Introduction
Carcinogenesis is considered to be a multi-stage
process involving the initiation, promotion and progression of
tumor cells, which may arise as a result of DNA damage, mutations,
clonal expansion of preneoplastic cells and dysregulation of
oncogenes and/or tumor suppressor genes.
Embryonic stem cells (ESCs) have self-renewal
potential and may be differentiated into lineage-specific cell
types, including hepatocytes (1).
In the liver, stem cells are located in the ductal plates in
fetuses and neonates and in the canals of Hering in infants and
adults (2). As stem cells have the
potential to survive, DNA damage induced by carcinogens may remain
as cell proliferation occurs. Furthermore, stem cells with a loss
of DNA repair function may be susceptible to malignant
transformation, either directly or through the emergence of
cancer-prone stem cells (3). Cell
proliferation at the time of carcinogen exposure may be pivotal for
the fixation of genotoxic injury as a heritable form (4).
It has been proposed that hepatocellular and ductal
carcinomas originate from liver stem cells and that enzyme-altered
foci and nodular changes are adaptive non-oncogenic responses to
the toxic effects of carcinogens (5). Liver tumors appear to be
hierarchically organized and sustained by a distinct subpopulation
of cancer stem cells (6). This
suggests that stem cells have a significant role in
hepatocarcinogenesis.
As a putative stem cell marker, the epithelial cell
adhesion molecule (EpCAM) is a membrane glycoprotein highly
expressed on the majority of cancer cells (7), although it is also expressed on the
majority of normal epithelial cells. In humans, EpCAM-positive
hepatocytes have been found to be rare in the early stages of liver
disease. However, they became increasingly prominent during the
later stages and are consistently arrayed around the periphery of
cords of keratin 19-positive hepatobiliary cells in the ductular
reaction (8). Human EpCAM-positive
hepatocellular carcinomas (HCCs) exhibit a distinct molecular
signature with the features of hepatic progenitor cells (HPCs),
including the presence of known stem cell/progenitor cell markers,
including cytokeratin 19 and c-Kit, whereas EpCAM-negative HCCs
exhibit a gene expression profile with the features of mature
hepatocytes (9). Thus, EpCAM
expression may be elevated during human liver tumor
progression.
EpCAM expression in mouse liver carcinogenesis has
not yet been reported. In the present study, we investigated the
expression of EpCAM in diethylnitrosamine (DEN)-induced hepatic
tumors. DEN is widely used in mouse liver cancer models (10) and has been used in several mouse
strains (11–14). Based on the finding that young
animals treated with DEN exhibited a higher incidence of liver
tumors than older animals (15),
we also assessed the effects of DEN on the expression of EpCAM and
proliferating cell nuclear antigen (PCNA) in hepatic cells. The
assessment was carried out at various developmental stages, from
ESCs to HPCs and hepatocyte-like cells (HCs).
Materials and methods
Animals and treatment
ICR mice (Koatec Inc., Pyungtaek, Korea) were housed
in a room maintained on a 12 h light/dark cycle and at a constant
temperature and humidity. The mice were allowed free access to a
pellet chow diet (Koatec Inc.) during the experiment. Male mice
were bred with females, yielding the F1 generation. Male F1 mice at
14 days of age were injected intraperitoneally with DEN (10 mg/kg
body weight; Sigma, St. Louis, MO, USA) dissolved in 0.9% saline.
Ten mice treated with DEN were sacrificed 36 weeks later and
hepatic masses were sampled for histopathological examination.
Histopathological examination of hepatic
tumors
The hepatic masses (n=71) were fixed in 10% neutral
phosphate-buffered formalin, embedded in paraffin, sectioned to a
thickness of 4 μm and stained with hematoxylin and eosin.
Tumor characteristics were classified based on histopathological
and cytological criteria.
Immunohistochemical analysis of EpCAM in
hepatic tumors
The avidin-biotin complex method was used to stain
EpCAM in 4-μm sections of liver tissues. The sections were
dewaxed in xylene, hydrated using a graded ethanol series and
boiled in sodium citrate buffer (pH 6.0) in an autoclave for 20
min. Then, they were sequentially treated with 0.3% hydrogen
peroxide, blocking buffer containing skimmed milk and the
anti-EpCAM antibody (ab32392; Abcam, Cambridge, MA, USA; diluted
1:400). The sections were washed with TBS-T and subjected to the
ABC-peroxidase procedure (ABC kit; Vector Laboratories, Burlingame,
CA, USA). As a negative control, skimmed milk was used instead of
the primary antibody.
