caffeine | 25 | 16 | 92 | 113 |
| Probst-Hensch | 1996 | AS-PCR | 213 | 228 | 226 | 258 |
| Ishibe | 2002 | PCR-RFLP | 64 | 79 | 98 | 110 |
| Tiemersma | 2004 | PCR-RFLP | 168 | 259 | 179 | 253 |
| Moslehi | 2006 | Taqman | 272 | 413 | 317 | 376 |
| Shin | 2008 | Taqman | 243 | 311 | 609 | 880 |
| Wang | 2011 | PCR-RFLP | 449 | 457 | 631 | 539 |
Test of heterogeneity
As shown in Table
III, the heterogeneity of the overall data was significant
since the P-value of the Q tests was <0.1 and thus the
random-effects model was used. However, when subgroup analysis with
regard to the source of the controls was conducted, no
heterogeneity in the hospital-based subgroup was found.
 | Table IIIMain results of the pooled data in
the meta-analysis. |
Table III
Main results of the pooled data in
the meta-analysis.
| Characteristic | Slow vs. rapid
acetylator variants
|
|---|
| Number of
cases/controls | OR | 95% CI | P-value for
Q-test |
|---|
| Overall | 3197/4681 | 1.04 | 0.90–1.21 | 0.043 |
| Smoking status | | | | |
| Never smoked | 387/481 | 0.92 | 0.53–1.58 | 0.051 |
| Have smoked | 665/561 | 1.31 | 1.04–1.64 | 0.795 |
| Source of
control | | | | |
|
Population-based | 1167/1382 | 0.96 | 0.66–1.39 | 0.019 |
