Introduction
Kupffer cells, the resident hepatic macrophages, are
key in hepatic fibrogenesis and are targets of proinflammatory
mediators (1–3). Kupffer cells are involved in the
liver fibrogenic processes via the production of cytokines and
growth factors which induce the myofibroblastic transformation of
hepatic stellate cells (HSCs) and also via the regulation of the
production of metalloproteinases and their inhibitors (4). In response to environmental signals,
Kupffer cells acquire special phenotypic characteristics with
diverse functions (5). Therefore,
Kupffer cell activation is best described as a wide spectrum of
gradual alterations to the cell phenotype, resulting from a complex
interplay of various activators and signaling pathways (6). It is well-established that the number
of macrophages increases during chronic liver injury and
fibrogenesis (7), although
detailed phenotypic characterizations of human intrahepatic
monocyte-derived cells are lacking at present.
Liver myofibroblasts (LMFs), which are principally
derived from activated HSCs (8),
are able to remodel the liver stroma in response to injury. Thus,
LMFs, which exhibit fibrogenic and contractile properties (9), are considered to be a major
fibrogenic hepatic cell type (10). Furthermore, LMFs are also involved
in promoting hepatic inflammation (11). LMFs accumulate in injured hepatic
areas, where they express cell adhesion molecules and secrete a
number of proinflammatory factors, including interleukin (IL)-6,
hepatocyte growth factor (HGF) and vascular endothelial growth
factor (VEGF), to support the adhesion and migration of
infiltrating lymphocytes (11). In
murine models of liver fibrosis, activated HSCs express the
coinhibitory molecule, B7-H4 which provides a signal to dampen
antigen-specific T cell responses to modulate T cell immunity
(12). Activated HSCs are also
able to control the development of T cell immunity in a non-major
histocompatibility complex (MHC)-restricted fashion by directly
interacting with T cells in a CD54-dependent manner (13). However, at present, it is unclear
how these findings from mouse models precisely relate to liver
diseases in humans.
The ability of lymphocytes and macrophages to
modulate the activation of stromal cells during immune responses is
well documented (2,14), although little is known about the
possible role of LMFs in the immune function of the human liver. In
particular, few studies have investigated whether LMFs shape the
phenotype and function of monocytes in liver disease. Since
sinusoids have numerous open pores, LMFs are also able to interact
with the sinusoid lumen, where antigen-presenting cells (APCs),
including dendritic cells (DCs) and liver macrophages or Kupffer
cells are present (15,16). Consistent with this property, a
previous study demonstrated that coculture with HSCs differentially
affected the expression of chemokine receptors and activation
markers of subsets of monocytes (17). These observations led us to
investigate whether LMFs secrete potent factors to regulate the
phenotype and function of monocytes within inflamed human
livers.
The phenotypes of Kupffer cells in cirrhotic livers
were observed in the present study and the association between LMFs
and Kupffer cells was investigated. Kupffer cells were shown to be
activated, with a concomitant increase in the expression of certain
surface molecules in fibrotic livers, while LMFs regulated the
phenotype and function of monocytes through specific soluble
factors. These results suggest that myofibroblasts are directly
involved in regulating the function of monocytes in the human liver
and that bidirectional interactions are present between the
monocytes and LMFs in the liver microenvironment.
Materials and methods
Specimens
Human specimens were obtained from patients
attending the Sun Yat-sen University affiliated hospitals following
approval by the ethics committee and receiving informed patient
consent. The cirrhotic liver tissues were obtained from patients
undergoing transplantation for alcoholic liver disease, hepatitis
B- or C-associated cirrhosis, drug-induced liver disease, Wilson’s
disease or cryptogenic cirrhosis. The liver tissues from patients
with hepatic hemangioma were used as the normal controls.
Tissue immunofluorescence
For the immunofluorescence analysis, the liver
tissues were cut into 5-μm sections which were subsequently
stained with polyclonal mouse anti-human CD68 (R&D systems,
Minneapolis, MN, USA) primary antibody and rabbit anti-human
α-smooth muscle actin (α-SMA) and fibroblast activation protein
(FAP) primary antibodies (Abcam, Cambridge, MA, USA) followed by
Alexa Fluor 488- or 568-conjugated goat anti-mouse IgG and Alexa
Fluor 568- or 488-conjugated goat anti-rabbit IgG (Invitrogen,
Carlsbad, CA, USA) secondary antibodies. The positive cells were
detected by confocal microscopy.
Isolation of LMFs and normal skin
fibroblasts
LMFs and normal skin fibroblasts were isolated as
described previously (18). The
LMFs and normal skin fibroblasts were passaged for 3–8 passage
doublings and were used for the subsequent experiments to minimize
the clonal selection and culture stress which may occur during
extended tissue culture.
