Introduction
Acute pancreatitis is an inflammatory lesion,
characterized by activation of pancreatic proenzymes with
autodigestion, proteolysis, edema, vascular damage, interstitial
hemorrhage, coagulation and fat necrosis. Severe acute pancreatitis
(SAP) is a dangerous and complicated disease with a high mortality
rate (1,2). SAP has various pathophysiological
manifestations, including cardiovascular, pulmonary, renal, central
nervous system and metabolic disorders (3–5).
Certain cases even develop rhabdomyolysis, which is a complication
rarely reported among the metabolic disorders induced by acute
pancreatitis (6,7).
Rhabdomyolysis is defined as injury of skeletal
muscle cells and leakage of its contents, including the enzyme
creatine kinase (CK), electrolytes and myoglobin. Rhabdomyolysis is
a severe pathological process caused by infection, diabetic
ketoacidosis, trauma, sedation drugs, muscle relaxants and
hyperthermia (8,9). Rhabdomyolysis induced by SAP belongs
to the non-traumatic type.
Alcohol abuse is associated with an increased risk
of acute pancreatitis and rhabdomyolysis. The pathophysiological
role of alcohol in the etiology and occurrence of acute
pancreatitis is complex, but increased oxidative stress, disruption
of cytosolic calcium homeostasis and changes in gene expression in
the pancreas appear to be involved (10,11).
Ethanol also causes rhabdomyolysis via disruption of adenosine
triphosphatase pump function, breakdown of the muscle membrane and
alteration of the sarcoplasmic reticulum (12). However, few studies have reported
the prognosis and association of SAP and rhabdomyolysis in alcohol
abuse patients.
In the present study, we summarize two cases of SAP
complicated by rhabdomyolysis in the Hepatobiliary Surgery
Intensive Care Unit of the PLA General Hospital, Beijing, China,
presenting with increased serum CK, increased myoglobin
concentration and acute renal failure. We investigated
characteristic clinical manifestations, laboratory findings and
treatment strategy of SAP to highlight the features of this
association.
Case reports
Case 1
Case history
A 30-year-old male presented with acute abdominal
pain and fever for 4 days after heavy alcohol consumption and was
admitted to the hospital. The patient had a history of chronic
alcoholism for 10 years. The patient also had diabetes mellitus and