|
Hospital-based | 2030/3299 | 1.05 | 0.90–1.23 | 0.172 |
Quantitative data synthesis
Table III lists the
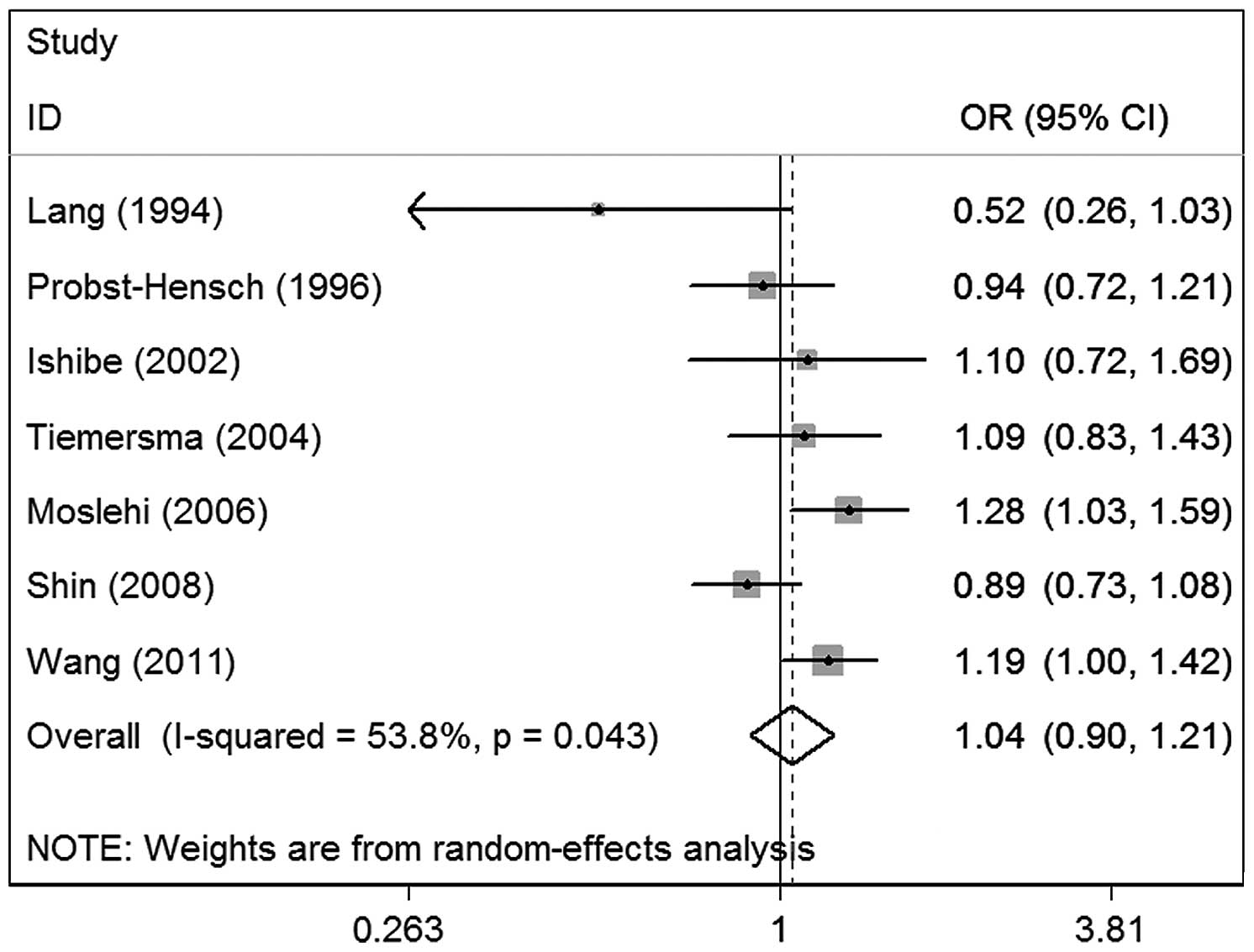
main results of the meta-analysis. The overall data from the seven
studies containing 3,197 cases and 4,681 controls revealed no
significant associations between NAT2 polymorphisms and
colorectal adenoma risk (OR, 1.04; 95% CI, 0.90–1.21; P=0.043 for
heterogeneity), suggesting that NAT2 polymorphisms may have
little association with colorectal adenoma risk (Fig. 2).
To further assess the possible impact of smoking and
the source of the controls on the results, relevant data were
extracted to conduct subgroup analyses. In the subgroup analysis of
the source of the controls, no significant associations were
observed in either the hospital-based group (OR, 1.05; 95% CI,
0.90–1.23; P=0.172 for heterogeneity) or the population-based group
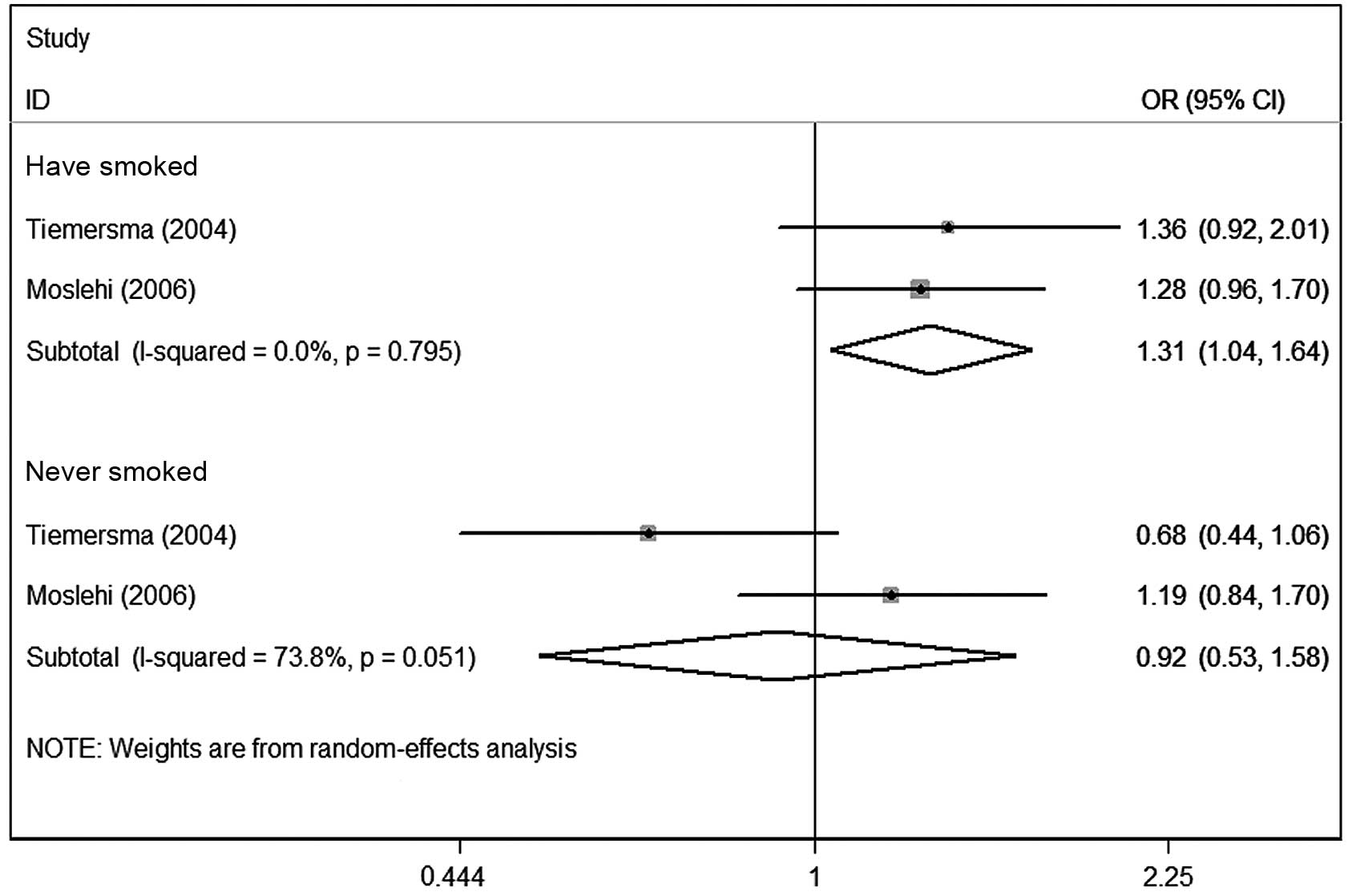
(OR, 0.96; 95% CI, 0.66–1.39; P=0.019 for heterogeneity; Fig. 3). In the smoking status subgroups,
the data showed that slow acetylator variants may be associated
with increased colonic adenoma risk in smokers (OR, 1.31; 95% CI,
1.04–1.64; P=0.795 for heterogeneity). Nevertheless, no marked