LMFs immunofluorescent staining
The LMFs were cultured on collagen-coated coverslips
(Corning, Inc., Corning, NY, USA) and were fixed with 1:1
acetone/methanol for 10 min, rinsed and prewetted with
phosphate-buffered saline (PBS)/10% fetal calf serum (FCS)/0.1%
sodium azide. Next, the cells were stained with antibodies against
fibroblast surface protein (FSP), vimentin, FAP, desmin, α-SMA,
fibronectin and immunoglobulin G (IgG; Abcam) in Tris-buffered
saline (pH 7.4) for 60 min. The cells were washed and incubated for
20 min in isotype-relevant goat anti-mouse
fluorescein-isothiocyanate (Invitrogen) and the nuclei were
counterstained with 4′,6′-diamidino-2-phenylindole hydrochloride
(Sigma, St. Louis, MO, USA). The images were viewed and assessed
using a fluorescence microscope (LEICA DMI 4000B) and analyzed
using the Leica Application software suite (version 4.0).
Isolation of monocytes
Peripheral blood mononuclear cells (PBMCs) were
isolated from the buffy coats derived from the blood of healthy
donors using Ficoll density gradients, as described previously
(19). The monocytes were selected
from the PBMCs using anti-CD14 magnetic beads (Miltenyi Biotec,
Bergisch Gladbach, Germany) and fresh tissue monocytes were
obtained as previously described (20). In brief, the liver biopsy specimens
(n=12) were cut into small sections and digested in RPMI-1640
medium (Sigma) supplemented with 0.05% collagenase IV (Sigma),
0.002% DNase I (Roche Diagnostics, Mannheim, Germany) and 20% FCS
(Hyclone Laboratories, Inc., Logan, UT, USA) at 37°C for 20 min.
The dissociated cells were filtered through a 150-μm mesh
and separated by Ficoll centrifugation. The mononuclear cells were
washed and resuspended in media supplemented with 1%
heat-inactivated FCS for the flow cytometry analysis.
Coculture of monocytes with LMFs or skin
fibroblasts
The monocytes were cultured in DMEM (Sigma) with 10%
FCS in 48-well flat-bottom microtiter plates (Corning,
2.5×105 cells per well) in the presence of either LMFs
or skin fibroblasts (monocyte/LMF or skin fibroblast ratio: 5/1).
At the indicated time intervals, the monocytes were harvested,
counted and analyzed.
Multiplex bead-based enzyme-linked
immunosorbent assay analysis of cell supernatants
Supernatants were generated by seeding
5×104 cells per well into 48-well plates in 500
μl phenol-red-free DMEM/1% bovine serum albumin (BSA),
containing 2 mmol/l L-glutamine, 60 μg/ml benzylpenicillin
and 100 μg/ml streptomycin (all purchased from Sigma). The
Multiplex bead-based enzyme-linked immunosorbent assay analysis of
the conditioned supernatants was performed using the Human 38-plex
antibody bead kit, the Human 11-plex antibody bead kit and the
Human 23-plex antibody bead kit (Millipore, Billerica, MA, USA) and
was analyzed using a Luminex plate reader and Milliplex analyst
software (Luminex 200 System).
Flow cytometry
The peripheral blood monocytes, liver monocytes and
LMFs were stained with fluorochrome-conjugated antibodies against
PD-L1, PD1, CD14, CD16, CD23, CD32, CD64, CD80, CD86, TLR2, TLR4,
CD166, CD90, CD29, CD73, CD13, CD44, CD105, CD31, CD45, CD34 and
control antibodies (BD Biosciences, Franklin Lakes, NJ, USA or
eBioscience, San Diego, CA, USA), according to the manufacturer’s
instructions. The cells were subsequently analyzed using multicolor
flow cytometry.
Statistical analysis
The results are expressed as the mean ± SEM.
Normality was tested using the Shapiro-Wilk test and the normally
distributed data were compared using paired t-tests for associated
samples, analysis of variance or independent t-tests. Non-normally
distributed data were compared using the Wilcoxon signed-ranks test
for associated samples or the Mann-Whitney U test for independent
samples. SPSS statistical software (version 13.0) was used for all
the statistical analyses. Unless otherwise specified, all the data
were analyzed using two-tailed tests and P<0.05 was considered
to indicate statistically significant differences.
Results
Kupffer cells are activated in fibrotic
livers and are in contact with LMFs
To identify the phenotypic features of Kupffer
cells, flow cytometry was used to analyze monocytes freshly
isolated from the tissues of 12 patients with cirrhosis undergoing
liver transplantation and liver tissues of 3 patients with hepatic
hemangioma as the normal controls. Compared with the normal
controls, the monocytes isolated from the fibrotic liver samples
had a significantly greater proportion of
CD32+CD14+ cells (17%; Fig. 1A and B, P<0.05) and expressed
significantly larger amounts of PD-L1 (42%; Fig. 1A and B, P<0.05). The results
also revealed that the expression levels of CD64, CD80 and TLR4 on
the monocytes were higher in the fibrotic livers than in the normal
liver tissues, although the increase in the absolute mean
fluorescence intensity (MFI) of the three subsets did not exhibit
statistically significant differences (Fig. 1B). The differences between the
phenotypes of the normal and cirrhotic liver monocytes indicate
that the fibrotic environment is capable of promoting the
differentiation of monocytes in situ.
