Introduction
Cancer of the brain and central nervous system
results in an estimated 142,000 mortalities per year, worldwide
(1). The prognosis is poor for
brain cancer patients, with 5-year survival rates of less than
one-third (2). There are also
indications that the incidences of glioma and meningioma have
increased over the past few decades (3). However, there are few
well-established risk factors for glioma and meningioma among
adults. Although exposure to ionizing radiation and rare inherited
genetic conditions, such as neurofibromatosis (4), are known to increase risk, these risk
factors only explain a small fraction of reported brain tumors
(5).
Diabetes mellitus (DM) is a serious and growing
health problem worldwide and is associated with severe acute and
chronic complications that negatively influence the quality of life
and survival of affected individuals (6). DM has been recognized as a
significant risk factor in several types of cancer, including
cancer of the breast, endometrium, pancreas and liver (7–10).
One mechanism to explain the correlation between DM and the risk of
cancer is based on the hypothesis that the effect of the insulin
and insulin-like growth factors (IGFs) axis triggers intracellular
signaling cascades with mitogenic and antiapoptotic effects
(11,12). Additionally, studies have found
that DM patients have greater oxidative damage to their DNA as
measured by the concentration of 8-hydroxy deoxyguanosine in
mononuclear cells (13).
Due to inconsistent reports on the correlation
between diabetes and brain tumor risk (14–26),
the purpose of this study was to summarize all available evidence
from published studies and to estimate the risk of brain tumors in
patients with diabetes following the meta-analysis of the published
studies. Available data were also analyzed according to the various
study characteristics.
Materials and methods
Study identification
The electronic databases of PubMed and Embase were
searched (up to May 24, 2012) using the following search terms:
‘diabetes’, ‘diabetes mellitus’, ‘DM’, ‘brain’, ‘CNS’, ‘Central
Nervous System’, ‘cancer’, ‘neoplasm’, ‘tumor’, ‘incidence’,
‘risk’, ‘occurrence’, ‘mortality’ and combinations of these terms.
All indexed studies were retrieved and we also reviewed the
reference lists of the identified publications to discover
additional pertinent studies. No language restrictions were
imposed. The literature search was carried out independently by two
investigators.
Inclusion and exclusion criteria
The selection criteria were that the study: i) was
published as an original article; ii) had DM as the exposure of
interest; iii) had brain tumor incidence or mortality as the
outcome of interest; iv) provided relative risk (RR), odds ratio
(OR), hazard ratio (HR) or standardized incidence/mortality rate
(SIR/SMR) with the corresponding 95% confidence intervals (CIs), or
presented original data from which to calculate them; and v) at
least took age as a confounding factor into consideration in the
calculation of RR and corresponding 95% CIs. When there was overlap
in the study populations between published papers, only the most
recent or complete study was included.
Data extraction
The following data from each included study were
extracted using a standardized data-collection protocol: the first
author’s name, country of origin, publication year, numbers of
cases and subjects, sample size, definition of the study
population, ascertainment of exposure and outcome, type of DM,
participant characteristics (gender composition), duration of
follow-up and variables adjusted for in the analysis. When several
risk estimates were presented, we used those adjusted for the
largest number of potential confounding factors. Data abstraction
was performed independently by two investigators and then
cross-checked.
Statistical analysis
A meta-analysis of brain tumor risk was conducted.
RRs were used as effect estimates. However, some studies reported
using OR, HR or SIR estimates. Due to the rare occurrence of brain
tumors, we assumed that all these measures would yield similar
effect estimates and they were considered equally in the overall
effect estimate. Summary RR estimates and the corresponding 95% CIs
were calculated for all studies combined and by subgroups using the
methods of DerSimonian and Laird with the assumptions of a
random-effects model that considered intra- and inter-study
variation (27). If studies
reported RRs for each gender or various types of DM, we calculated
a pooled RR and its corresponding 95% CI to determine the overall
effect. Statistical heterogeneity between studies was evaluated
using Cochran’s Q test and the I2 statistic (28). For the Q statistic, P<0.10 was
considered to indicate statistically significant heterogeneity, and
a value of I2>50% was also considered to indicate
significant heterogeneity (29).