associations were observed in the never smoked subgroup (OR, 0.92;
95% CI, 0.53–1.58; P=0.051 for heterogeneity; Fig. 4).
An attempt was made to extract relevant data
concerning meat and alcohol consumption to use in the subgroup
analysis, but insufficient data were available.
Sensitivity analysis
In order to compare the differences and evaluate the
sensitivity of the meta-analyses, the results of the fixed-effects
model for the overall data were also reported. The combined OR and
95% CI were 1.07 and 0.97–1.17, respectively, similar to the
results of the random-effects model, suggesting that the
meta-analyses were stable. Additionally, one-way sensitivity
analysis (30) was also used to
evaluate the stability of the meta-analysis. The statistical
significance of any of the results was not altered by the omission
of any single study, suggesting that the data in this meta-analysis
were relatively stable and credible.
Bias diagnostics
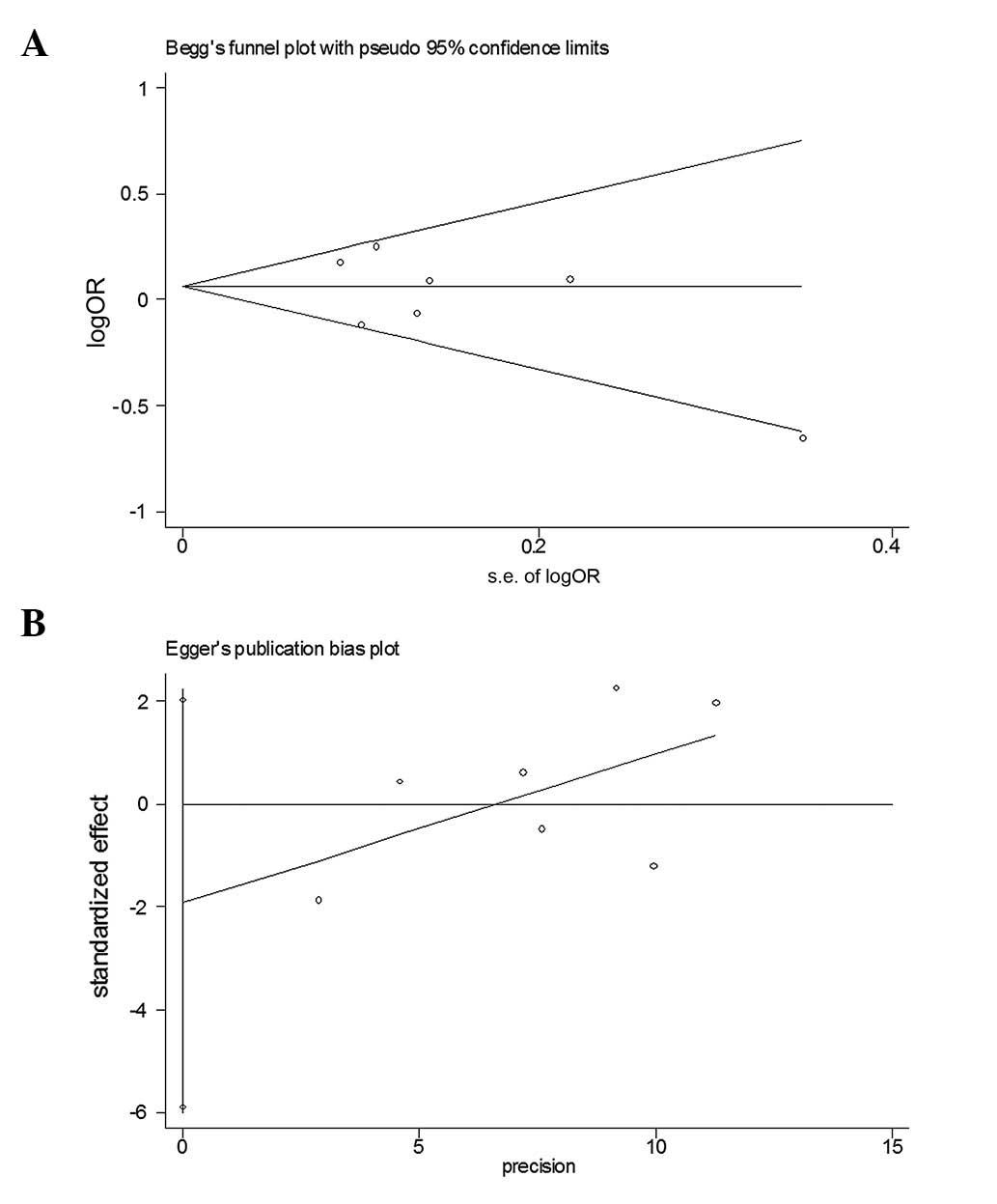
A funnel plot was created to assess possible
publication biases (Fig. 5A).
Egger’s linear regression test was then used to assess the symmetry
of the plot (Fig. 5B). The data
suggested that the funnel plot was symmetrical (t=−1.25,
P>0.05), indicating that the results of the meta-analyses were
relatively stable and that publication bias had little effect on
the results.
Discussion
In the present study, the overall data of the
meta-analyses showed that the NAT2 polymorphism may not have
a significant association with colorectal adenoma risk.
Nevertheless, in subgroup analysis, slow acetylator variants may
modify the colorectal adenoma susceptibility of individuals who
have a history of smoking.
Possible correlations of NAT2 polymorphisms
with cancer risk were evaluated by the meta-analyses. Previously,
NAT2 polymorphisms have been indicated to be associated with
an increased susceptibility to prostate cancer and laryngeal cancer
in Asian populations (31,32). However, meta-analyses with regard
to lung and gastric cancer did not reveal any marked associations
(33,34). Similarly, meta-analyses published
in 2002, 2006 and 2012 failed to demonstrate a significant
association between NAT2 polymorphisms and colorectal cancer
risk (35–37). In the present study, the overall
data also failed to reveal a significant association of the
NAT2 variants with colorectal adenoma, in line with the
meta-analyses concerning colorectal cancer.