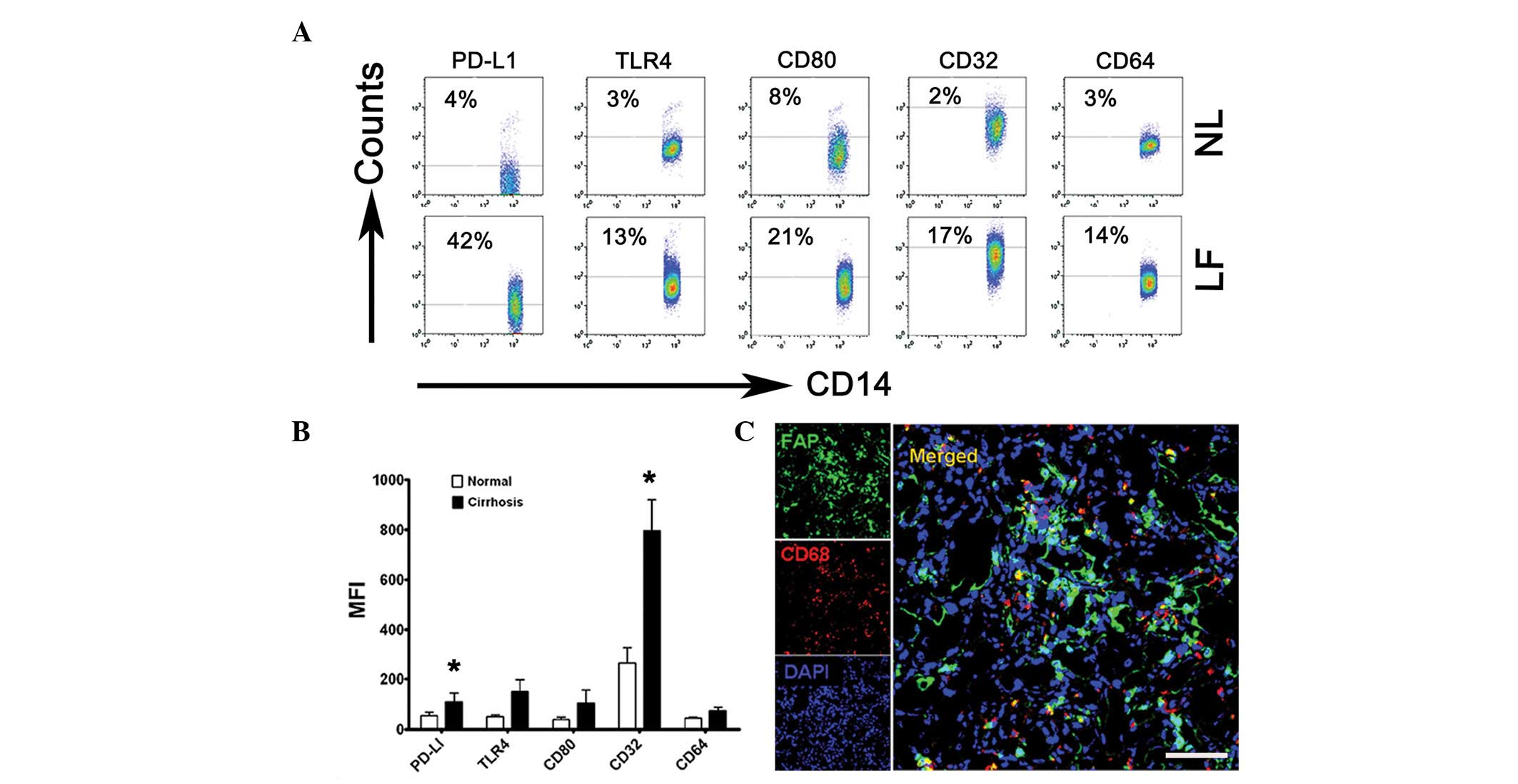 | Figure 1Kupffer cells were activated in the
cirrhotic livers and were in contact with LMFs. (A and B) Flow
cytometry analysis of PD-L1, TLR4, CD80, CD32 and CD64 expression
on freshly isolated monocytes from NL (from 3 patients with hepatic
hemangioma) and LF (from 12 patients with liver failure). (A) The
percentage of expression of PD-L1, TLR4, CD80, CD32 and CD64 on
CD14+ monocytes and (B) the MFI of these molecules are
shown. The data in (A) are representative dot plots of ≥7
individuals from >5 independent experiments; (B) shows the
statistical analysis of these samples. The results are expressed as
the mean ± SEM. Significant differences in comparison with normal
livers are indicated (*P<0.05). (C) Analysis of LMF
(FAP+) and Kupffer cell (CD68+) distribution
in cirrhotic liver samples by confocal microscopy. The micrographs
show the contact of the LMFs and Kupffer cells; 1 out of 10
representative micrographs is shown. Bar, 200 μm. LMF, liver
myofirbroblast; NL, normal livers; LF cirrhotic livers; MFI, mean
fluorescence intensity; FAP, fibroblast activating protein. |
Since Kupffer cells are critical for initiating and
maintaining HSC responses, the association between Kupffer cells
and LMFs in the cirrhotic livers was subsequently investigated,
with particular attention to the microlocalization of the cells.