Potential sources of heterogeneity were explored by meta-regression
analysis. Funnel plots and Begg’s test were used to assess the
potential publication bias (30).
Statistical analyses were carried out with STATA version 11.0
(StataCorp, College Station, TX, USA). All tests were two-sided.
P<0.05 was considered to indicate statistically significant
differences.
Results
Search results and study
characteristics
A total of 13 studies, including the entire Danish
population, 5,107,506 other participants and over 2,206 cases of
brain tumors, were found to match our inclusion criteria. Of these
13 studies, 4 were conducted in the Asia-Pacific region, 2 in the
United States and 7 in Europe. Characteristics of the studies
included in the meta-analysis are shown in Table I.
 | Table ICharacteristics of cohort studies of
diabetes and brain tumor incidence and mortality. |
Table I
Characteristics of cohort studies of
diabetes and brain tumor incidence and mortality.
| First author
(Refs.) | Source and
starting-ending year | Study design | No. of
participants | Diabetes
assessment | Outcome
ascertainment | Cases | Type of DM | FU (years) | Confounding
factors |
|---|
| Adami HO (14) | Sweden 1965–1983 | Cohort | 51,008 (M/F) | Medical records | Cancer registry | 66 | No assessment | Mean 5.2 | 1,5 |
| Wideroff L (15) | Denmark
1977–1989 | Cohort | 109,581 (M/F) | Discharge
diagnosis | Cancer registry | 159 | No assessment | Mean 5.7 | 1,2,5,11 |
| Zendehdel K (16) | Sweden 1965–1999 | Cohort | 29,187 (M/F) | Discharge
diagnosis | Cancer registry | 32 | Type 1 | Mean 14.4 | 1,2,5,11 |
| Coughlin SS (17) | USA 1982–1998 | Cohort | 1,056,243 (M/F) | Self-report | Mortality