severe fatty liver which was also observed in family members.
Physical examination. The results of physcial
examination were as follows: body temperature, 38°C; pulse rate,
160 bpm; respiratory rate, 35 bpm; and blood pressure, 110/70 mmHg.
The conjunctivae were neither icteric nor anemic. The lungs were
clear and heart sounds were normal. Neither hepatosplenomegaly nor
edema were identified. Palpation revealed a distended abdomen and
muscle tenderness on the left side. The chest roentgenogram and
electrocardiogram were normal. Laboratory tests on admission
indicated pancreatitis with elevated levels of serum pancreatic
enzymes. Serum biochemical indicators are listed in Table I. The Ranson score at admission was
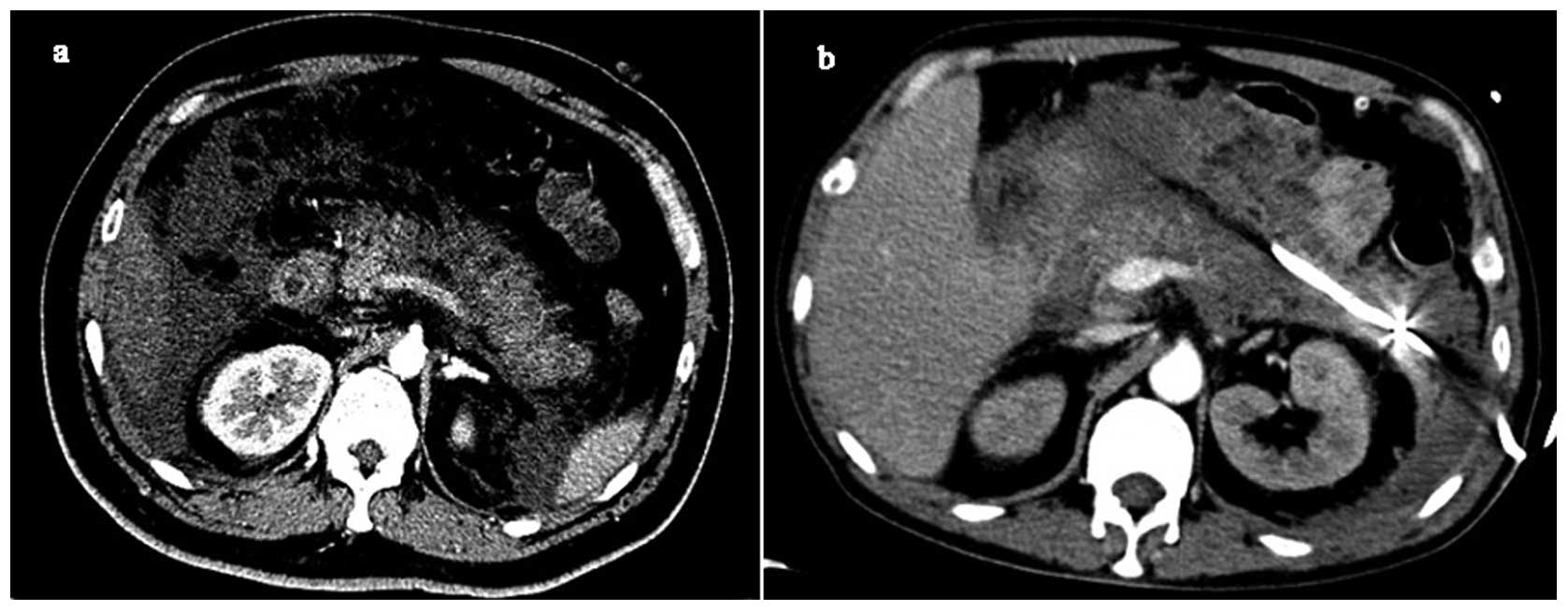
1. Ultrasonography and computed tomography (CT) of the abdomen
revealed marked swelling and pancreatic effusion (Fig. 1a). An enhanced CT scan demonstrated
necrosis in the pancreas and the Balthazar CT scan was grade E.
 | Table IPathological parameters. |
Table I
Pathological parameters.
| Indicators
(units) | Case 1 | Case 2 |
|---|
| Peak CK (U/l) | 338,800 | 19,820.1 |
| Peak CK-MB
(ng/ml) | 29.6 | 19.4 |
| Myoglobin
(μg/l) | 7,315.0 | 2,219.0 |
| Amylase (U/l) | 1,091.9 | 1,707.7 |
| Lipase (U/l) | 4,093.9 | 1,678.1 |
| LDH (U/l) | 21,378.2 | 2,010.6 |
| ALT (U/l) | 3,006.5 | 2,114.2 |
| AST (U/l) | 14,578.1 | 12,046.0 |
| BUN (mmol/l) | 26.9 | 21.8 |
| Cr
(μmol/l) | 358.1 | 389.6 |
| Ca (mmol/l) | 1.54 | 1.10 |
| P (mmol/l) | 2.81 | 2.26 |
| K (mmol/l) | 6.3 | 5.5 |
| WBCs
(×109/l) | 20.3 | 15.1 |
| Hb (g/l) | 83.0 | 71.0 |
| Plts
(×109/l) | 42.0 | 76.0 |
| PT (sec) | 24.1 | 19.5 |
| aPTT (sec) | 42.8 | 39.3 |
| PTA (%) | 24.0 | 21.0 |
| Fib (g/l) | 1.93 | 2.25 |
| INR | 2.5 | 1.9 |
Diagnosis. Based on these results, we
diagnosed the patient with a case of fulminating acute
pancreatitis, due to alcohol intake and secondary multiple organ
failure (MOF).
Treatment strategy. Avoidance of food and
water, gastrointestinal suction, neutralization of acid, restraint
of pancreatic enzyme treatment, anti-inflammatory drugs, fluid
replacement and organ function support were the chosen treatment
strategies for this patient. Five hours after admission, the
patient developed early signs of shock that included respiratory
difficulty, decreased SaO2 and blood pressure, and
oliguria. Immediate salvage measures were performed.