Using confocal microscopy, the Kupffer cells (mainly expressing
CD68) were demonstrated to be in close contact with the LMFs
(highly expressing FAP), suggesting that the LMFs may regulate the
function of Kupffer cells via certain signals (Fig. 1C). Collectively, these data
indicate that Kupffer cells are activated in the inflamed livers of
patients with cirrhosis and are in contact with LMFs.
Phenotype of LMFs from fibrotic
livers
A total of 7 primary LMF cell lines were established
from patients with cirrhosis and the cell phenotypes were analyzed
by immunofluorescence and cytofluorimetric analyses. As shown in
Fig. 2, the cultures were of high
purity, with characteristic spindle-shaped cells that expressed the
fibroblast markers fibronectin, α-SMA, FAP, desmin, FSP, vimentin,
CD166, CD90, CD29, CD73, CD13, CD44 and CD105. Additionally,
staining for CD31, CD45 and CD34 was used to exclude contamination
with endothelial, epithelial and hematopoietic cells. As the
expression of FAP has been reported to be a distinctive feature of
activated fibroblasts (21), the
phenotypic profile suggests an activated state for the LMFs.
Notably, no differences were observed in the phenotype of the LMF
subsets between the different underlying etiologies of cirrhosis
(data not shown), suggesting that the qualitative changes in the
LMF compartment represent a somewhat uniform response during
fibrogenesis.
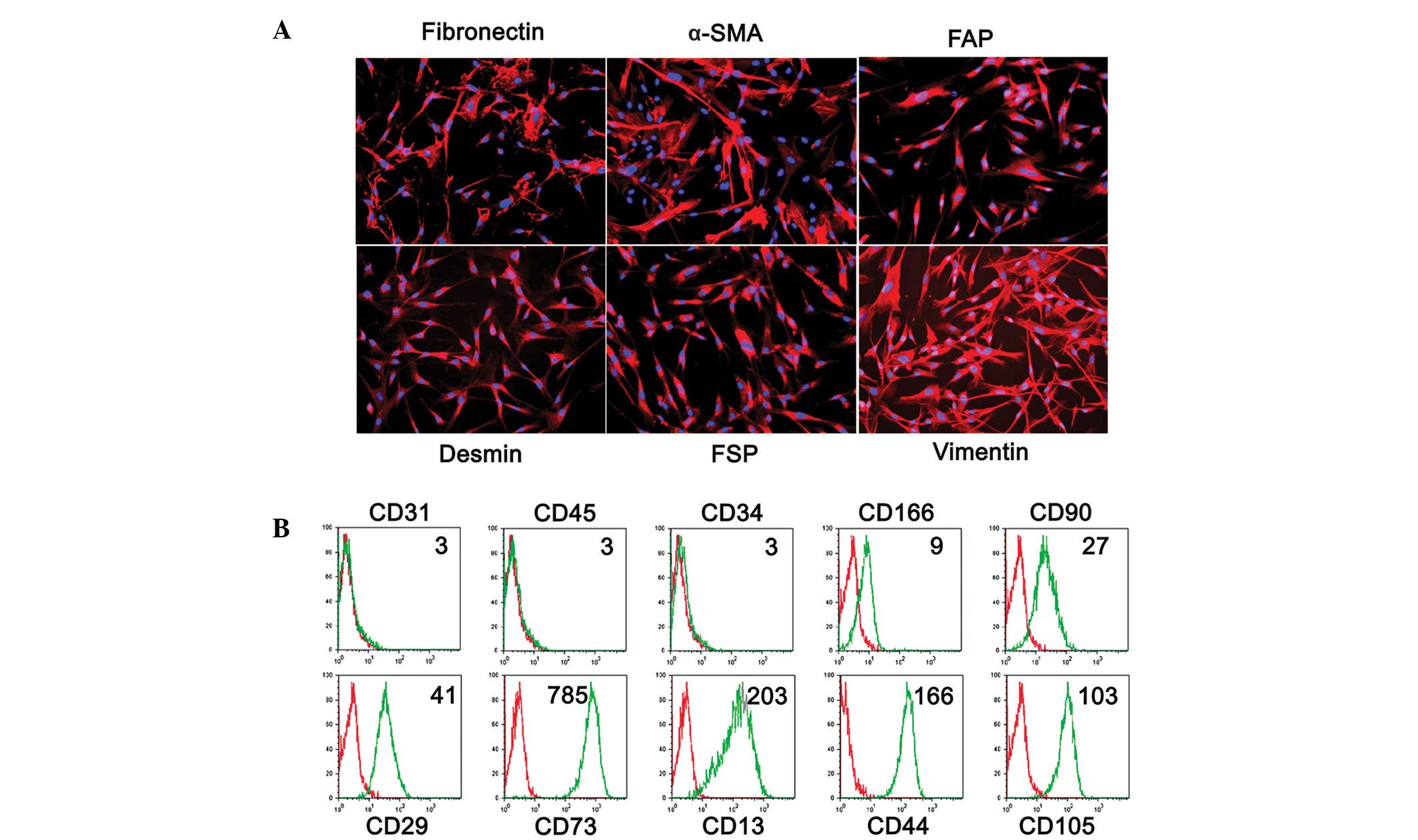 | Figure 2Phenotypic characterization of LMFs
isolated from human liver tissues. (A) Immunofluorescent staining
of LMFs isolated from a representative sample of cirrhotic livers
with anti-α-SMA, fibronectin, FSP, vimentin, desmin and FAP. (B)
The surface markers of the LMFs cultured for 3–5 population
doublings were determined by flow cytometry. The red lines
represent LMFs stained with the control antibodies (Isotype) and
the green lines represent LMFs stained with the indicated
antibodies (Antibody). Representative data of the MFI of LMFs are
shown. The purity of the LMFs was confirmed using the endothelial,
epithelial and hematopoietic markers, CD31, CD45 and CD34. The
samples collected were the same as in Fig. 1. The data shown are representative
of ≥7 individuals from >5 independent experiments. LMF, liver
myofibroblast; α-SMA, α-smooth muscle actin; FSP, fibroblast
surface protein; FAP, fibroblast activating protein; MFI, mean
fluorescence intensity. |
LMFs and skin fibroblasts promote
monocyte activation in vitro
To investigate the possible effect of LMFs on
monocytes, the cells were cultured together. Monocytes, freshly
