Introduction
Whether chaperones play a role in the resistance of
human cancer cells to anticancer agents such as radiation and
chemical compounds is a valuable question. Previously, we found
that glucose-regulated protein 78 (GRP78) and heat shock protein 27
(HSP27) have a protective role against ultraviolet ray C (UVC)
lethality in RSa cells, which are an established human cell line
with high UVC sensitivity (1,2).
Compared with the RSa-derived UVC-resistant cell line
APr-1, RSa cells expressed lower amounts of GRP78 and
HSP27. Using RSa and APr-1 cells transfected with siRNA
and/or cDNA for GRP78 and HSP27, cellular amounts of
these chaperones were suggested to be causally associated with
cellular susceptibility to UVC lethality.
In addition to RSa cells, we previously established
KT cells, which are a UVC-sensitive human cell line (3). Notably, both RSa and KT cells also
show high sensitivity to cell proliferation inhibition by human
interferon-β (HuIFN-β) (3).
Coordination between cellular sensitivity to UVC and HuIFN was
observed in various human cell lines, but the mechanisms underlying
the coordination remain unclear (4–6).
However, cellular chaperone metabolism may be the key for
clarifying these mechanisms. Notably, UVC-sensitive cells,
pretreated with HuIFN-β and then irradiated with UVC, become
resistant to UVC lethality (5,6);
however, the role of the chaperones in the HuIFN-β effect has not
been studied.
In the present study, the cellular amounts of GRP78
and HSP27 were examined in KT cells. KT cells are derived from
breast cancer tissue (3),
therefore we used MCF-7 cells, another breast cancer-derived cell
line, to compare the expression levels of the chaperones as well as
to examine the UVC and HuIFN-β susceptibility of these cells.
Materials and methods
Cells and culture conditions
KT cells and their UVC and HuIFN-β susceptibility
have been previously investigated (3). MCF-7 human breast cancer cells
(HTB-26) were obtained from the American Type Culture Collection
(Manassas, VA, USA). KT and MCF-7 cells were cultured in Eagle’s
minimal essential medium (Nissui, Tokyo, Japan) supplemented with
5% (v/v) calf serum (Invitrogen, Carlsbad, CA, USA) and 10% (v/v)
fetal bovine serum (Roche Diagnostics GmbH, Mannheim, Germany),
respectively. All cells were grown at 37°C in a humidified
atmosphere containing 5% CO2 and subcultured upon
reaching semi-confluency.
UVC irradiation
UVC was generated from a 6-W National germicidal
lamp (Matsushita Electronic Industrial, Co., Osaka, Japan). The UVC
intensity was 1.0 J/m2/sec, which was measured by the UV
radiometer, UVR-254 (Tokyo Kogaku Kikai, Co., Ltd., Tokyo, Japan).
The cells were irradiated with UVC immediately after the medium was
removed and then reincubated for an appropriate time, as previously
described (7). Mock-irradiated
cells were treated in the same manner but without irradiation.
HuIFN-β treatment
HuIFN-β was purchased from PeproTech, Inc. (Rocky
Hill, NJ, USA). Twenty-four hours after plating the cells in
dishes, HuIFN-β was added to the medium of each dish and cultured
as described elsewhere (8).
Cell survival assays
The sensitivity of cells to UVC lethality was
measured using a colony survival assay, as previously reported
(2). Briefly, cells were plated in
100-mm dishes (1×103 cells/dish) and irradiated with UVC
at the indicated doses 24 h after plating. Fourteen days after UVC
irradiation, colonies were stained and their numbers were
estimated. The survival fraction at each dose was expressed as a
percentage of the mock-irradiated cell number.
D0, which is the UVC dosage required to reduce
the colony survival from any point in the exponential portion of
the surviving fraction curve to 37% of that point, was
calculated.
Cell sensitivity to HuIFN-β was measured by the
viability assay using methylthiazol tetrazolium (MTT), as
previously described (1). Briefly,
cells were plated into 96-well plates (1.3×104
cells/well) and incubated for 3 days. After incubation, living
cells were assayed by incubation with 0.5 mg/ml MTT (Sigma, St.