Intra-abdominal pressure reached 13 cmH2O which
continued to rise. A drainage tube was inserted into the abdominal
cavity to decrease the intra-abdominal pressure. On the second day
after admission, patient laboratory data revealed the following:
serum CK level 22,160 U/l, lactate dehydrogenase (LDH) level
4,916.2 U/l, glutamic-pyruvic transaminase (ALT) level 3,006.5 U/l
and glutamic-oxalacetic transaminase (AST) level 6,578 U/l. The
Ranson score at 48 h was 4 (PaO2 increased to 83 mmHg in
6 liters of O2; blood urea, 18 mmol/l; serum calcium,
1.65 mmol/l). On the third day, two additional abdominal drainage
tubes were inserted. However, the abdominal pressure remained high
(34 mmHg) after drainage. The effluent of blood filtration was
pink. Serum potassium and myoglobin levels were elevated. CK and
LDH levels were 15,180 U/l and 17,130 U/l, respectively. These
parameters indicated the presence of hemolysis and rhabdomyolysis.
Plasma exchange was then carried out. On the fourth day after
admission, the peak CK level was 338,800 U/l; and the LDH level was
21,378.2 U/l. On the fifth day after admission, the patient’s
condition markedly deteriorated, with blood pressure severely
lowered and the presence of metabolic acidosis and disseminated
intravascular coagulation (DIC). The patient’s family members
requested to stop treatment and therapy was terminated.
Case 2
Case history
A 58-year-old male was admitted to the PLA General
Hospital for abdominal pain for 2 weeks and the pain was becoming
more severe over 2 days. The patient had a history of heavy alcohol
consumption prior to the onset of these symptoms.
Physical examination. Physical examination
revealed the following: body temperature, 36.5°C; pulse rate, 100
bpm; respiratory rate, 30 bpm; and blood pressure, 138/87 mmHg.
Abdominal distension, muscle tension and tenderness were observed.
Laboratory findings on admission showed serum amylase to be 1,707.7
U/l and lipase 1,678.1 U/l (Table
I). A CT scan revealed stones in the gall bladder, a pancreatic
swelling, obvious effusion and marked necrosis (Fig. 1b).
Diagnosis. Based on these results, a
diagnosis of fulminating acute pancreatitis, gall bladder lithiasis
and rheumatoid arthritis was made.
Treatment strategy. Avoidance of food and
water, gastrointestinal suctioning, neutralization of acid, control
of pancreatic enzyme incretion, anti-inflammatory drugs, fluid
replacement and organ function support were the selected treatment
methods used. We performed endotracheal intubation and mechanical
ventilation on the first day. On the second day, the patient’s
laboratory data showed that the serum CK level peaked at 19,820.1
U/l; and the CK-MB level was 19.4 ng/ml. The abdominocentesis fluid
was dark red blood. A drainage tube was inserted into the abdominal
cavity. On the third day, the patient was treated with blood
filtration, abdominal cavity pressure monitor and anti-infection
treatments. Seven days after admission, the patient developed
pancreatic encephalopathy. At eleven days after admission, we
performed a back peritoneal incision and inserted a drainage tube.
Thirteen days after admission the abdominal drainage tube presented
with blood. The patient’s hemoglobin level decreased and
coagulation function deteriorated. Transfusion and operation were
carried out to open the abdomenal cavity for exploration and to
remove the necrotic tissue around the pancreas to insert an
abdominal drainage tube. After operation, the patient’s renal and
coagulation function worsened, manifesting in anuria and errhysis
from the incision and the abdominal drainage tube. Red blood cells
(RBCs), fresh frozen plasma (FFP) and platelets were administered
via transfusion. On the third day after operation, the patient had
abdominal bleeding, MOF, decreased blood glucose, severe metabolic
acidosis and hypotention. The patient succumbed on the fourth day
after operation.
Discussion
Severe acute pancreatitis (SAP) is a dangerous and
complicated disease with a high mortality rate. The severity of SAP
is assessed by the Ranson score, serious complications, organ
failure and/or laparotomy (13,14).
