Introduction
Chronic heart failure (CHF) is an ongoing,
progressive syndrome characterized by the impairment of cardiac
function and an increase in neurohormonal activity (1). B-type natriuretic peptide (BNP) is a
neurohormone primarily secreted by the cardiac ventricles which is
now used as a diagnostic marker for CHF since it is easy to obtain
and may be detected rapidly (2).
BNP is a natural antagonist of the renin-angiotensin-aldosterone
system (RAAS). It not only decreases systemic vascular resistance
and central venous pressure, but also decreases blood volume and
cardiac output (3). Since the
levels of BNP are correlated with left ventricular dysfunction,
measurement of BNP may help clinicians diagnose and evaluate heart
failure (HF) rapidly. BNP measurement has become a routine part of
CHF diagnosis (4).
It is well known that a high proportion of CHF
patients have diabetes mellitus (DM), and the prognosis is worse
for coronary heart disease patients who have DM than for those who
do not (5,6). The Hoorn Study revealed that BNP
levels are associated with markers of left ventricular diastolic
function and changes in left ventricular mass. The association of
BNP with left ventricular diastolic function appears to be
particularly strong in individuals with DM (7). Other studies indicated that BNP was
not only associated with left ventricular abnormalities but also
with cardiovascular disease and mortality risk in DM patients
(8,9). Therefore, when BNP is used to assess
cardiovascular disease (CVD) risk in CHF patients, the presence or
absence of DM should be taken into account (10,11).
Meanwhile, certain studies demonstrated that the level of BNP in
CHF patients with DM was higher than in CHF patients without DM,
but provided no relevant evidence from strict control-based
research (12,13).
The aim of the current study was to accurately
investigate the influence of DM on the level of BNP in CHF
patients, using a scaling method based on parameters including age,
hypertension, LVEF, LVW and NYHA degree.
Materials and methods
Subjects
A total of 559 CHF inpatients from our hospital were
enrolled in this study, including 276 patients with coronary heart
disease, 234 with hypertensive heart disease and 49 with dilated
cardiomyopathy diagnosed by coronary angiography. The study was
conducted in accordance with the declaration of Helsinki and with
approval from the Ethics Committee of Nanchang University. Written
informed consent was obtained from all participants. The subjects
were divided into a non-DM group and a DM group which included 384
and 175 patients, respectively. Blood samples for blood routine
tests and biochemical tests of all subjects were drawn in the
morning, following a 12 h fast. Plasma BNP was measured using a
microplate luminometer reader (Centro LB 960; Berthold Technologies
GmbH & Co., Bad Wildbad, Germany) and a Shionoria BNP kit
(Shionogi Company, Osaka, Japan). As the minimum and maximum
detectable concentrations were 0.1 and 4,000 ng/l respectively, BNP
levels <0.1 ng/l were counted as 0.1 ng/l and BNP levels
>4,000 ng/l were counted as 4,000 ng/l in this study. The above
tests were performed in our clinical laboratory, and inter- and
intra-batch coefficient of variations were controlled within 5.5
and 3.5%, respectively.
The left ventricular ejection fraction (LVEF) and
the average thickness of the septal and posterior walls of the left
ventricle (LVW) were detected by echocardiography. The cardiac
function grade was assessed according to the NYHA criterion. Body
weight, body height and seated blood pressure were measured and
body mass index (BMI) was calculated. History of smoking and
aspirin use were recorded.
Hypertension was divided into 3 grades based on
blood pressure levels: grade 1 was systolic blood pressure (SBP)
140–159 mmHg and/or diastolic blood pressure (DBP) 99–90 mmHg;
grade 2 was SBP 160–179 mmHg and/or DBP 100–109 mmHg; and grade 3
was SBP ≥180 mmHg and/or DBP ≥110 mmHg. Patients under DM
treatment, or with fasting plasma glucose >7.0 mmol/l and/or 2 h
postprandial plasma glucose >11.0 mol/l, were classified as
having DM.