isolated from the blood of unrelated healthy donors, were
cocultured with various LMF cell lines for 3 or 6 days. The
expression of surface molecules on the monocytes (including PD-L1,
TLR4, CD80, CD32 and CD64) was subsequently analyzed by flow
cytometry. The results showed that the LMFs were potent at
promoting the activation of the monocytes which exhibited
phenotypic features similar to the monocytes isolated from the
cirrhotic livers (Fig. 3A and B).
It is notable that, although these molecules were significantly
upregulated in the monocytes following exposure to the LMFs for 3
days of coculture, the expression became more evident at day 6
(Fig. 3A). Similar to the LMFs,
normal skin fibroblasts also affected the phenotype of monocytes
and although the modulation effect of the LMFs appeared to be
stronger than that of the normal skin fibroblasts, no statistically
significant difference was observed (Fig. 3B). Therefore, the same effect may
exist in the activation of monocytes exposed to fibroblasts of
various origins.
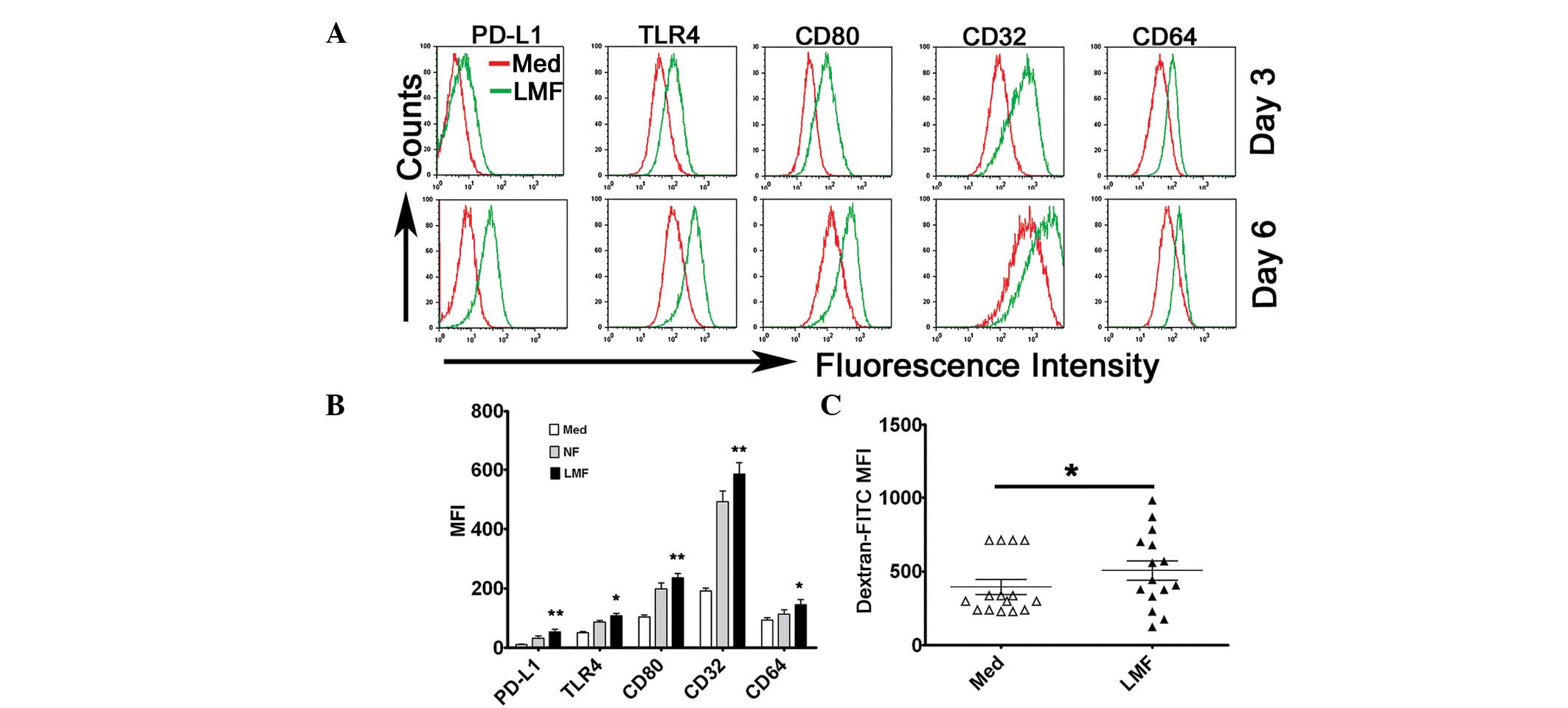 | Figure 3LMFs regulated PD-L1, TLR4, CD80, CD32
and CD64 expression in monocytes. (A and B) Monocytes were Med or
cocultured with NF or LMFs for different time periods. The
histograms are representative of 6 separate experiments. (B)
Statistical analysis of the MFI with regard to the expression of
the surface markers, PD-L1, TLR4, CD80, CD32 and CD64, on the
monocytes following 6 days of coculture. (C) The monocytes were
left untreated or pretreated for 6 days with the LMFs and were
subsequently incubated for 30 min with FITC-dextran at the
indicated concentrations (ng/ml). The endocytotic function of the
monocytes was assessed by flow cytometry. The values in (B) and (C)
represent the mean ± SEM of 6 separate experiments.
*P<0.05 and **P< 0.01 indicate
significant differences from the untreated monocytes (B and C).
LMF, liver myofibroblast; Med, untreated; NF, normal skin
fibroblasts. |
To investigate the endocytotic ability of the
monocytes following pretreatment with the LMFs further,
FITC-dextran was supplied for 30 min in the coculture system. On
days 3 and 6, the LMF-exposed monocytes demonstrated significant
increases in dextran endocytosis (Fig.
3C). Moreover, no correlation was observed for the ability of
the LMFs to augment the monocytic response and the different
underlying etiologies of cirrhosis.
To obtain further insight into how the LMFs or skin
fibroblasts were able to modulate the monocytic response, a method
was designed to determine whether the enhancing effect was due to
diffusible factors or required direct cell-to-cell interactions.