A previous meta-analysis suggested that smoking
affected the formation and aggressiveness of colon adenomas
(38). Another meta-analysis
suggested that smoking may interact with certain gene variants,
such as the NQO1 genetic variant (39), in the development of colorectal
adenomas. Thus, we attempted to extract relevant data from the
primary literature to conduct a subgroup analysis. The data showed
that slow acetylator variants may be associated with an increased
adenoma risk in individuals who have a history of smoking. The data
indicate possible interactions between smoking with slow acetylator
variants in the pathological mechanisms of colorectal adenoma. The
underlying mechanisms by which NAT2 polymorphisms affect
colon adenomas in individuals with a history of smoking are not
fully understood. The NAT2 gene is located on chromosome
8p22, encodes a 290-amino acid protein (40) and catalyzes the detoxification
and/or activation of aromatic and heterocyclic amine carcinogens by
two pathways. This metabolic reaction may result in detoxification
by N-acetylation or bioactivation by O-acetylation which is often
proceeded by CYP450 hydroxylation (41). Polymorphisms of NAT2 may
lead to differences in the rate of arylamine metabolism and
consequently cancer risk (42).
Individuals with a history of smoking and NAT2 genetic
variants who are exposed to cigarette toxins, such as nickel
sulphate and benzo(b)fluoranthene, may have a reduced
detoxification ability, thus resulting in an increased risk of
colon adenoma as well as cancer. However, only two of the studies
provided sufficient data concerning smoking status for subgroup
analysis. Biases may be noted due to the limited number of selected
studies and sample sizes. Therefore, these results should be
interpreted with care.
Subgroup analyses of the source of the controls also
failed to reveal significant associations in studies using
hospital- or population-based controls. Since hospital-based
controls may not always truly represent the general population,
particularly when the controls have any disease-related conditions
relevant to the studied genotypes, inherent selection biases may
exist. Further well-designed studies, including population-based
controls with strict matching criteria, are required to reduce such
possible biases.
Inter-study heterogeneity was observed in this
study. Thus, the random-effects model was used. Subgroup analyses
were then conducted. As expected, the heterogeneities of the
subgroups were reduced or removed. The data suggested that the
source of the controls and smoking status may partly contribute to
the heterogeneities. Additionally, other factors, including age,
gender, ethnicity and the prevalence of lifestyle factors, may also
generate heterogeneities.
Publication bias is an important factor that should
be considered in a meta-analysis. In the present study, a funnel
plot and Egger’s test were used to assess publication biases. The
results indicated that publication biases exerted little effect on
the overall results, suggesting that the meta-analysis was robust
and credible.
Certain limitations may be included in this study.
Firstly, in this meta-analysis, the majority of published studies
and papers written in English were searched. However, it is
possible that some related published or unpublished studies that
may have met the inclusion criteria were missed. Thus, it is
inevitable that publication biases may exist in the results.
Secondly, all the studies concerned mixed ethnicities, with the
exception of one study by Tiemersma (28) involving a Caucasian population.
Therefore, subgroup analysis concerning ethnicity was not conducted
and we were unable to assess the possible effects of ethnic
variation on the results. Further studies conducted on separated
ethnicities in various areas are required. Thirdly, subgroup
analyses of age, gender, alochol use and meat consumption were not
performed since insufficient information was available in the
primary literature. Gene-gene and gene-environment interactions
should also be considered in the further investigations.
In conclusion, although no significant associations
of NAT2 genetic variations with the overall risk of
colorectal adenoma were revealed, statistically significant
findings were noted in individuals with a history of smoking,
suggesting that NAT2 genetic variations may modify the
colorectal adenoma risk of smokers. Further investigations are
required to confirm this conclusion.
References
|
1
|
Toyomura K, Yamaguchi K, Kawamoto H, et
al: Relation of cigarette smoking and alcohol use to colorectal
adenomas by subsite: the self-defense forces health study. Cancer
Sci. 95:72–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robertson DJ, Sandler RS, Haile R, et al:
Fat, fiber, meat and the risk of colorectal adenomas. Am J
Gastroenterol. 100:2789–2795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma S, Iwasaki M, Kunieda C, et al:
Development of a quantitative food frequency questionnaire for
assessing food, nutrient, and heterocyclic aromatic amines intake
in Japanese Brazilians for a colorectal adenoma case-control study.
Int J Food Sci Nutr. 60(Suppl 7): 128–139. 2009. View Article : Google Scholar
|
|
4
|
Mansoor-ul-Haq and Faisal N: Familial
adenomatous polyposis. J Coll Physicians Surg Pak. 21:46–48.