Based on the recorded data, parameters relevant to
BNP were collected to calculate the total score for each patient
(Table I). The highest score was
14 points and lowest was 4 points. The correlation curves of BNP
with the score were constructed for the DM and non-DM groups.
 | Table IScoring of the HF-associated
parameters. |
Table I
Scoring of the HF-associated
parameters.
| Score
|
|---|
| Related
parameters | 1 | 2 | 3 |
|---|
| Age (y) | ≤60 | 60–80 | ≥80 |
| NYHA | II | III | IV |
| LVEF (%) | ≥50 | 40–50 | ≤40 |
| LVW (mm) | ≤11 | 11–13 | ≥13 |
| Hypertension | Grade 1 | Grade 2 | Grade 3 |
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software package. All data were expressed as the mean ± standard
deviation (SD). We used an independent sample t-test between the
two groups and linear correlation analysis was performed. A p-value
of <0.05 was considered to indicate a statistically significant
difference.
Results
Table II provides
the general characteristics of the two groups. There were no
differences in age, proportion of male subjects, number of smokers
or blood pressure level between the DM and non-DM groups. However,
white blood cell count, fasting blood glucose and the percentage of
aspirin users in the DM group were higher than those in the non-DM
group. No significant differences in the echocardiographic index,
including LVEF and LVW, were identified between the two groups.
However, the percentage of atrial fibrillation was lower in the DM
than in the non-DM group.
 | Table IISelected characteristics of the two
groups. |
Table II
Selected characteristics of the two
groups.
| CHF with DM
(n=175) | CHF without DM
(n=384) |
|---|
| Age (years) | 68.3±10.6 | 69.5±10.3 |
| Male [N (%)] | 102 (58.2) | 233 (60.6) |
| BMI
(kg/m2) | 25.6±3.5a | 23.3±3.4 |
| Smoking history [N
(%)] | 62 (35.4) | 125 (32.5) |
| Systolic blood
pressure (mmHg) | 142.3±28.6 | 139.2±24.7 |
| Diastolic blood
pressure (mmHg) | 80.6±16.1 | 78.0±13.9 |
| Aspirin use [N
(%)] | 50 (28.5)a | 71 (18.4) |
| NYHA II:III:IV
(N) | 84:71:20 | 189:159:36 |
| Atrial fibrillation
[N (%)] | 25 (14.2)a | 90 (23.4) |
| Laboratory
examinations | | |
| WBC
(109/l) | 8.4±4.8a | 6.9±2.7 |
| RBC
(1012/l) | 4.1±0.7 | 4.1±0.6 |
| HB (g/l) | 121.3±20.8 | 121.9±19.5 |
| RDW (%) | 14.0±1.3 | 13.9±1.3 |
| PLT
(109/l) | 189.8±68.1 | 184.5±65.1 |
| CR (μmol/l) | 92.8±48.7 | 89.3±41.2 |
| FBG (mmol/l) | 8.1±3.6a | 4.87±0.8 |
| Echocardiogram
index | | |
| LVEF (%) | 55.6±12.9 | 57.2±13.2 |
| LVW (mm) | 10.1±1.3 | 10.2±1.4 |
Generally, the plasma BNP level was higher in the DM
group than in the non-DM group (1143.7±94.0 vs. 884.3±57.0 ng/l,
P<0.05). Based on the NYHA grade, the plasma BNP levels of the 3
DM subgroups were higher than those of the 3 non-DM subgroups.
Statistical significance was observed with NYHA II (92.5±72.6 vs.
369.6±25.4 ng/l, P<0.05) and III (1603.8±152.6 vs. 1213.9±76.5
ng/l, P<0.05), but not with NYHA IV (2362.1±186.5 vs.
2249.9±157.0 ng/l, P>0.05; Fig.
1).