registry | 87 | No assessment | Mean 14.7 | 1,4,7,8,9,10,
13,14,15,16 |
| Jee SH (18) | Korea 1992–2002 | Cohort | 829,770 (M) | Self-report or blood
glucose level | Cancer registry and
records | NA | No assessment | Mean 10 | 1,3,8,9 |
| Swerdlow AJ (19) | UK 1972–1993 | Cohort | 28,900 (M/F) | Discharge
diagnosis | Cancer registry | 16 | Type 1 and 2 | Mean 18 | 1,2,5,17 |
| Chodick G (20) | Israel 2000–2010 | Cohort | 100,595 (M/F) | Self-report or blood
glucose level | Cancer registry | 12 | No assessment | Mean 8 | 1,6,7,19,26 |
| Hemminki K (21) | Sweden 1964–2007 | Cohort | 125,126 (M/F) | Medical records | Cancer registry | 304 | Type 2 | Median 15 | 1,2,6,18,20,29 |
| Shu X (22) | Sweden
1964–2006 | Cohort | 24,052 (M/F) | Discharge
diagnosis | Cancer
registry | 20 | Type 1 | Mean 18.3 | 2,6,27,28 |
| Atchison EA
(23) | USA 1969–1996 | Cohort | 594,815 (M) | Discharge
diagnosis | Cancer
registry | 527 | No assessment | Mean 10.5 | 1,2,4,18,23 |
| Lam EK (24) | Asia Pacific region
NA | Cohort | 367,361 (M/F) | Self report,
diagnosis or blood glucose level | NA | 168 | No assessment | Median 4 | 1 |
| Carstensen B
(25) | Denmark
1995–2009 | Cohort | The Danish
population (M/F) | Medical
records | Cancer
registry | 418 | No assessment | NA | 1,2,5,12,21,22 |
| Lo SF (26) | Taiwan
1996–2009 | Cohort | 1,790,868
(M/F) | Medical
records | Cancer
registry | 397 | Type 2 | Median 3.5 | 1,2,23,24,25 |
Quantitative data synthesis
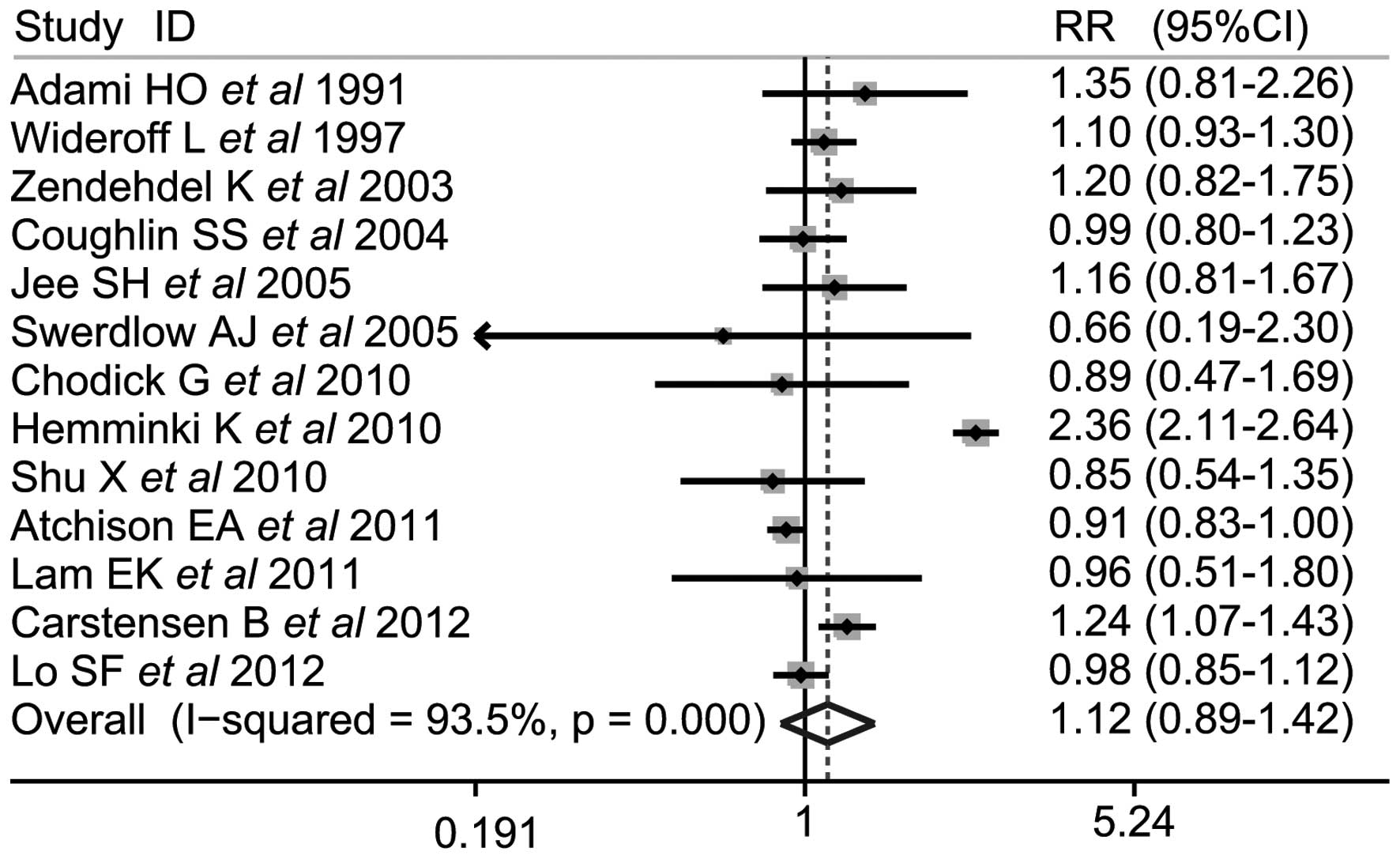
The combined results based on all studies
demonstrated that there was a there was a similar correlation
between DM and brain tumor risk (SRR, 1.12; 95% CI, 0.89–1.42; Q,
185.57; P<0.001, I2, 93.5%; Fig. 1).