The immune complexes were visualized using the
chromogen 3,3′-diaminobenzidine tetrahydrochloride (DAB). The
sections were counterstained with hematoxylin to facilitate their
examination under a light microscope.
Double staining of EpCAM and PCNA in
hepatic tumors
The sections were dewaxed in xylene, hydrated using
a graded ethanol series and boiled in a sodium citrate buffer (pH
6.0) in an autoclave for 20 min. Then they were sequentially
treated with 0.3% hydrogen peroxide, blocking buffer containing
horse serum and anti-PCNA antibody (M879; Dako, Carpinteria, CA,
USA; diluted 1:500) for 1 h. The sections were washed with TBS and
incubated with HRP-polymer (MRT621; Biocare Medical, Concord, CA,
USA). The immune complexes were visualized using DAB.
For concomitant labeling of EpCAM, the sections were
washed with TBS, blocked with goat serum, incubated with anti-EpCAM
antibody (ab32392, Abcam) for 1 h and then incubated with
AP-polymer (RMR625; Biocare Medical) for 30 min. The sections were
washed with TBS, treated with Vulcan Fast Red (FR805H; Biocare
Medical), washed again with TBS, counterstained with hematoxylin
and viewed under a light microscope.
Culture of mouse ESCs and differentiation
of hepatic lineage cells
Mouse NVRQS-11F ESCs were cultured on mitomycin
C-treated mouse embryonic fibroblasts (feeder cells) grown on 0.1%
gelatin-coated dishes in Dulbecco’s modified Eagle’s medium
(Millipore, Billerica, MA, USA), supplemented with 15% fetal bovine
serum (Hyclone, Rockville, MD, USA), 2 mM L-glutamine (Millipore),
0.1% non-essential amino acids (Invitrogen, Carlsbad, CA, USA), 1%
penicillin-streptomycin (Millipore) and 10 ng/ml mouse leukemia
inhibitory factor (Millipore).
To differentiate the ESCs into hepatic lineage
cells, we used the culture conditions for differentiating ESCs into
HPCs and HCs reported by Zhou et al(16).
DEN treatment of ESCs, HPCs and HCs
To determine a non-cytotoxic concentration of DEN,
ESCs were treated with DEN at concentrations of 0–90 mM for 24 h.
Cell viability was estimated by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay and the fluorescence intensity was analyzed using an
ArrayScan VTI HCS Reader (Thermo Scientific, Rockford, IL, USA).
Subsequently, ESCs (day 0), HPCs (day 22 of differentiation) and
HCs (day 40 of differentiation) were treated with four
concentrations of DEN (0, 1, 5 and 15 mM; G1, G2, G3, G4,
respectively) for 24 h.
EpCAM and PCNA mRNA expression in ESC,
HPCs and HCs
RNA was isolated from cultured cells using an
easy-spin Total RNA Extraction kit (Intron Biotechnology,
Scottsdale, AZ, USA), dissolved in DEPC-treated distilled water and
stored at −80°C until use. RNA concentrations were measured using a
UV/Vis Spectrophotometer (DU730; Beckman Coulter, Miami, FL, USA).
The quality of the isolated RNA was assessed using an Agilent 2100
Bioanalyzer (Agilent, Palo Alto, CA, USA) and an Agilent RNA 6000
Nano kit (Agilent).
EpCAM and PCNA mRNA expression was determined by
relative quantitative real-time PCR in 96-well optical plates using
an ABI 7500 Real Time PCR System (Applied Biosystems, Foster City,
CA, USA). Assay-on-Demand TaqMan probes (Applied Biosystems) were
used to measure EpCAM and PCNA mRNA (Table I). A master mix was created
containing the following: 6.25 μl water, 1.25 μl
forward primer (9 μM) and reverse primer (9 μM), 2.5
μl probe mixture (2.5 μM) and 12.5 μl TaqMan
PCR 2X master mixture (Applied Biosystems). Reverse transcribed
total RNA (40 ng in 5 μl) was added as the PCR template.
 | Table IProbe sequences. |
Table I
Probe sequences.
| Assay ID | Probe sequence | Gene symbol | Amplicon size
(bp) |
|---|
| Mm00493214_m1 |
TTGAAAAAGATGTGAAGGGGGAGTC | EpCAM | 95 |
| Mm00448100_g1 |
CAACTTGGAATCCCAGAACAGGAGT | PCNA | 117 |
The following PCR conditions were used: initial
activation of uracyl-N-glycosylase at 50°C for 2 min;
activation of AmpliTaq Gold at 95°C for 10 min; and 45 cycles of
denaturation at 95°C for 15 sec and annealing/extension at 60°C for
1 min. During PCR, the amplified products were continuously
monitored by measuring the fluorescence emission. All PCR assays
were performed in triplicate.