Correlations between BNP and
parameters
The linear regression analysis in 559 CHF patients
revealed a negative correlation between plasma BNP level and LVEF
(r=−0.511, P<0.05), but positive correlations between BNP level
and NYHA (r=0.438, P<0.05), age (r=0.214, P<0.05) and LVW
thickness (r=0.182, P<0.05).
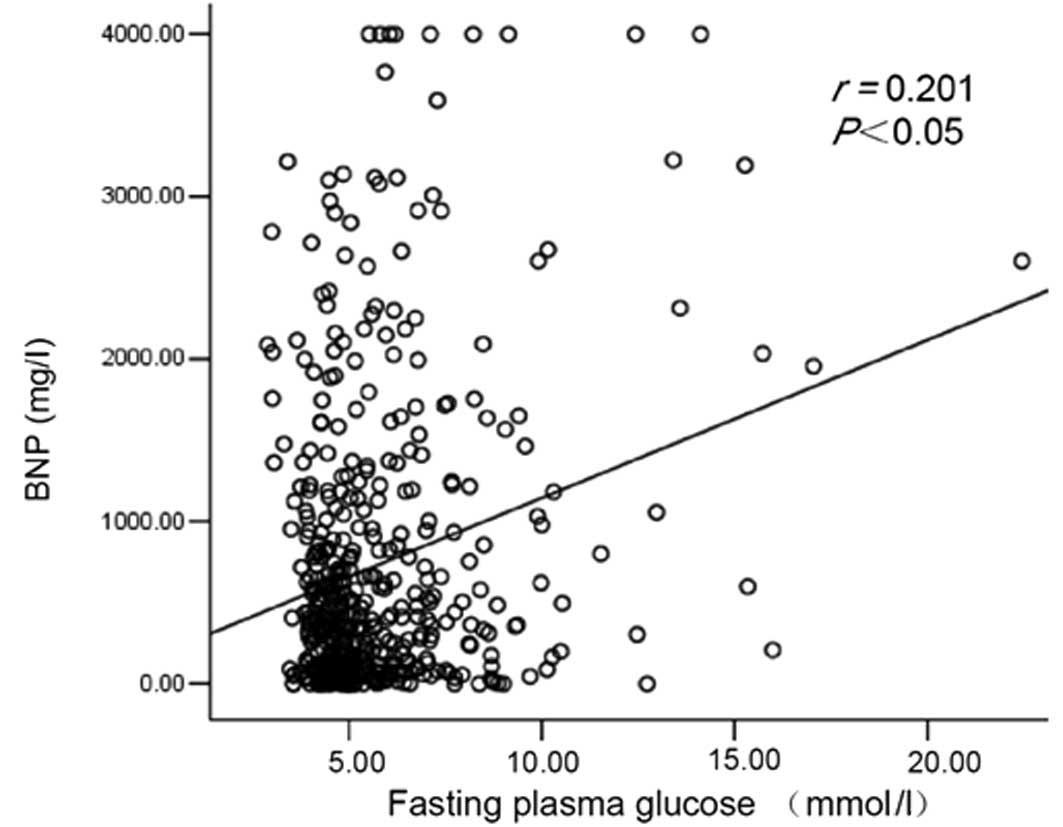
There was also a positive correlation between plasma
BNP and fasting plasma glucose levels (r=0.201, P<0.05; Fig. 2).