We then conducted subgroup meta-analyses by gender,
geographical region, types of DM and level of adjustments. A
statistically significant positive correlation was detected between
DM and brain tumor risk in females (SRR, 1.242; 95% CI,
1.026–1.502) but not in males (SRR, 1.024; 95% CI, 0.938–1.119),
and there was clear heterogeneity in the analysis of female
subjects (P=0.035; I2, 55.8%). Furthermore, positive
associations were observed between diabetes and brain tumor risk in
the diabetes assessment by self-report and by blood glucose level
groups (SRR, 1.136; 95% CI, 1.017–1.268). However, no differences
were found in brain tumor risk with diabetes between strata in the
geographic region, types of diabetes, the number of cases,
population size, duration of follow-up and level of confounding
factors (Table II).
 | Table IISubgroup analysis of relative risks
for the association of diabetes with brain tumor risk. |
Table II
Subgroup analysis of relative risks
for the association of diabetes with brain tumor risk.
| | | Heterogeneity
|
|---|
| Study | Studies | RR (95% CI) | Q | P-value |
I2(%) |
|---|
| Total | 13 | 1.121
(0.887–1.417) | 185.57 | <0.001 | 93.5 |
| Gender | | | | | |
| Male | 8 | 1.024
(0.938–1.119) | 9.82 | 0.278 | 18.5 |
| Female | 6 | 1.242
(1.026–1.502) | 13.58 | 0.035 | 55.8 |
| Geographic
region | | | | | |
| Asia Pacific | 4 | 0.995
(0.879–1.417) | 0.87 | 0.834 | 0 |
| Europe | 7 | 1.257
(0.888–1.779) | 88.87 | <0.001 | 93.2 |
| North
America | 2 | 0.922
(0.846–1.005) | 0.48 | 0.487 | 0 |
| Types of
diabetes | | | | | |
| Type 1 | 3 | 1.04
(0.803–1.348) | 15.21 | 0.033 | 54 |
| Type 2 | 3 | 1.177
(0.525–2.638) | 97.13 | <0.001 | 97.9 |
| No
assessment | 8 | 1.061
(0.939–1.198) | 15.21 | 0.033 | 54 |
| Diabetes
assessment | | | | | |
| Self-report or
blood glucose level | 6 | 1.136
(1.017–1.268) | 4.41 | 0.492 | 0 |
| Medical diagnosis
or records | 7 | 1.186
(0.831–1.693) | 179.77 | <0.001 | 96.7 |
| Population
size | | | | | |
| ≥300,000 | 6 | 1.027
(0.909–1.161) | 12.92 | 0.024 | 61.3 |
| <300,000 | 7 | 1.192
(0.79–1.8) | 77.94 | <0.001 | 92.3 |
| Cases among
subjects | | | | | |
| ≥150 | 6 | 1.199
(0.844–1.704) | 177.8 | <0.001 | 97.2 |
| <150 | 6 | 1.023
(0.873–1.199) | 3.17 | 0.674 | 0 |
| Follow-up time
(years) | | | | | |
| ≥10 | 7 | 1.13
(0.733–1.741) | 170.96 | <0.001 | 96.5 |
| <10 | 5 | 1.034
(0.935–1.143) | 2.44 | 0.656 | 0 |
| Level of considered
confounding factors | | | | | |
| ≥5 | 6 | 1.168
(0.809–1.685) | 180.17 | <0.001 | 97.2 |
| <5 | 7 | 1.099
(0.969–1.245) | 2.92 | 0.819 | 0 |
Meta-regression analyses were conducted to
investigate the sources of heterogeneity between studies according
to the above subgroups, however, we did not find a significant
source of heterogeneity. Subsequently, we investigated whether the
source of heterogeneity was a single study using a meta-regression
analysis and found that the study by Hemminki et al
(21) explained 88.91%
heterogeneity.