MOF presents in 93% of SAP cases (15). SAP was found to lead to a 10–30%
mortality rate in the majority of prospective series (16,17).
In the present study, laboratory test and CT scan results showed
that serum amylase and lipase levels were increased and the
pancreas displayed swelling, obvious effusion and necrosis. These
clinical symptoms supported the diagnosis of SAP.
Obstruction of the common bile duct by stones (38%)
and alcohol abuse (36%) are the most frequent causes of acute
pancreatitis. In the present study, gall bladder lithiasis was
detected by CT scan in case 2, but this symptom was not diagnosed
as the main cause of SAP. Alcohol abuse is the second most frequent
cause of acute pancreatitis. Acute pancreatitis develops in 10% of
chronic alcohol abusers (>80 g daily intake). The present
patients admitted to extreme alcohol consumption before acute
abdominal pain occurred. SAP was considered the result of multiple
pathogenic factors in the present cases, but alcohol abuse might be
the critical direct cause. A previous study reported that alcohol
plays an important role in the onset of both acute pancreatitis and
rhabdomyolysis (18). In the
present cases, alcohol might be contributed to both the onset and
progression of SAP complicated by rhabdomyolysis.
Acute rhabdomyolysis is a complication rarely seen
in acute pancreatitis. Several studies have described cases of an
association between acute pancreatitis and rhabdomyolysis.
Nankivell and Gillies reported, in a retrospective study, that
asymptomatic rhabdomyolysis occurs in 14/548 (2.6%) of patients
with acute pancreatitis (15).
Another report showed that its prevalence varied from 2 to 7%
(19). However, the incidence of
rhabdomyolysis in SAP has not been extensively reported. In the
present study, we reported two patients with severe pancreatitis
developing non-traumatic rhabdomyolysis with CPK >10,000 IU/l.
CK is the most sensitive indicator of muscle cell injury. There is
diagnostic value for rhabdomyolysis when the serum CK level is
elevated five times or more than the normal concentration. In this
report, patient peak CK was elevated 3–5 days after the onset of
pancreatitis and all exceeded 10,000 IU/l. Serum or urine myoglobin
is another rhabdomyolysis indicator. In this report, serum
myoglobin concentrations were elevated in all cases and all
exceeded twice the upper normal limit. Both cases developed marked
myoglobinuria. Myocardial infarction was excluded.
Pezzilli et al measured the serum and urine
myoglobin concentrations and demonstrated a close correlation
between the severity of acute pancreatitis and rhabdomyolysis
(19). Pezzilli et al
suggested that renal failure that follows acute pancreatitis is
partly due to rhabdomyolysis and elevated serum concentrations of
myoglobin. Rhabdomyolysis-related kidney failure is associated with
a poor prognosis for patients with SAP. Hypocalcemia is another
poor prognostic indicator in acute pancreatitis. When pancreatitis
and rhabdomyolysis coexist, hypocalcemia is common (93%), severe
and prolonged. Rhabdomyolysis may aggravate hypocalcemia.
Therefore, rhabdomyolysis should be worthy of attention if patients
are observed to have secondary worsening of renal function and/or
profound hypocalcemia.
The treatment strategy for SAP-induced
rhabdomyolysis is administration of full fluid resuscitation in
order to maintain adequate urine output. Initial fluid requirements
were more than 6 liters in the first 48 h. If patients still
suffered oliguria after fluid resuscitation, blood purification
treatment including plasma exchange or perfusion should be
immediately performed. Maintaince of homeostasis and prevention of
complications are also necessary.
The condition of the patients with SAP complicated
by rhabdomyolysis deteriorated rapidly. Patients with a higher
Ranson score and organ failure score often have a longer average
intensive care unit stay, poor treatment outcome and high mortality
rate. In the present cases, although standard treatment of organ
support was utilized, patients were unable to recover from the
severe circumstances. Intra-abdominal hypertension and
rhabdomyolysis are difficult to control. All patients developed MOF
and, ultimately, succumbed. A study reported that the mortality of
patients with SAP and rhabdomyolysis was approximately 79%
(15). The prognostic significant
of rhabdomyolysis during acute pancreatitis is currently being
debated. Based on the present cases, we conclude that alcohol
abuse-induced SAP complicated by rhabdomyolysis demonstrated a poor
prognosis and high mortality.