After purified monocytes were cultured in conditioned medium from
LMFs or skin fibroblasts, it was demonstrated that the supernatant
from the fibroblasts also effectively induced the activation of
monocytes, suggesting that certain soluble factors were secreted to
modulate the monocytes (data not shown).
Taken together, these results suggest that LMFs are
critical in maintaining the activation of monocytes in the fibrotic
liver environments of humans.
LMFs and skin fibroblasts may regulate
the phenotype and function of monocytes through types of cytokines,
chemokines and growth factors
The aforementioned observations indicate that the
LMFs may supply locally acting paracrine cues which induce monocyte
activation within the fibrotic liver environment. To understand
this crosstalk more thoroughly, in vitro cocultures of
monocytes and LMFs/skin fibroblasts were established and their
conditioned media were screened for levels of various cytokines,
chemokines and growth factors using the Multiplex bead-based
enzyme-linked immunosorbent assay. Notably, the levels of the
majority of the cytokines, chemokines and growth factors in the
cocultures were higher than those produced by the monocytes alone
(Fig. 4).
Discussion
Over the past decade, considerable research has been
focused on Kupffer cell-mediated liver injury. Kupffer cells are
the best-characterized targets of lipopolysaccharide (LPS) in the
liver (22,23) where they are crucial in hepatic
fibrogenesis through the enhancement of HSC activation (24,25).
However, little is known concerning whether LMFs affect the
differentiation and function of Kupffer cells. The present study
demonstrates that the LMFs from cirrhotic livers modulate the
phenotype and function of monocytes which may represent a novel
link between inflammation and fibrosis in the liver.
The liver consists of hepatic parenchyma and a large
proportion of nonparenchymal cells (NPCs), including sinusoidal
endothelial cells, Ito cells and dedicated hepatic macrophages
(Kupffer cells) (26). Kupffer
cells are important in the normal physiology and homeostasis of the
liver and participate in the acute and chronic responses to toxic
compounds. The direct or indirect activation of Kupffer cells by
toxic agents results in the release of an array of inflammatory
mediators, growth factors and reactive oxygen species and this
activation appears to modulate hepatocyte injury. In the present
study, the Kupffer cells in diseased livers were observed to
exhibit activated phenotypes with increased expression of PD-L1,
CD80, CD32, CD64 and TLR4 (Fig.