A sensitivity analysis was carried out by omitting
one study at a time and calculating the pooled RRs for the
remainder of studies. The study by Hemminki et al (21) appeared to have a strong influence
on the meta-analysis estimate of effect. After this study was
excluded, we found that the pooled SRR changed slightly (SRR,
1.038; 95% CI, 0.950–1.134). We did not observe notable changes
when the other studies were omitted.
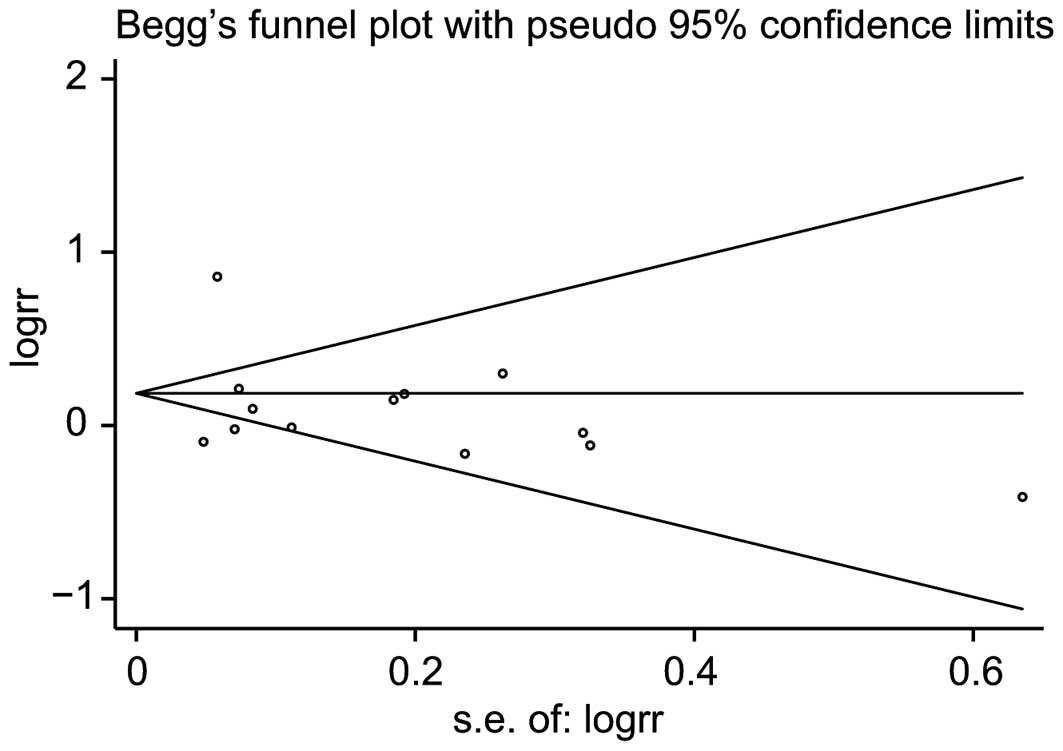
In the publication bias test the shape of the funnel
plots appeared to be symmetrical for all studies investigating DM
and the risk of brain tumors (Fig.
2). Begg’s test did not suggest any evidence of publication
bias (P=0.669).
Discussion
In this meta-analysis, we revealed that DM was
associated with a 12% increased risk of brain tumors, however, this
correlation was not statistically significant. However, a
significant positive correlation between diabetes and brain tumor
risk was observed in females, but not in males. This result was
independent of the geographic region, type of diabetes, number of
cases, population size, duration of follow-up and the level of
confounding factors.