Louis, MO, USA) for 4 h, followed by measurement of absorbance.
Cell viability was calculated by the absorbance as a percentage of
the mock-treated cells (1).
Immunoblotting analysis
Cells were washed twice with phosphate-buffered
saline and whole cells were lysed with SDS sample buffer and boiled
for 5 min. Whole-cell lysates were separated in a 10% SDS gel and
immunoblotting analysis was performed as described elsewhere
(2). GRP78 protein was detected
with mouse anti-GRP78 monoclonal antibody (SPA-827; dilution,
1:1,000; StressGen, Victoria, BC, Canada), and HSP27 protein was
detected with mouse anti-HSP27 monoclonal antibody (mH3; dilution,
1:1,000) (9,10). The antigen-antibody complexes were
incubated with horseradish peroxidase-conjugated anti-mouse IgG
antibody (Amersham Biosciences, Buckinghamshire, UK), followed by
the ECL system reaction (Amersham Biosciences). Protein levels of
actin were also analyzed as the loading control, using mouse
anti-actin antibody (dilution, 1:20,000; ICN Biomedicals, Inc.,
Costa Mesa, CA, USA). The intensity of protein signals was
quantified by the MultiGauge Ver2.2 image analyzing software (Fuji
Photo Film Co., Ltd., Tokyo, Japan) and expressed as a value
relative to actin.
Construction of expression vectors
Full-length human HSP27 cDNA was prepared
according to a previously reported method (2). Briefly, the cDNA was ligated into
pQE-30 plasmid (Qiagen, Germantown, MD, USA) using the SacI
and PstI restriction sites. The construction was confirmed
by sequencing and was then digested with EcoRI and
HindIII. To construct a His-tagged HSP27 (His-HSP27)
expression vector [His-HSP27/pcDNA3.1(-)], the digested fragment
was gel-purified and then ligated into pcDNA3.1(-) (Invitrogen),
using the EcoRI and HindIII restriction sites. The
construct was confirmed by sequencing.
Plasmid transfection
Cells grown to 60–80% confluency in 35-mm dishes
were transiently transfected with the indicated expression plasmids
using Lipofectamine™ LTX reagent (Invitrogen) and Plus™ reagent
(Invitrogen) according to the manufacturer’s instructions.
GRP78 knockdown
Duplex small interfering RNA (siRNA) with Stealth
modification against human GRP78 (GRP78 siRNA) was
synthesized based on the nucleotide sequence (Invitrogen), as
previously described (11).
Stealth RNAi negative control duplex (NC siRNA), with a GC content
similar to that of the above Stealth RNAi, was used as a negative
control. The siRNAs (100 nM) were transfected into cells for 6 h
using Lipofectamine™ 2000 (Invitrogen) according to the
manufacturer’s instructions, as described elsewhere (12). Two days after transfection, the
cells were harvested and used for immunoblotting analysis and cell
survival assays.
Statistical analysis
Statistical analysis was performed using the
Student’s t-test with the StatView software (version 4.5; Abacus
Concepts Inc., Berkeley, CA, USA).
Results
Discrepancy in UVC sensitivity between
MCF-7 and KT cells
The sensitivity of MCF-7 and KT cells to UVC-induced
cell death was examined by the colony survival assay. KT cells
showed a higher UVC sensitivity than MCF-7 cells, with
D0 values of 2.3 and 5.5 J/m2,
respectively (Fig. 1).
Discrepancy in expression levels of GRP78
and HSP27 in MCF-7 and KT cells
To evaluate chaperones associated with the high
sensitivity of KT cells to UVC lethality, the expression levels of
GRP78 and HSP27 were examined in MCF-7 and KT cells by western blot
analysis. The GRP78 expression level was higher in KT than in MCF-7
cells (Fig. 2A and B), while the
HSP27 expression level was lower in KT than in MCF-7 cells
(Fig. 2A and B).