1). Notably, these activated Kupffer cells were in close
contact with LMFs, suggesting that such monocytes may actually be
modulated by LMFs. This theory is supported by the subsequent
finding that the phenotype and function of monocytes were
correlated with the LMFs in coculture (Fig. 3).
LMFs originate principally from activated HSCs.
However, in fibrotic disease, subpopulations arise from other
sources, such as bone-marrow precursors (27–29).
Since it is hypothesized that myofibroblasts isolated from tissues
express imprinted phenotypes that are stable in culture (30), the behavior of these cells in
vitro is likely to reflect their function in
vivo(31). Differentiated LMFs
isolated directly from diseased human livers were studied. The
isolated myofibroblasts were positive for fibronectin, α-SMA, FAP,
desmin, FSP, vimentin, CD166, CD90, CD29, CD73, CD13, CD44 and
CD105, whereas the characteristic markers of epithelial,
endothelial or hematopoietic cells, including CD31, CD45 and CD34,
were negative. There were no consistent differences that
characterized the LMFs isolated from the various diseased livers
and all the LMFs expressed the same types of markers (Fig. 2). Consistent with the results of
the present study, other investigators have reported that LMF
preparations from various diseased livers expressed similar
patterns of proinflammatory cytokines and chemokines (11).
Monocytes are versatile, plastic cells that respond
to environmental signals through diverse functional programs
(32,33). Other investigators have
demonstrated that LMFs secrete potent lymphocyte chemotactic
factors when stimulated by proinflammatory cytokines. The present
study provides evidence that certain cytokines, chemokines and
growth factors exist in LMFs and skin fibroblast coculture systems
with monocytes. These soluble factors may promote monocytes
activation. Therefore, LMFs may represent a novel mechanism which
modulates monocyte immunity. Moreover, further detail was provided
on these soluble factors using the Multiplex bead-based
enzyme-linked immunosorbent assay and the results of the present
study are likely to aid future studies.
The skin fibroblasts were as effective as the LMFs
at inducing the activation of monocytes, suggesting that
fibroblasts, which are numerous in the body, may represent an
underrated cell population that is actively involved in
immunomodulatory functions. However, the mechanisms involved in the
activation of monocytes may differ between the LMFs and skin
fibroblasts for a number of the distinct soluble factors in the
coculture systems.
The observation that LMFs modulate the phenotype and
function of Kupffer cells provides a mechanism whereby LMFs may
determine the immune status within the inflamed liver. There is a
fine-tuned collaborative interaction between immune cells and LMFs
in liver microenvironments. Indeed, bidirectional interactions
between LMFs and Kupffer cells may function as an ‘amplification
loop’ to enhance inflammation further in the liver, thereby
extending the role of the LMFs in liver disease from fibrogenesis
to an active role in regulating inflammation. Although these
regulatory loops must be studied in more detail, the present study
provides novel insights into the mechanisms underlying hepatic
inflammation and particularly the role of LMFs as proinflammatory
elements. Together with previous studies indicating that HSCs act
as antigen-presenting cells (34),
the findings of the present study suggest that LMFs are central to
the pathogenesis of liver disease and may be important therapeutic
targets for reversing liver inflammation.
Acknowledgements
The authors would like to thank Dr
Tuan-Jie Li (the First Affiliated Hospital of An Hui Medical
University) for technical assistance with the isolation of liver
myofibroblasts and Dong-Ming Kuang (State Key Laboratory of
Biocontrol, Sun Yat-sen University) for the critical reading of the
manuscript. The present study was supported by National Science and
Technology Major Project (2012ZX10002007), the National Natural
Science Foundation of China (No. 81202319, 30971356), the National
Key Basic Research Program of China (No. 2007CB513006) and the
Natural Science Fund of Guangdong Province (No.
S2012040008104).
References
|
1
|
Naito M, Hasegawa G, Ebe Y and Yamamoto T:
Differentiation and function of Kupffer cells. Med Electron
Microsc. 37:16–28. 2004. View Article : Google Scholar
|
|
2
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-beta
signaling and hepatic fibrosis. Nat Med. 13:1324–1332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heymann F, Hammerich L, Storch D, et al:
Hepatic macrophage migration and differentiation critical for liver
fibrosis is mediated by the chemokine receptor C-C motif chemokine
receptor 8 in mice. Hepatology. 55:898–909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolios G, Valatas V and Kouroumalis E:
Role of Kupffer cells in the pathogenesis of liver disease. World J
Gastroenterol. 12:7413–7420. 2006.PubMed/NCBI
|
|
5
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bilzer M, Roggel F and Gerbes AL: Role of
Kupffer cells in host defense and liver disease. Liver Int.
26:1175–1186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heymann F, Trautwein C and Tacke F:
Monocytes and macrophages as cellular targets in liver fibrosis.