The expression levels of the target genes were
normalized to mouse GAPDH mRNA and were presented as relative
expression. The expression of the genes was normalized to GAPDH,
using the comparative Ct method. The cycle number at
which the fluorescence signal of the target product was detectable
(threshold cycle, Ct) was normalized against the
Ct of GAPDH, to give ΔCt. The expression of
the genes relative to a reference was calculated as
2−ΔΔCt, where ΔΔCt referred to the difference
between the ΔCt values of the test group and the reference.
Statistical analysis
Data were analyzed using the Student’s t-test
with JMP software (SAS Institute, Cary, NC, USA). For all
comparisons, P<0.05 was considered to indicate a statistically
significant difference.
Results
Histopathological examination of hepatic
masses
Histo-pathological examination of the 71 hepatic
masses showed that 48 samples were adenomas and 23 were
adenocarcinomas. The tumor tissues exhibited nuclear pleomorphism
and alteration of cellular structure, with or without fatty liver
and inflammatory cell infiltration.
Immunohistochemical examination of
EpCAM
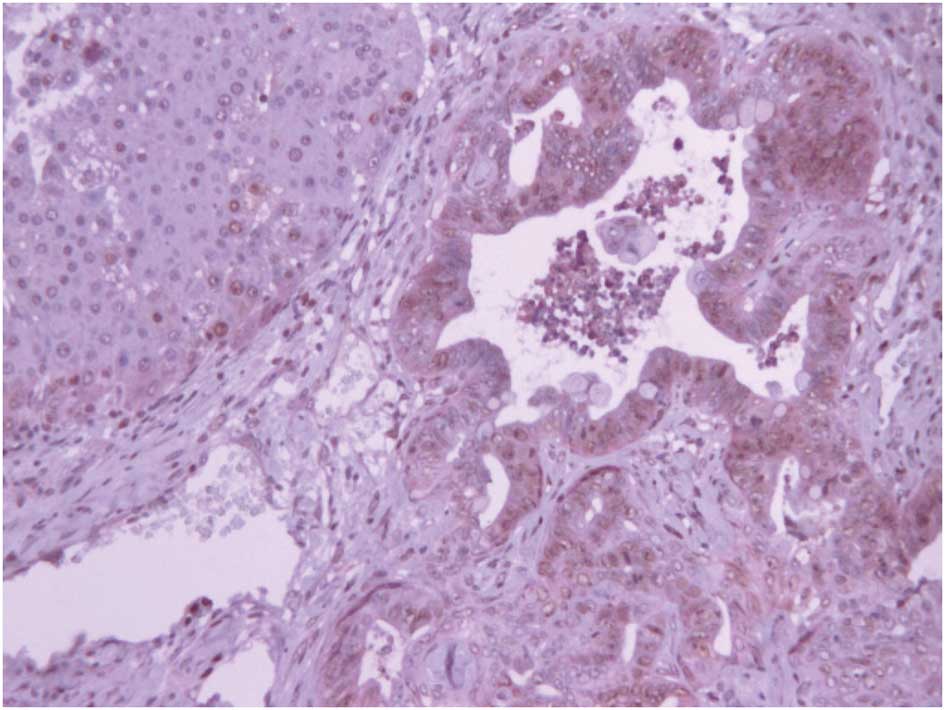
EpCAM was immunohistochemically detected mainly in
hepatic tumor cells, with some expression in the surrounding
visually normal cells, and exhibited a cytoplasmic staining pattern
(Fig. 1). EpCAM expression was
similar in benign and malignant tumors.
Double staining of EpCAM and PCNA
Double staining showed that EpCAM and PCNA were
co-expressed in numerous tumor cells, particularly in dysplastic
ductal tumor cells (Fig. 2). PCNA
showed nuclear staining and EpCAM exhibited cytoplasmic
staining.