2011.
|
|
5
|
Wilschut JA, Habbema JD, Ramsey SD, Boer
R, Looman CW and van Ballegooijen M: Increased risk of adenomas in
individuals with a family history of colorectal cancer: results of
a meta-analysis. Cancer Causes Control. 21:2287–2293. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhuo W, Wang Y, Zhuo X, et al: CYP1A1 and
GSTM1 polymorphisms and oral cancer risk: association studies via
evidence-based meta-analyses. Cancer Invest. 27:86–95. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hein DW: N-acetyltransferase 2 genetic
polymorphism: effects of carcinogen and haplotype on urinary
bladder cancer risk. Oncogene. 25:1649–1658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou W, Liu G, Thurston SW, et al: Genetic
polymorphisms in N-acetyltransferase-2 and microsomal epoxide
hydrolase, cumulative cigarette smoking, and lung cancer. Cancer
Epidemiol Biomarkers Prev. 11:15–21. 2002.PubMed/NCBI
|
|
9
|
Bolt HM, Selinski S, Dannappel D,
Blaszkewicz M and Golka K: Re-investigation of the concordance of
human NAT2 phenotypes and genotypes. Arch Toxicol. 79:196–200.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hein DW and Doll MA: Accuracy of various
human NAT2 SNP genotyping panels to infer rapid, intermediate and
slow acetylator phenotypes. Pharmacogenomics. 13:31–42. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taja-Chayeb L, González-Fierro A,
Miguez-Muñoz C, et al: Acetylator status and N-acetyltransferase 2
gene polymorphisms; phenotype-genotype correlation with the
sulfamethazine test. Pharmacogenet Genomics. 21:894–901. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Munafò MR, Clark TG and Flint J: Assessing
publication bias in genetic association studies: evidence from a
recent meta-analysis. Psychiatry Res. 129:39–44. 2004.PubMed/NCBI
|
|
13
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raimondi S, Botteri E, Iodice S, Lowenfels
AB and Maisonneuve P: Gene-smoking interaction on colorectal
adenoma and cancer risk: review and meta-analysis. Mutat Res.
670:6–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Potter JD, Bigler J, Fosdick L, et al:
Colorectal adenomatous and hyperplastic polyps: smoking and
N-acetyltransferase 2 polymorphisms. Cancer Epidemiol Biomarkers
Prev. 8:69–75. 1999.PubMed/NCBI
|
|
16
|
Moonen H, Engels L, Kleinjans J and Kok T:
The CYP1A2-164A→C polymorphism (CYP1A2*1F) is associated with the
risk for colorectal adenomas in humans. Cancer Lett. 229:25–31.
2005.
|
|
17
|
Bunschoten A, Tiemersma E, Schouls L and
Kampman E: Simultaneous determination of polymorphism in
N-acetyltransferase 1 and 2 genes by reverse line blot
hybridization. Anal Biochem. 285:156–162. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crabtree MD, Fletcher C, Churchman M, et
al: Analysis of candidate modifier loci for the severity of colonic
familial adenomatous polyposis, with evidence for the importance of
the N-acetyl transferases. Gut. 53:271–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin HJ, Probst-Hensch NM, Hughes NC, et
al: Variants of N-acetyltransferase NAT1 and a case-control study
of colorectal adenomas. Pharmacogenetics. 8:269–281. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Probst-Hensch NM, Haile RW, Ingles SA, et
al: Acetylation polymorphism and prevalence of colorectal adenomas.
Cancer Res. 55:2017–2020. 1995.PubMed/NCBI
|
|
21
|
Voskuil DW, Kampman E, Grubben MJ, et al:
Meat consumption and preparation, and genetic susceptibility in
relation to colorectal adenomas. Cancer Lett. 114:309–311. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tiemersma EW, Voskuil DW, Bunschoten A, et
al: Risk of colorectal adenomas in relation to meat consumption,
meat preparation, and genetic susceptibility in a Dutch population.