Relationship between GRP78 expression and
UVC-sensitivity of KT cells
To investigate the role of the high levels of GRP78
expression in KT cells compared with MCF-7 cells, we knocked down
the expression of GRP78 in KT cells by transfection with siRNA for
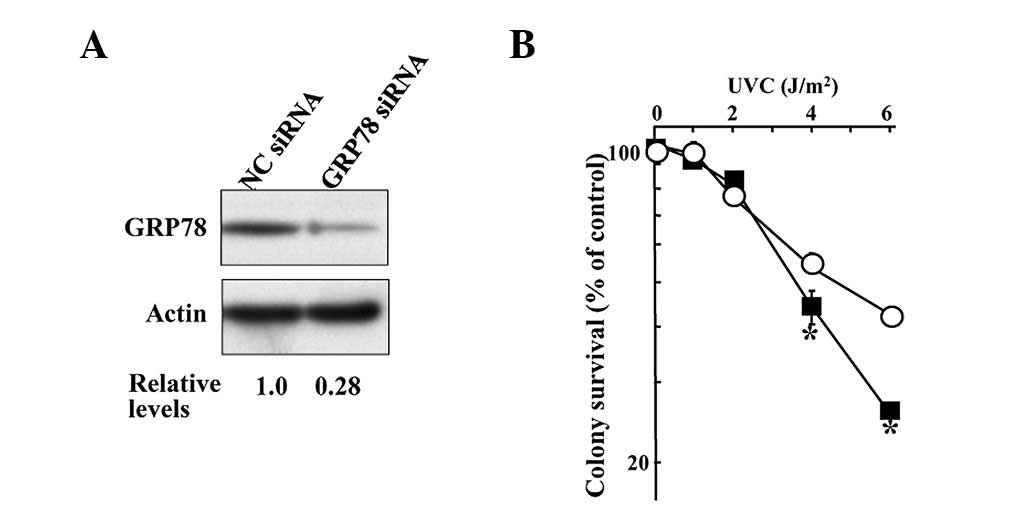
GRP78. The transfectants with GRP78 siRNA
demonstrated lower GRP78 protein expression than control KT cells
transfected with NC siRNA (Fig.
3A). Compared with the NC siRNA-expressing cells, at UVC
irradiation of up to 2 J/m2 the GRP78
siRNA-expressing cells showed the same sensitivity to UVC-induced
cell death, although they showed higher sensitivity when the UVC
irradiation was higher than 4 J/m2 (Fig. 3B).
Involvement of HSP27 expression in
cellular susceptibility to UVC of KT cells
To investigate whether low levels of HSP27 are
causally associated with the high UVC susceptibility of KT cells,
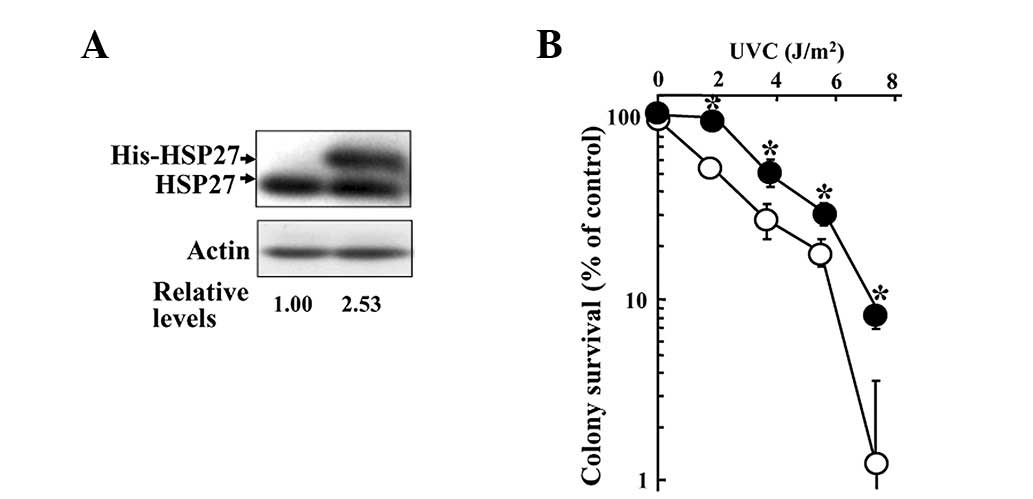
we induced HSP27 overexpression in KT cells by transfection with
His-HSP27/pcDNA3.1(-). The transfectants exhibited higher
expression of the His-HSP27 protein than the control KT cells that
were transfected with an empty vector (Fig. 4A). In addition, the colony survival
assay demonstrated that the His-HSP27-expressing cells showed lower
sensitivity to UVC-induced cell death than the control cells
(Fig. 4B).