EpCAM and PCNA mRNA expression in ESCs,
HPCs and HCs
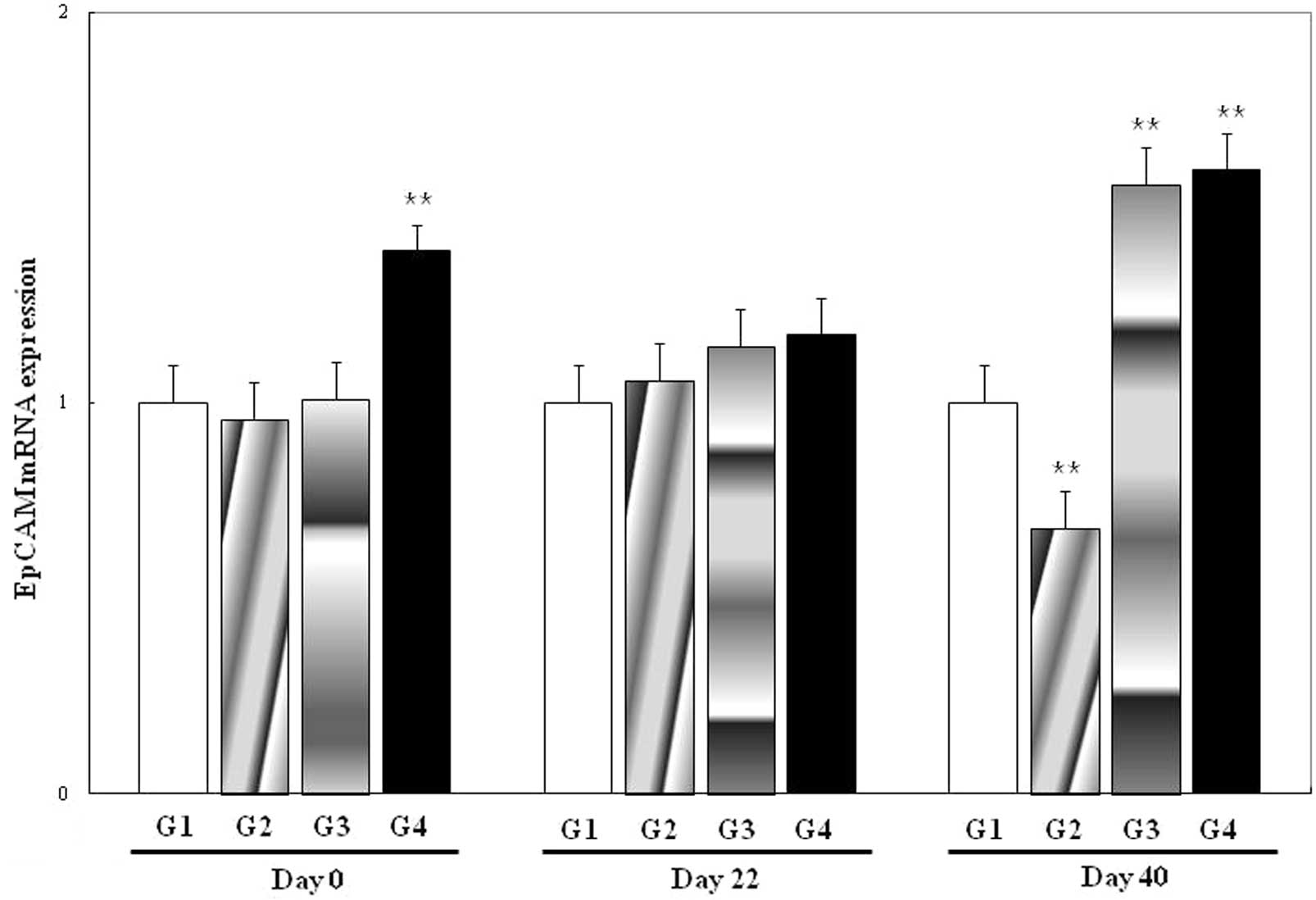
The expression of EpCAM mRNA was significantly
different for G4 at day 0 (P<0.01) and for G2, G3 and G4 at day
40 (P<0.01) compared with the control (G1) at the corresponding
time-points (Fig. 3). There were
no differences for G2 or G3 at day 0, or for G2, G3 or G4 at day
22.
PCNA mRNA expression was significantly different for
G3 and G4 at day 0 (P<0.01), for G2, G3 and G4 at day 22
(P<0.01) and for G2 at day 40 (P<0.01) compared with G1 at
the corresponding time-points (Fig.
4). There were no differences for G2 at day 0 and for G3 or G4
at day 40.