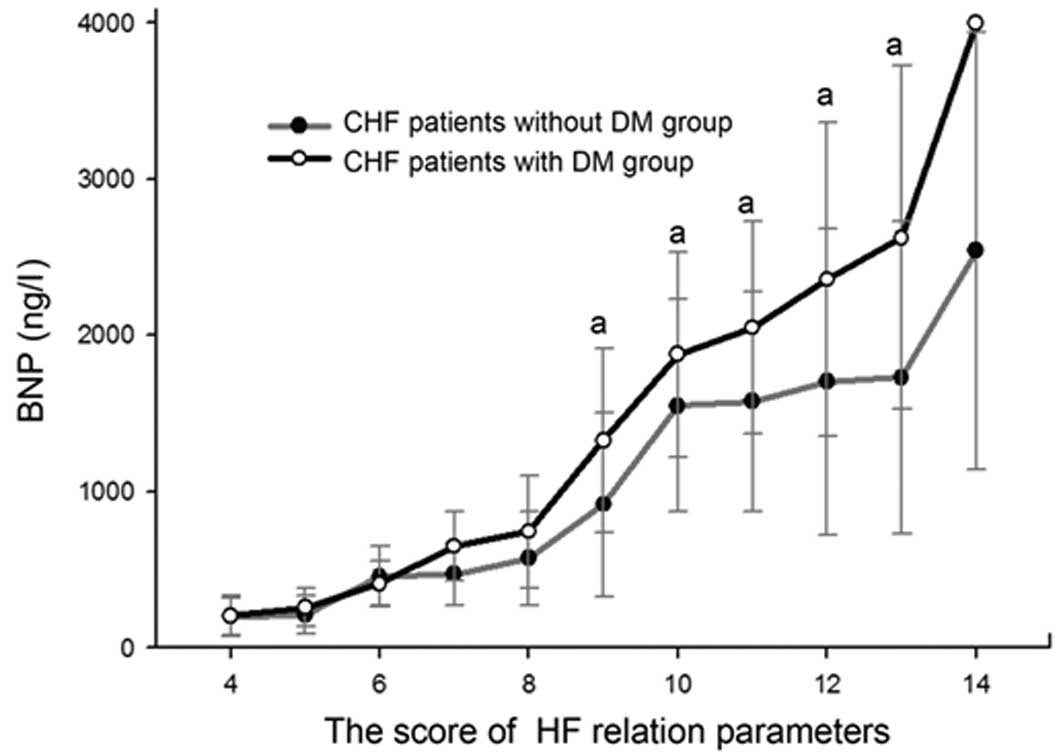
Scores of BNP-related parameters
The BNP-score curve clearly suggests that, for all
patients, plasma BNP levels elevated as the score increased. The
two curves separate at 7 points, after which the curve of the DM
group is higher than the curve of the non-DM group. The plasma BNP
levels of the subgroups with scores of between 9 and 13 points were
significantly higher in the DM group than in the corresponding
subgroups in the non-DM group. The positive correlation of plasma
BNP level with the score was stronger in the DM group (r=0.523)
compared with the non-DM group (r=0.436). The plasma BNP levels of
the two patients with a total score of 14 points in the DM group
were >4,000 ng/l (Fig. 3).
Discussion
BNP is mainly secreted by ventricular muscle cells,
and is directly related to ventricular volume change and
ventricular wall tension. A number of studies have demonstrated
that the level of plasma BNP may sensitively and specifically
reflect the ventricular function and positively correlates with
NYHA cardiac grades (14).
However, various factors, including obesity and
renal insufficiency, may affect the BNP level in CHF patients.
Recently, some studies showed that the level of BNP in CHF patients
with DM was higher than in CHF patients without DM; however, most
of those studies were simple intergroup comparisons rather than
strict control-based research (12,13,15).
In the current study, a total score of the
HF-associated parameters was evaluated for each patient according
to age, hypertension, LVEF, LVW and NYHA degree. The purpose of
this score was to accurately explore the influence of DM on the
level of BNP in CHF patients at the same HF degree.
Our study not only demonstrated that the BNP level
in CHF patients increased as the NYHA degree increased but also
demonstrated that higher BNP levels were observed in the 3 NYHA
subgroups with DM compared with the corresponding subgroups without
DM. However, no significant difference was found in the NYHA IV
subgroup. One reason for this may be the small number of subjects
in that subgroup. Another possible reason is that the reported
upper limit of BNP concentration was 4,000 ng/l, which
underestimates the value of BNP in the DM subgroup, as there were
more patients with BNP higher than 4,000 ng/l in the DM
subgroup.