Cancer Causes Control. 15:225–236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishibe N, Sinha R, Hein DW, et al: Genetic
polymorphisms in heterocyclic amine metabolism and risk of
colorectal adenomas. Pharmacogenetics. 12:145–150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lang NP, Butler MA, Massengill J, et al:
Rapid metabolic phenotypes for acetyltransferase and cytochrome
P4501A2 and putative exposure to food-borne heterocyclic amines
increase the risk for colorectal cancer or polyps. Cancer Epidemiol
Biomarkers Prev. 3:675–682. 1994.
|
|
25
|
Moslehi R, Chatterjee N, Church TR, et al:
Cigarette smoking, N-acetyltransferase genes and the risk of
advanced colorectal adenoma. Pharmacogenomics. 7:819–829. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Probst-Hensch NM, Haile RW, Li DS, et al:
Lack of association between the polyadenylation polymorphism in the
NAT1 (acetyltransferase 1) gene and colorectal adenomas.
Carcinogenesis. 17:2125–2129. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shin A, Shrubsole MJ, Rice JM, et al: Meat
intake, heterocyclic amine exposure, and metabolizing enzyme
polymorphisms in relation to colorectal polyp risk. Cancer
Epidemiol Biomarkers Prev. 17:320–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tiemersma EW, Bunschoten A, Kok FJ, Glatt
H, de Boer SY and Kampman E: Effect of SULT1A1 and NAT2 genetic
polymorphism on the association between cigarette smoking and
colorectal adenomas. Int J Cancer. 108:97–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Yamamoto JF, Caberto C, et al:
Genetic variation in the bioactivation pathway for polycyclic
hydrocarbons and heterocyclic amines in relation to risk of
colorectal neoplasia. Carcinogenesis. 32:203–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tobias A: Assessing the influence of a
single study in the meta-analysis estimate. Stata Techn Bull.
47:15–17. 1999.
|
|
31
|
Gong C, Hu X, Gao Y, Cao Y, Gao F and Mo
Z: A meta-analysis of the NAT1 and NAT2 polymorphisms and prostate
cancer: a huge review. Med Oncol. 28:365–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ying XJ, Dong P, Shen B, Wang J, Wang S
and Wang G: Possible association of NAT2 polymorphism with
laryngeal cancer risk: an evidence-based meta-analysis. J Cancer
Res Clin Oncol. 137:1661–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui D, Wang Z, Zhao E, Ma J and Lu W: NAT2
polymorphism and lung cancer risk: a meta-analysis. Lung Cancer.
73:153–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhong X, Hui C, Xiao-Ling W, Yan L and Na
L: NAT2 polymorphism and gastric cancer susceptibility: a
meta-analysis. Arch Med Res. 41:275–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye Z and Parry JM: Meta-analysis of 20
case-control studies on the N-acetyltransferase 2 acetylation
status and colorectal cancer risk. Med Sci Monit. 8:CR558–565.
2002.PubMed/NCBI
|
|
36
|
Borlak J and Reamon-Buettner SM:
N-acetyltransferase 2 (NAT2) gene polymorphisms in colon and lung
cancer patients. BMC Med Genet. 7:582006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang LQ, Zhou JN, Wang J, et al: Absence
of association between N-acetyltransferase 2 acetylator status and
colorectal cancer susceptibility: based on evidence from 40
studies. PLoS One. 7:e324252012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Botteri E, Iodice S, Raimondi S,
Maisonneuve P and Lowenfels AB: Cigarette smoking and adenomatous
polyps: a meta-analysis. Gastroenterology. 134:388–395. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou JY, Shi R, Yu HL, Zheng WL and Ma WL:
Association of NQO1 Pro187Ser polymorphism with the risks for
colorectal cancer and colorectal adenoma: a meta-analysis. Int J
Colorectal Dis. 27:1123–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brockton N, Little J, Sharp L and Cotton
SC: N-acetyltransferase polymorphisms and colorectal cancer: a HuGE
review. Am J Epidemiol. 151:846–861. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu L, Wagner CR and Hanna PE:
Isoform-selective inactivation of human arylamine
N-acetyltransferases by reactive metabolites of carcinogenic
arylamines. Chem Res Toxicol. 22:1962–1974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Walker K, Ginsberg G, Hattis D, Johns DO,
Guyton KZ and Sonawane B: Genetic polymorphism in
N-acetyltransferase (NAT): population distribution of NAT1 and NAT2
activity. J Toxicol Environ Health B Crit Rev. 12:440–472. 2009.
View Article : Google Scholar : PubMed/NCBI
|