The null link between a history of diabetes and the
risk of brain tumors is particularly notable since one of the
hypothesized mechanisms for this association is via insulin
resistance with secondary hyperinsulinemia. Hyperinsulinemia has
been shown to increase the concentration of bio-available IGF-1 by
reducing the concentration of IGF-binding proteins(31). The insulin and IGF axes are crucial
in cell proliferation and apoptosis and thus may affect
carcinogenesis(31,32). IGFs also exert an important role in
the differentiation, proliferation and apoptosis of brain cells in
early brain development and this may be a biologically feasible
mechanism for an association between brain tumor risk and
diabetes(33). However, it was
demonstrated that although high concentrations of IGF-I are
positively correlated with the risk of low-grade gliomas and
acoustic neuromas, they are not correlated with the risk of
high-grade gliomas and meningiomas(34).
Increased circulating insulin levels have a number
of indirect effects, including decreasing the hepatic synthesis and
blood levels of sex hormone-binding globulin, leading to increases
in bio-available estrogen levels in males and females (12). Findings suggest that female sex
hormones are protective against glioma (35). Since gliomas, including some of the
most lethal types of cancer, account for over 80% of brain and
central nervous system cancers (36), this may be a plausible mechanism to
explain the null link between a history of diabetes and the risk of
brain tumors when the large proportion of gliomas in brain tumor
cases is taken into consideration.
In the subgroup analysis stratified by gender, our
results showed that diabetes was associated with a significantly
increased risk of brain tumors in females. Increased levels of
insulin in blood circulation have been shown to induce an increase
in the level of bio-available testosterone in females but not in
males (12). Furthermore, obese
males have lower levels of testosterone (37). There is evidence that testosterone
stimulates cell growth and local production of IGF-I and IGF-I-R
(38). This evidence may provide a
plausible explanation for the positive correlation of brain tumor
risk with diabetes in females, but not in diabetic males.
The strengths of the present study are as follows:
i) our meta-analysis was based on 13 studies, the majority of which
were prospective studies, thereby minimizing the possibility of
recall or selection bias; ii) all included studies evaluated
multiple potential confounding factors, some of which were
considered to be risk factors for cancer, such as alcohol use,
smoking and body mass index; iii) the varied populations of the
studies expanded on prior observational studies by permitting
additional subgroup evaluation (e.g., by gender, geographic region,
type of DM and sources of population).
As with any meta-analysis of observational studies,
there are several potential limitations to the results of this
meta-analysis. Firstly, significant heterogeneity existed across
studies, throwing some doubt on the reliability of the summary RR
estimates. Although we found the main source of heterogeneity to be
one study, we are unable to account for how this study differed
from the others. Secondly, the majority of studies included in this
meta-analysis did not distinguish between type 1 and type 2 DM.
This non-differential misclassification may distort the magnitude
of the association between DM and the risk of brain tumors.
Thirdly, the history of DM may also reflect other factors
associated with an unhealthy lifestyle, such as smoking, heavy
alcohol consumption and obesity. Such unhealthy lifestyles have
generally been associated with an increased risk of cancer.
However, some authors did not adjust for those risk factors.
Fourthly, the status of DM was self-reported in some studies. This
may contribute to bias in the diabetes assessments. Additionally,
the majority of studies did not consider the role of anti-diabetic
drugs on the occurrence and mortality of brain tumors. This role
may also contribute to bias. Finally, as in any meta-analysis, it
is possible that an observed association is the result of
publication bias, since small studies with null results tend not to
be published. However, the results obtained from the funnel plot
analysis and formal statistical tests did not provide evidence for
such bias.
In summary, the results of this meta-analysis
suggest a non-significant association between diabetes and the risk
of brain tumors. However, compared with non-diabetic patients,
diabetic females may have a slightly increased risk of brain
tumors, whereas this was not the case in diabetic males. It should
be noted that this meta-analysis does not provide firm evidence of
any association between DM and brain tumor risk. Future studies are
required to determine the role of a history of DM in brain tumor
incidence or mortality.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Brenner H: Long-term survival rates of
cancer patients achieved by the end of the 20th century: a period
analysis. Lancet. 360:1131–1135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deltour I, Johansen C, Auvinen A,
Feychting M, Klaeboe L and Schüz J: Time trends in brain tumour
incidence rates in Denmark, Finland, Norway, and Sweden, 1974–2003.