In this study, a BNP-score curve was introduced for
quantitative evaluation of the extent of HF. Positive correlations
between the score and BNP levels were observed in the DM group and
in the non-DM group. The two curves began to separate at 7 points,
after which the curve of the DM group was persistently above that
of the non-DM group. Among the subgroups with scores of between 9
and 13 points, the plasma BNP levels were significantly higher in
the DM group than in the corresponding subgroups in the non-DM
group. These results further demonstrate that DM is a promoting
factor for higher plasma BNP levels in CHF patients. Research has
shown that BNP levels of coronary heart disease patients with DM
are significantly high compared with those of coronary heart
disease patients without DM.
The underlying mechanism for the higher BNP level in
CHF patients with DM is not clear; however, it may involve an
increase in BNP formation and a decrease in degradation (21).
A previous study has suggested that the kidneys may
secrete natriuretic peptides that aid BNP degradation, such as
neutral endopeptidase (NEP) (17).
In patients with renal impairment, NEP secretion declined and BNP
degradation subsequently decreased. Liu et al found that BNP
levels in DM patients with renal failure were significantly higher
than those in DM patients with normal renal function (18). Siebenhofer et al confirmed
that plasma N-terminal pro-brain Natriuretic Peptide (NT-pro BNP)
levels in DM patients with an abnormal urinary albumin excretion
rate (UAER >20 μg/min) was higher than in DM patients with a
normal UAER (19). Although UAER
was not measured in the current study, as the parameters concerning
renal function were similar between the DM group and the non-DM
group, the elevated BNP may not be due to decreased excretion of
BNP, and mostly due to increased formation.
Certain studies suggest that atrial fibrillation
(AF) may be a cause of the increased BNP level (20). Since the DM group had a lower
percentage of patients with AF than the non-DM group, AF is not a
cause of the higher BNP level in the DM group. As the
echocardiographic parameters such as LVEF and LVW were similar
between the DM group and the non-DM group, the increased BNP
formation unlikely to be caused by more severe cardiac
remodeling.
Based on the positive correlation between the
fasting glucose and BNP levels in this study, it is reasonable to
speculate that higher plasma glucose levels may accelerate BNP
secretion. Higher levels of plasma glucose may induce a hypertonic
state, causing cell dehydration and an increase in blood volume.
All these changes might increase the ventricular tension and
therefore raise the BNP level. Moreover, in patients with DM,
symptoms were often accompanied by vascular lesions more frequently
found in coronary heart disease patients. Coronary heart disease
may not only cause myocardial ischemia, hypoxia, necrosis and
fibrosis, but also increase ventricular wall tensions and promote
BNP secretion (14). Additionally,
the DM patients often had cardiac autonomic dysfunction. A previous
study has confirmed that an elevated BNP level is correlated with
cardiac autonomic dysfunction (21).
Since the plasma BNP level was significantly higher
in the DM group than in the non-DM group at the same HF score, when
plasma BNP level is used to evaluate HF in a clinical environment,
the factor of DM should be taken into consideration.
Acknowledgements
This study was supported by a grant
from the Chinese National Science and Technology Plan (2008
BAI68B02).
References
|
1
|
Dhingra R and Vasan RS: Diabetes and the
risk of heart failure. Heart Fail Clin. 8:125–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen HH and Burnett JC: Natriuretic
peptides in the pathophysiology of congestive heart failure. Curr
Cardiol Rep. 2:198–205. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palazzuoli A, Caputo M, Calabrò A and Nuti
R: Clinical impact of BNP and other emerging biomarkers in heart
failure evaluation and management. Minerva Cardioangiol.