Involvement of HSP27 in cellular HuIFN-β
susceptibility of KT cells
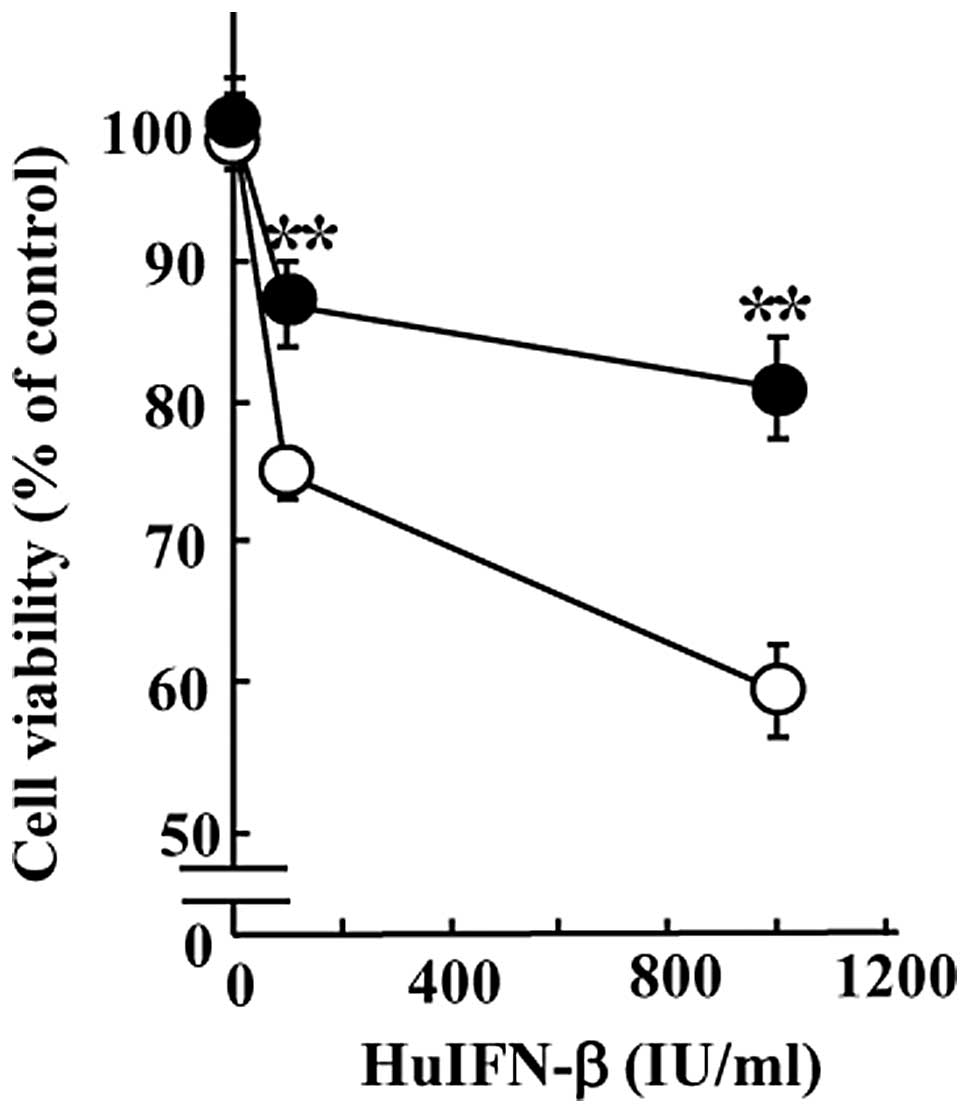
To evaluate the involvement of HSP27 in HuIFN
susceptibility of KT cells, we used the MTT assay to determine the
viability of His-HSP27-expressing cells 48 h after HuIFN-β
treatment in comparison with control cells transfected with an
empty vector. The former cells showed increased viability compared
with the latter cells (Fig. 5).