J Natl Cancer Inst. 101:1721–1724. 2009.
|
|
4
|
Martuza RL, Seizinger BR, Jacoby LB,
Rouleau GA and Gusella JF: The molecular biology of human glial
tumors. Trends Neurosci. 11:22–27. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McKinney PA: Brain tumours: incidence,
survival, and aetiology. J Neurol Nerosurg Psychiatry. 75(Suppl 2):
ii12–ii17. 2004.PubMed/NCBI
|
|
6
|
Vigneri P, Frasca F, Sciacca L, Pandini G
and Vigneri R: Diabetes and cancer. Endocr Relat Cancer.
16:1103–1123. 2009. View Article : Google Scholar
|
|
7
|
Heidemann C, Boeing H, Pischon T,
Nöthlings U, Joost HG and Schulze MB: Association of a diabetes
risk score with risk of myocardial infarction, stroke, specific
types of cancer, and mortality: a prospective study in the European
Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam
cohort. Eur J Epidemiol. 24:281–288. 2009. View Article : Google Scholar
|
|
8
|
Saltzman BS, Doherty JA, Hill DA,
Beresford SA, Voigt LF, Chen C and Weiss NS: Diabetes and
endometrial cancer: an evaluation of the modifying effects of other
known risk factors. Am J Epidemiol. 167:607–614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ben Q, Cai Q, Li Z, Yuan Y, Ning X, Deng S
and Wang K: The relationship between new-onset diabetes mellitus
and pancreatic cancer risk: a case-control study. Eur J Cancer.
47:248–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang CS, Yao WJ, Chang TT, Wang ST and
Chou P: The impact of type 2 diabetes on the development of
hepatocellular carcinoma in different viral hepatitis statuses.
Cancer Epidemiol Biomarkers Prev. 18:2054–2060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frasca F, Pandini G, Sciacca L, Pezzino V,
Squatrito S, Belfiore A and Vigneri R: The role of insulin
receptors and IGF-I receptors in cancer and other diseases. Arch
Physiol Biochem. 114:23–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin CS, Moon BS, Park KS, Kim SY, Park
SJ, Chung MH and Lee HK: Serum 8-hydroxy-guanine levels are
increased in diabetic patients. Diabetes Care. 24:733–737. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adami HO, McLaughlin J, Ekbom A, Berne C,
Silverman D, Hacker D and Persson I: Cancer risk in patients with
diabetes mellitus. Cancer Causes Control. 2:307–14. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wideroff L, Gridley G, Mellemkjaer L, Chow
WH, Linet M, Keehn S, Borch-Johnsen K and Olsen JH: Cancer
incidence in a population-based cohort of patients hospitalized
with diabetes mellitus in Denmark. J Natl Cancer Inst.
89:1360–1365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zendehdel K, Nyrén O, Ostenson CG, Adami
HO, Ekbom A and Ye W: Cancer incidence in patients with type 1
diabetes mellitus: a population-based cohort study in Sweden. J
Natl Cancer Inst. 95:1797–1800. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coughlin SS, Calle EE, Teras LR, Petrelli
J and Thun MJ: Diabetes mellitus as a predictor of cancer mortality
in a large cohort of US adults. Am J Epidemiol. 159:1160–1167.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jee SH, Ohrr H, Sull JW, Yun JE, Ji M and
Samet JM: Fasting serum glucose level and cancer risk in Korean men
and women. JAMA. 293:194–202. 2005. View Article : Google Scholar
|
|
19
|
Swerdlow AJ, Laing SP, Qiao Z, Slater SD,
Burden AC, Botha JL, Waugh NR, Morris AD, Gatling W, Gale EA,
Patterson CC and Keen H: Cancer incidence and mortality in patients
with insulin-treated diabetes: a UK cohort study. Br J Cancer.