60:183–194. 2012.PubMed/NCBI
|
|
4
|
Oremus M, Raina PS, Santaguida P, et al: A
systematic review of BNP as a predictor of prognosis in persons
with coronary artery disease. Clin Biochem. 41:260–265. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Bibra H and St John Sutton M: Impact
of diabetes on post-infarction heart failure and left ventricular
remodeling. Curr Heart Fail Rep. 8:242–251. 2011.PubMed/NCBI
|
|
6
|
Shen WF: An intriguing association between
congestive heart failure and diabetes mellitus. Chin Med J.
123:643–645. 2010.PubMed/NCBI
|
|
7
|
van den Hurk K, Alssema M, Kamp O, et al:
Slightly elevated B-type natriuretic peptide levels in a non-heart
failure range indicate a worse left ventricular diastolic function
in individuals with, as compared with individuals without, type 2
diabetes: the Hoorn Study. Eur J Heart Fail. 12:958–965. 2010.
|
|
8
|
Aguiar VB, Ochiai ME, Cardoso JN, et al:
Relationship between depression, BNP levels and ventricular
impairment in heart failure. Arq Bras Cardiol. 95:732–737.
2010.PubMed/NCBI
|
|
9
|
From AM, Scott CG and Chen HH: The
development of heart failure in patients with diabetes mellitus and
pre-clinical diastolic dysfunction a population-based study. J Am
Coll Cardiol. 55:300–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelder JC, Cowie MR, McDonagh TA, et al:
Quantifying the added value of BNP in suspected heart failure in
general practice: an individual patient data meta-analysis. Heart.
97:959–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horwich TB and Fonarow GC: Glucose,
obesity, metabolic syndrome, and diabetes relevance to incidence of
heart failure. J Am Coll Cardiol. 55:283–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boonman LJ, Rutten FH, Cramer MJ, et al:
Early recognition of heart failure in patients with diabetes type 2
in primary care. A prospective diagnostic efficiency study. BMC
Public Health. 21:479–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Igarashi M, Jimbu Y, Hiram A and Tominaga
M: Characterization of plasma brain natriuretic peptide level in
patients with type 2 diabetes. Endocr J. 52:352–362.
2005.PubMed/NCBI
|
|
14
|
Albertini JP, Cohen R, Valensi P, Sachs RN
and Charniot JC: B-type natriuretic peptide, a marker of
asymptomatic left ventricular dysfunction in type 2 diabetic
patients. Diabetes Metab. 34:355–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beer S, Golay S and Bardy D: Increased
plasma levels of N-terminal brain natriuretic peptide in type 2
diabetic patients with vascular complications. Diabetes Metab.
31:567–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clerico A, Vittorini S and Passino C:
Circulating forms of the b-type natriuretic peptide prohormone:
pathophysiologic and clinical considerations. Adv Clin Chem.
58:31–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marchant K: Diabetes and chronic kidney
disease: a complex combination. Br J Nurs. 17:356–361. 2008.
View Article : Google Scholar
|
|
18
|
Liu C, Agnes G, Corcuff JB, Bordenave L,
Gin H and Rigalleau V: B-type natriuretic peptide in diabetes
mellitus: the influence of chronic renal failure and food. Diabetes
Care. 28:7522005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siebenhofer A, Ng LL, Plank J, Berghold A,
Hödl R and Pieber TR: Plasma NT-pro BNP in type 1 diabetic patients
with and without diabetic nephropathy. Diabet Med. 20:535–539.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koitabashi T, Inomata T, Niwano S, et al:
Distinguishable optimal levels of plasma B-type natriuretic peptide
in heart failure management based on complicated atrial
fibrillation. Int Heart J. 46:453–464. 2005. View Article : Google Scholar
|
|
21
|
Yufu K, Takahashi N, Nakagawa M, Hara M,
Saikawa T and Yoshimatsu H: Brain natriuretic peptide and cardiac
autonomic function in type 2 diabetic patients. Diabetes Res Clin
Pract. 72:12–19. 2006. View Article : Google Scholar : PubMed/NCBI
|