The viability of His-HSP27-expressing cells after treatment with
HuIFN-β was similar to MCF-7 cells treated with the same interferon
dose (Table I).
 | Table IEffects of HSP27 overexpression on
HuIFN-susceptibility of KT cells. |
Table I
Effects of HSP27 overexpression on
HuIFN-susceptibility of KT cells.
| | Viability (% of
control) after HuIFN-β treatment
|
|---|
| | HuIFN-β (IU/ml)
|
|---|
| Cells | Transfection | 100 | 1000 |
|---|
| MCF-7 | Mock | 91.6 | 78.1 |
| KT | Mock | 81.0 | 67.2 |
| KT | pcDNA3.1(-) | 74.9 | 67.3 |
| KT |
His-HSP27/pcDNA3.1(-) | 90.1 | 84.2 |
Involvement of HSP27 in HuIFN-β-induced
resistance of KT cells to UVC lethality
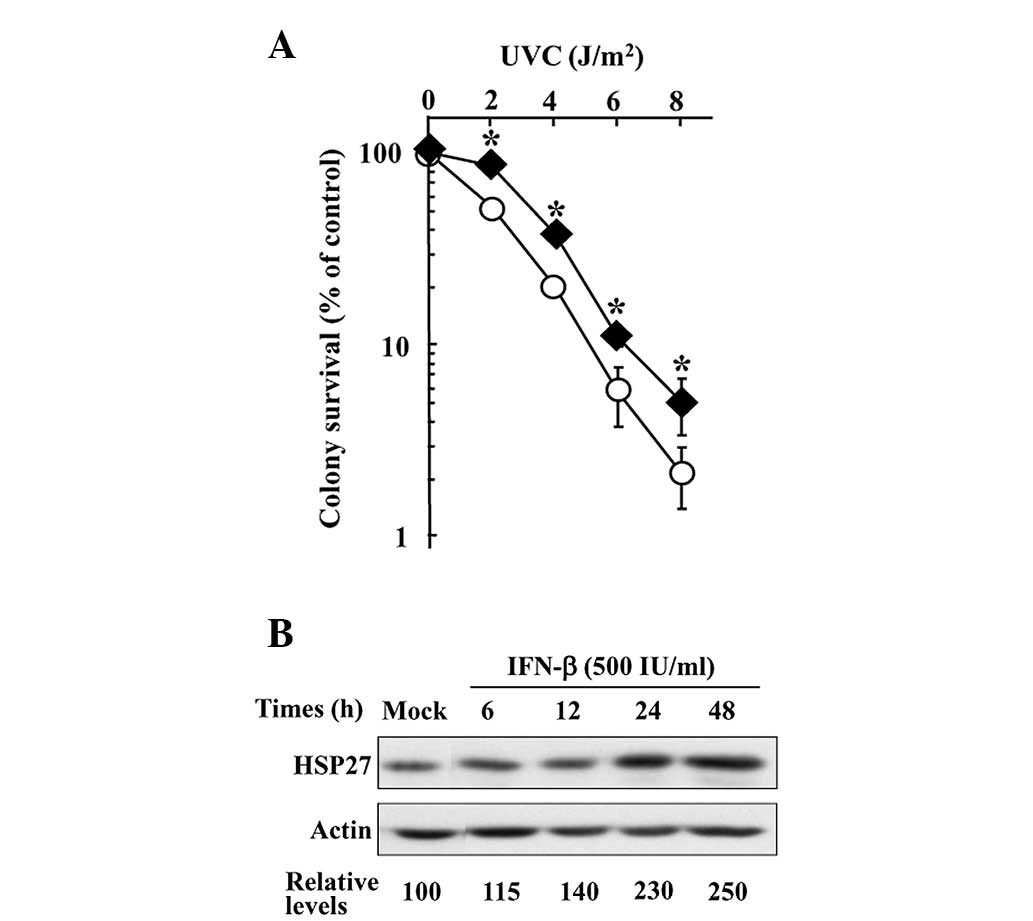
The susceptibility of HuIFN-β-pretreated KT cells to
UVC lethality compared with mock cells without HuIFN-β pretreatment
was tested using the colony survival assay. Following pretreatment
with 50 IU/ml HuIFN-β for 24 h, the cells showed slightly higher
colony survival after UVC irradiation compared with the mock cells
(Fig. 6A).