Discussion
In the present study, EpCAM expression was increased
in DEN-induced tumors and was associated with PCNA. Although EpCAM
expression was also detected in surrounding visually normal cells,
its expression was stronger in hepatic tumor cells. It has been
reported that EpCAM-positive HCCs express stem cell and progenitor
cell markers (9). Double staining
revealed that EpCAM and PCNA were co-expressed in numerous tumor
cells, suggesting that EpCAM-positive tumor cells may have the
potential to proliferate.
Previously, EpCAM was identified as an additional
marker of cancer-initiating cells (7) and it was detected in the cytoplasm of
hepatic stem cells and in the plasma membranes of hepatoblasts
(2). EpCAM-positive cells in the
rat liver were shown to be bipotential adult hepatic epithelial
progenitors (17). These findings
indicate that EpCAM may be involved in the early stages of liver
carcinogenesis.
To investigate the role of EpCAM in cancer
initiation, we examined its expression in hepatic cells at various
stages of differentiation. Hepatic differentiation of mouse ESCs
may be induced in a stepwise manner by adding several specific
growth factors following embryoid body formation (18) and functional hepatocytes may be
generated using chemically defined culture conditions (19). In the present study, mouse
NVRQS-11F ESCs were efficiently differentiated into hepatic lineage
cells, HPCs and HCs. This multi-step generation of HCs from ESCs
resembles in vivo hepatogenesis and ESC-derived
hepatogenesis may be useful as a novel integrative model for
hepatocarcinogenesis or for the hepatic toxicity evaluation of a
number of chemicals.
DEN treatment enhanced EpCAM expression in ESCs,
although EpCAM expression may be lost as the progeny of adult liver
stem cells differentiate toward HPCs. However, DEN treatment
induced the upregulation of EpCAM in HCs. This indicates that
carcinogen treatment altered EpCAM expression in cells at each
stage of differentiation.
Notably, DEN treatment increased PCNA expression at
days 0 and 22, but not at day 40. This may be significant, since
any proliferative cell in the liver may be susceptible to
neoplastic transformation at the time of carcinogen exposure. As
there was a time lag between increased EpCAM expression and cell
proliferation, additional stem cell proteins may be involved.
Further studies are also warranted to investigate the role of other
stem cell markers in the context of hepatocarcinogenesis.
It has been hypothesized that stem cells and/or
progenitor cells are transformed into cancer stem cells (20) by a process involving the
dysregulation of stem cell self-proliferation (21). Considering that the induction of
hepatic tumors by DEN treatment in animals was dependent on age at
carcinogen treatment (22), there
may be more hepatic stem and/or progenitor cells in young animals.
Generally, compared with older animals, young animals are more
sensitive to chemical-induced carcinogenesis (23). Hepatic tumors have been generated
in young animals upon the administration of only a single injection
of DEN (22) and ∼1% of cells
treated with a tumor promoter developed into altered hepatic foci
(24).
In the present study, low-dose DEN treatment
decreased cell proliferation at day 40. Although it is not known
why cell proliferation was not observed at day 40 following middle-
and high-dose DEN treatment, it may be that the differentiation
stage of hepatic cells is a significant factor in cellular
proliferation caused by carcinogen treatment. Further studies are
required to investigate the effects of various hepatocarcinogens on
hepatic cells at various stages of differentiation.
In summary, EpCAM and PCNA expression was increased
in DEN-induced tumors and the expression of EpCAM and PCNA in ESCs,
HPCs and HCs was modulated by DEN treatment. This study contributes
to cancer research by clarifying EpCAM expression in hepatic tumors
and during the early stages of hepatocarcinogenesis.
Acknowledgements
The authors would like to thank Ms. He
Jin Jung, Sung Chan Kim, Ji Ae Seo and Eun-Joo Park for their
technical assistance. This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(2010-0003829).
References
|
1
|
Rambhatla L, Chiu CP, Kundu P, Peng Y and
Carpenter MK: Generation of hepatocyte-like cells from human
embryonic stem cells. Cell Transplant. 12:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner R, Lozoya O, Wang Y, Cardinale V,
Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D and
Reid LM: Human hepatic stem cell and maturational liver lineage
biology. Hepatology. 53:1035–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kenyon J and Gerson SL: The role of DNA
damage repair in aging of adult stem cells. Nucleic Acids Res.
35:7557–7565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alison MR: Liver stem cells: implications
for hepatocarcinogenesis. Stem Cell Rev. 1:253–260. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sell S and Dunsford HA: Evidence for the
stem cell origin of hepatocellular carcinoma and
cholangiocarcinoma. Am J Pathol. 134:1347–1363. 1989.PubMed/NCBI
|
|
6
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van der Gun BT, Melchers LJ, Ruiters MH,
de Leij LF, McLaughlin PM and Rots MG: EpCAM in carcinogenesis: the
good, the bad or the ugly. Carcinogenesis. 31:1913–1921.