Acknowledgements
We appreciate the support from Chinese
Army twelve-five project (number: CWS11J109).
References
|
1
|
de Beaux AC, Palmer KR and Carter DC:
Factors influencing morbidity and mortality in acute pancreatitis;
an analysis of 279 cases. Gut. 37:121–126. 1995.PubMed/NCBI
|
|
2
|
Fenton-Lee D and Imrie CW: Pancreatic
necrosis: assessment of outcome related to quality of life and cost
of management. Br J Surg. 80:1579–1582. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balthazar EJ: Acute pancreatitis:
assessment of severity with clinical and CT evaluation. Radiology.
223:603–613. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Casas JD, Díaz R, Valderas G, Mariscal A
and Cuadras P: Prognostic value of CT in the early assessment of
patients with acute pancreatitis. AJR Am J Roentgenol. 182:569–574.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dugernier TL, Laterre PF, Wittebole X, et
al: Compart-mentalization of the inflammatory response during acute
pancreatitis: correlation with local and systemic complications. Am
J Respir Crit Care Med. 168:148–157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ali M and Abdalla H: Salmonella
typhi infection complicated by rhabdomyolysis, pancreatitis and
polyneuropathy. Arab J Nephrol Transplant. 4:91–93. 2011.
|
|
7
|
Khan FY, Al-Ani A and Ali HA: Typhoid
rhabdomyolysis with acute renal failure and acute pancreatitis: a
case report and review of the literature. Int J Infect Dis.
13:e282–e285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parekh R, Care DA and Tainter CR:
Rhabdomyolysis: advances in diagnosis and treatment. Emerg Med
Pract. 14:1–15. 2012.
|
|
9
|
Gabow PA, Kaehny WD and Kelleher SP: The
spectrum of rhabdomyolysis. Medicine (Baltimore). 61:141–152. 1982.
View Article : Google Scholar
|
|
10
|
Palmieri VO, Grattagliano I and Palasciano
G: Ethanol induces secretion of oxidized proteins by pancreatic
acinar cells. Cell Biol Toxicol. 23:459–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siech M, Zhou Z, Zhou S, et al:
Stimulation of stellate cells by injured acinar cells: a model of
acute pancreatitis induced by alcohol and fat (VLDL). Am J Physiol
Gastrointest Liver Physiol. 297:G1163–G1171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Antoon JW and Chakraborti C:
Corticosteroids in the treatment of alcohol-induced rhabdomyolysis.
Mayo Clin Proc. 86:1005–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lellouche N, Bruneel F, Troche G, et al: A
rare cause of rhabdomyolysis: acute pancreatitis. Gastroenterol
Clin Biol. 27:1172–1174. 2003.(In French).
|
|
14
|
Abdul-Ghaffar NU and el-Sonbaty MR:
Pancreatitis and rhabdomyolysis associated with
lovastatin-gemfibrozil therapy. J Clin Gastroenterol. 21:340–341.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nankivell BJ and Gillies AH: Acute
pancreatitis and rhabdomyolysis: a new association. Aust N Z J Med.
21:414–417. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knochel JP: Rhabdomyolysis and
myoglobinuria. Annu Rev Med. 33:435–443. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piaścik M, Rydzewska G, Milewski J, et al:
The results of severe acute pancreatitis treatment with continuous
regional arterial infusion of protease inhibitor and antibiotic: a
randomized controlled study. Pancreas. 39:863–867. 2010.
|
|
18
|
Nakano S, Mugikura M, Endoh M, Ogami Y and
Otsuki M: Acute pancreatitis with diabetic ketoacidosis associated
with hypermyoglobinemia, acute renal failure, and DIC. J
Gastroenterol. 31:623–626. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pezzilli R, Billi P, Cappelletti O,
Barakat B and Miglio F: Rhabdomyolysis and acute pancreatitis. J
Gastroenterol Hepatol. 14:168–171. 1999. View Article : Google Scholar
|