92:2070–2075. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chodick G, Heymann AD, Rosenmann L, Green
MS, Flash S, Porath A, Kokia E and Shalev V: Diabetes and risk of
incident cancer: a large population-based cohort study in Israel.
Cancer Causes Control. 21:879–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hemminki K, Li X, Sundquist J and
Sundquist K: Risk of cancer following hospitalization for type 2
diabetes. Oncologist. 15:548–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shu X, Ji J, Li X, Sundquist J, Sundquist
K and Hemminki K: Cancer risk among patients hospitalized for Type
1 diabetes mellitus: a population-based cohort study in Sweden.
Diabet Med. 27:791–797. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Atchison EA, Gridley G, Carreon JD,
Leitzmann MF and McGlynn KA: Risk of cancer in a large cohort of
U.S. veterans with diabetes. Int J Cancer. 128:635–643. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lam EK, Batty GD, Huxley RR, Martiniuk AL,
Barzi F, Lam TH, Lawes CM, Giles GG, Welborn T, Ueshima H,
Tamakoshi A, Woo J, Kim HC, Fang X, Czernichow S and Woodward M;
Asia Pacific Cohort Studies Collaboration: Associations of diabetes
mellitus with site-specific cancer mortality in the Asia-Pacific
region. Ann Oncol. 22:730–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carstensen B, Witte DR and Friis S: Cancer
occurrence in Danish diabetic patients: duration and insulin
effects. Diabetologia. 55:948–958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo SF, Chang SN, Muo CH, Chen SY, Liao FY,
Dee SW, Chen PC and Sung FC: Modest increase in risk of specific
types of cancer types in type 2 diabetes mellitus patients. Int J
Cancer. Apr 17–2012.(Epub ahead of print).
|
|
27
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giovannucci E: Insulin, insulin-like
growth factors and colon cancer: a review of the evidence. J Nutr.
131(11 Suppl): S3109–S3120. 2001.PubMed/NCBI
|
|
32
|
Adachi Y, Li R, Yamamoto H, Min Y, Piao W,
Wang Y, Imsumran A, Li H, Arimura Y, Lee CT, Imai K, Carbone DP and
Shinomura Y: Insulin-like growth factor-I receptor blockade reduces
the invasiveness of gastrointestinal cancers via blocking
production of matrilysin. Carcinogenesis. 30:1305–1313. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Russo VC, Gluckman PD, Feldman EL and
Werther GA: The insulin-like growth factor system and its
pleiotropic functions in brain. Endocr Rev. 26:916–943. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rohrmann S, Linseisen J, Becker S, et al:
Concentrations of IGF-I and IGFBP-3 and brain tumor risk in the
European Prospective Investigation into Cancer and Nutrition.
Cancer Epidemiol Biomarkers Prev. 20:2174–2182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cowppli-Bony A, Bouvier G, Rué M, Loiseau
H, Vital A, Lebailly P, Fabbro-Peray P and Baldi I: Brain tumors
and hormonal factors: review of the epidemiological literature.
Cancer Causes Control. 22:697–714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Central Brain Tumor Registry of the United
States (CBTRUS): Statistical Report: Primary Brain Tumors in the
United States, 2000–2004. CBTRUS; 2008, http://www.cbtrus.org/index.htmluri.
|
|
37
|
Field AE, Colditz GA, Willett WC, Longcope
C and McKinley JB: The relation of smoking, age, relative weight,
and dietary intake to serum adrenal steroids, sex hormones, and sex
hormone-binding globulin in middle-aged men. J Clin Endocrinol
Metab. 79:1310–1316. 1994.PubMed/NCBI
|
|
38
|
Maor G, Segev Y and Phillip M:
Testosterone stimulates insulin- like growth factor-I and
insulin-like growth factor-I-receptor gene expression in the
mandibular condyle - a model of endochondral ossification.
Endocrinology. 140:1901–1910. 1999.PubMed/NCBI
|