To determine whether the expression levels of HSP27
increase in HuIFN-β-treated KT cells, comparative analysis of the
expression between HuIFN-β and mock treatment was performed by
western blot analysis (Fig. 6B).
The HSP27 expression levels in cells treated with 500 IU/ml HuIFN-β
for >24 h increased by >2-fold compared with the mock-treated
cells (Fig. 6B).
Discussion
In the present study, the relative roles of HSP27
and GRP78 chaperones in the cellular susceptibility to UVC and
HuIFN lethality were investigated between two human breast cancer
cell lines, MCF-7 and KT cells.
It appears likely that GRP78 plays a role in the
susceptibility of human cells to DNA damaging agents, e.g., a
protective role against UVC-induced cell death, possibly via
enhancement of the DNA-nucleotide excision repair ability (1). Previously, we suggested that the
protective role of GRP78 is associated with its constitutively
expressed levels rather than the UVC-induced expression levels, and
that GRP78 is involved in regulating the metabolism of nucleotide
excision repair enzymes. KT cells, examined here, demonstrated
higher levels of GRP78 expression than MCF-7 cells (Fig. 2), although the former cells were
more sensitive to UVC lethality than the latter cells (Fig. 1). Thus, GRP78 expression levels do
not appear to be associated with the UVC-sensitivity discrepancy
between KT and MCF-7 cells, and the involvement of GRP78 expression
in the UVC resistance of KT cells was unclear. However, KT cells
transfected with GRP78 siRNA showed a decreased capacity of
colony survival after UVC irradiation greater than 4
J/m2 compared with the NC siRNA transfectants (Fig. 3B). Therefore, GRP78 expression
levels may be partially associated with the UVC resistance of KT
cells, although the resistance is low.
HSP27 appears to be another chaperone responsible
for the UVC resistance of human cells (2). The UVC resistance may be due to
increased DNA-repair capacity, including removal of UVC-induced
damage, thymine dimers (6-4) and photoproducts (2). As HSP27-overexpressing KT cells
showed increased levels of colony survival (Fig. 4A and B), HSP27 may play a role in
the increased UVC resistance of KT cells via the modulation of
DNA-repair mechanisms. However, the UVC-induced DNA-repair capacity
of KT cells appears to be normal (3). Recently, we observed that Annexin II,
an HSP27-binding protein, confers UVC-resistance to human cells
(13,14). Therefore, it is necessary to
further investigate the mechanisms underlying the involvement of
HSP27 and/or its binding protein in UVC resistance.
Yonekura et al (15) reported that HuIFN-γ, but not
HuIFN-α, downregulates HSP27 expression and suppresses the negative
regulation of cell death in cells derived from oral squamous cell
carcinoma. However, in our study, HuIFN-β treatment was found to
upregulate HSP27 expression in KT cells (Fig. 6B), possibly leading to the
increased resistance of KT cells to HuIFN-β (Fig. 5) and UVC lethality (Fig. 6A). Previously, we reported that
HuIFN-α treatment results in a similar increased UVC resistance of
human UVC-sensitive cells (5).
Therefore, HuIFN-α and -β may act differently than HuIFN-γ at the
point of cell survival modulation against UVC. In addition, HSP27
may play a role in cellular HuIFN-β susceptibility in association
with increased resistance of the cells to HuIFN-β lethality
(Table I).
Acknowledgements
This study was partly supported by
grants-in-aid from the following organizations: the Miso Central
Institute of Japan, the Smoking Research Foundation, the Tokyo
Foundation for a Better Environment, the Ministry of Health, the
Labour and Welfare for the Intractable Diseases Treatment Research
Program and the Japan Society for the Promotion of Science
(Japan).