Inflamm Allergy Drug Targets. 8:307–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henderson NC and Iredale JP: Liver
fibrosis: cellular mechanisms of progression and resolution. Clin
Sci (Lond). 112:265–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reeves HL and Friedman SL: Activation of
hepatic stellate cells - a key issue in liver fibrosis. Front
Biosci. 7:d808–d826. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View
Article : Google Scholar
|
|
11
|
Holt AP, Haughton EL, Lalor PF, Filer A,
Buckley CD and Adams DH: Liver myofibroblasts regulate infiltration
and positioning of lymphocytes in human liver. Gastroenterology.
136:705–714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chinnadurai R and Grakoui A: B7-H4
mediates inhibition of T cell responses by activated murine hepatic
stellate cells. Hepatology. 52:2177–2185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schildberg FA, Wojtalla A, Siegmund SV, et
al: Murine hepatic stellate cells veto CD8 T cell activation by a
CD54-dependent mechanism. Hepatology. 54:262–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muhanna N, Horani A, Doron S and Safadi R:
Lymphocyte-hepatic stellate cell proximity suggests a direct
interaction. Clin Exp Immunol. 148:338–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Braet F and Wisse E: Structural and
functional aspects of liver sinusoidal endothelial cell fenestrae:
a review. Comp Hepatol. 1:12002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomson AW and Knolle PA:
Antigen-presenting cell function in the tolerogenic liver
environment. Nat Rev Immunol. 10:753–766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zimmermann HW, Seidler S, Nattermann J, et
al: Functional contribution of elevated circulating and hepatic
non-classical CD14CD16 monocytes to inflammation and human liver
fibrosis. PLoS One. 5:e110492010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
19
|
Zheng L, He M, Long M, Blomgran R and
Stendahl O: Pathogen-induced apoptotic neutrophils express heat
shock proteins and elicit activation of human macrophages. J
Immunol. 173:6319–6326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Unitt E, Rushbrook SM, Marshall A, et al:
Compromised lymphocytes infiltrate hepatocellular carcinoma: the
role of T-regulatory cells. Hepatology. 41:722–730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
22
|
Schwabe RF, Seki E and Brenner DA:
Toll-like receptor signaling in the liver. Gastroenterology.
130:1886–1900. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seki E, Tsutsui H, Nakano H, et al:
Lipopolysaccharide-induced IL-18 secretion from murine Kupffer
cells independently of myeloid differentiation factor 88 that is
critically involved in induction of production of IL-12 and
IL-1beta. J Immunol. 166:2651–2657. 2001. View Article : Google Scholar
|
|
24
|
Duffield JS, Forbes SJ, Constandinou CM,
et al: Selective depletion of macrophages reveals distinct,
opposing roles during liver injury and repair. J Clin Invest.
115:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rivera CA, Bradford BU, Hunt KJ, et al:
Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment
or dietary glycine. Am J Physiol Gastrointest Liver Physiol.
281:G200–G207. 2001.PubMed/NCBI
|
|
26
|
Roberts RA, Ganey PE, Ju C, Kamendulis LM,
Rusyn I and Klaunig JE: Role of the Kupffer cell in mediating
hepatic toxicity and carcinogenesis. Toxicol Sci. 96:2–15. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cassiman D, Libbrecht L, Desmet V, Denef C
and Roskams T: Hepatic stellate cell/myofibroblast subpopulations
in fibrotic human and rat livers. J Hepatol. 36:200–209. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Magness ST, Bataller R, Yang L and Brenner
DA: A dual reporter gene transgenic mouse demonstrates
heterogeneity in hepatic fibrogenic cell populations. Hepatology.
40:1151–1159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Forbes SJ, Russo FP, Rey V, Burra P, Rugge
M, Wright NA and Alison MR: A significant proportion of
myofibroblasts are of bone marrow origin in human liver fibrosis.
Gastroenterology. 126:955–963. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Buckley CD, Pilling D, Lord JM, Akbar AN,
Scheel-Toellner D and Salmon M: Fibroblasts regulate the switch
from acute resolving to chronic persistent inflammation. Trends
Immunol. 22:199–204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Minicis S, Seki E, Uchinami H, Kluwe J,
Zhang Y, Brenner DA and Schwabe RF: Gene expression profiles during
hepatic stellate cell activation in culture and in vivo.
Gastroenterology. 132:1937–1946. 2007.PubMed/NCBI
|
|
32
|
Geissmann F, Manz MG, Jung S, Sieweke MH,
Merad M and Ley K: Development of monocytes, macrophages, and
dendritic cells. Science. 327:656–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuang DM, Zhao Q, Peng C, et al: Activated
monocytes in peritumoral stroma of hepatocellular carcinoma foster
immune privilege and disease progression through PD-L1. J Exp Med.
206:1327–1337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Viñas O, Bataller R, Sancho-Bru P, et al:
Human hepatic stellate cells show features of antigen-presenting
cells and stimulate lymphocyte proliferation. Hepatology.
38:919–929. 2003.PubMed/NCBI
|


