2010.PubMed/NCBI
|
|
8
|
Yoon SM, Gerasimidou D, Kuwahara R,
Hytiroglou P, Yoo JE, Park YN and Theise ND: Epithelial cell
adhesion molecule (EpCAM) marks hepatocytes newly derived from
stem/progenitor cells in humans. Hepatology. 53:964–973. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamashita T, Forgues M, Wang W, Kim JW, Ye
Q, Jia H, Budhu A, Zanetti KA, Chen Y, Qin LX, Tang ZY and Wang XW:
EpCAM and alpha-fetoprotein expression defines novel prognostic
subtypes of hepatocellular carcinoma. Cancer Res. 68:1451–1461.
2008. View Article : Google Scholar
|
|
10
|
Fausto N and Campbell JS: Mouse models of
hepatocellular carcinoma. Semin Liver Dis. 30:87–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diwan BA, Rice JM, Ward JM, Ohshima M and
Lynch PH: Inhibition by phenobarbital and lack of effect of
amobarbital on the development of liver tumors induced by
N-nitrosodiethylamine in juvenile B6C3F1 mice. Cancer Lett.
23:223–234. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jang JJ, Weghorst CM, Henneman JR, Devor
DE and Ward JM: Progressive atypia in spontaneous and
N-nitrosodiethylamine-induced hepatocellular adenomas of C3H/HeNCr
mice. Carcinogenesis. 13:1541–1547. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tamano S, Merlino GT and Ward JM: Rapid
development of hepatic tumors in transforming growth factor alpha
transgenic mice associated with increased cell proliferation in
precancerous hepatocellular lesions initiated by
N-nitrosodiethylamine and promoted by phenobarbital.
Carcinogenesis. 15:1791–1798. 1994. View Article : Google Scholar
|
|
14
|
Jensen MR, Factor VM, Fantozzi A, Helin K,
Huh CG and Thorgeirsson SS: Reduced hepatic tumor incidence in
cyclin G1-deficient mice. Hepatology. 37:862–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vesselinovitch SD, Koka M, Mihailovich N
and Rao KV: Carcinogenicity of diethylnitrosamine in newborn,
infant, and adult mice. J Cancer Res Clin Oncol. 108:60–65. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou QJ, Xiang LX, Shao JZ, Hu RZ, Lu YL,
Yao H and Dai LC: In vitro differentiation of hepatic progenitor
cells from mouse embryonic stem cells induced by sodium butyrate. J
Cell Biochem. 100:29–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yovchev MI, Grozdanov PN, Zhou H, Racherla
H, Guha C and Dabeva MD: Identification of adult hepatic progenitor
cells capable of repopulating injured rat liver. Hepatology.
47:636–647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamazaki T, Iiboshi Y, Oka M, Papst PJ,
Meacham AM, Zon LI and Terada N: Hepatic maturation in
differentiating embryonic stem cells in vitro. FEBS Lett.
497:15–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Touboul T, Hannan NR, Corbineau S,
Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H,
Pedersen R, Di Santo J, Weber A and Vallier L: Generation of
functional hepatocytes from human embryonic stem cells under
chemically defined conditions that recapitulate liver development.
Hepatology. 51:1754–1765. 2010. View Article : Google Scholar
|
|
20
|
Wicha MS, Liu S and Dontu G: Cancer stem
cells: an old idea - a paradigm shift. Cancer Res. 66:1883–1890;
discussion 1895–1886, 2006.
|
|
21
|
Al-Hajj M and Clarke MF: Self-renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang JS, Wanibuchi H, Morimura K, Gonzalez
FJ and Fukushima S: Role of CYP2E1 in diethylnitrosamine-induced
hepatocarcinogenesis in vivo. Cancer Res. 67:11141–11146. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gray R, Peto R, Brantom P and Grasso P:
Chronic nitrosamine ingestion in 1040 rodents: the effect of the
choice of nitrosamine, the species studied, and the age of starting
exposure. Cancer Res. 51:6470–6491. 1991.PubMed/NCBI
|
|
24
|
Pitot HC, Dragan YP, Teeguarden J, Hsia S
and Campbell H: Quantitation of multistage carcinogenesis in rat
liver. Toxicol Pathol. 24:119–128. 1996. View Article : Google Scholar : PubMed/NCBI
|