References
|
1
|
Zhai L, Kita K, Wano C, Wu Y, Sugaya S and
Suzuki N: Decreased cell survival and DNA repair capacity after UVC
irradiation in association with down-regulation of GRP78/BiP in
human RSa cells. Exp Cell Res. 305:244–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wano C, Kita K, Takahashi S, Sugaya S,
Hino M, Hosoya H and Suzuki N: Protective role of HSP27 against
UVC-induced cell death in human cells. Exp Cell Res. 298:584–592.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki N, Inaba N, Sugano I, Umehara S,
Murakami T and Takakubo Y: Establishment and characterization of a
human cell strain, KT, with high sensitivity to UV-killing and to
cell proliferation inhibition by interferon. Jpn J Cancer Res.
79:1184–1192. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki N, Watanabe I, Nishimaki J, Fuse A,
Sugita K, Sekiya S, Takakubo Y and Terao K: Increased resistance to
the anticellular effect of interferon in an ultraviolet
light-resistant human cell line, UVr-1. J Gen Virol. 67:651–661.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki N, Suzuki H, Kojima T, Sugita K,
Takakubo Y and Okamoto S: Effects of human interferon on cellular
response to UV in UV-sensitive human cell strains. Mutat Res.
198:207–214. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugita K, Suzuki N, Higuchi Y, Kita K,
Suzuki Y and Lehmann A: Enhancement of XPG mRNA expression by human
interferon-beta in Cockayne syndrome cells. Mutat Res. 408:67–72.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Isogai E, Ishijima S, Sonoda T, et al:
Protease activation following UV irradiation is linked to
hypomutability in human cells selected for resistance to
combination of UV and antipain. Mutat Res. 403:215–222. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka T, Sugaya S, Kita K, et al:
Inhibition of cell viability by human IFN-β is mediated by
microRNA-431. Int J Oncol. 40:1470–1476. 2012.
|
|
9
|
Okubo MA, Chiba S, Nishikata T, Matsuno A
and Hosoya H: Generation and characterization of a monoclonal
antibody, mH1, raised against mitotic HeLa cells. Dev Growth
Differ. 41:381–389. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hino M, Kurogi K, Okubo MA, Murata-Hori M
and Hosoya H: Small heat shock protein 27 (HSP27) associates with
tubulin/microtubules in HeLa cells. Biochem Biophys Res Commun.
271:164–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang X, Ren Q, Chen SP, Tong XB, Dong M,
Sugaya S, Tanaka T, Kita K and Suzuki N: UVC mutagenicity is
suppressed in Japanese miso-treated human RSa cells, possibly via
GRP78 expression. Biosci Biotechnol Biochem. 75:1685–1691. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin YH, Kita K, Sun Z, Tong XB, Nie H and
Suzuki N: The roles of HSP27 and Annexin II in resistance to
UVC-induced cell death: comparative studies between the human
UVC-sensitive and -resistant cell lines, RSa and APr-1.
Biosci Biotec Biochem. 73:1318–1322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tong XB, Kita K, Karata K, Zhu C, Sugaya
S, Ichimura Y, Satoh M, Tomonaga T, Nomura F, Jin YH and Suzuki N:
Annexin II, a novel HSP27-interacted protein, is involved in
resistance to UVC-induced cell death in human APr-1
cells. Photochem Photobiol. 84:1455–1461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kita K, Sugita K, Chen SP, Suzuki T,
Sugaya S, Tanaka T, Jin YH, Satoh T, Tong XB and Suzuki N:
Extracellular recombinant annexin II confers UVC resistance and
increases the Bcl-xL to Bax protein ratios in human UVC-sensitive
cells. Radiat Res. 176:732–742. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yonekura N, Yokota S, Yonekura K, Dehari
H, Arata S, Kohama G and Fujii N: Interferon-gamma downregulates
Hsp27 expression and suppresses the negative regulation of cell
death in oral squamous cell carcinoma lines. Cell Death Differ.
10:313–322. 2003. View Article : Google Scholar : PubMed/NCBI
|