Introduction
Angiogenesis is the process of new blood vessel
formation. It is an important process in the growth of malignant
tumors as solid tumors must develop an angiogenic phenotype which
promotes the establishment of an expanding vascular network for the
delivery of oxygen and other nutrients (1). The predominant regulator of tumor
angiogenesis is vascular endothelial growth factor (VEGF) (2,3)
which is the only angiogenic factor known to be present throughout
the entire tumor lifecycle (2,4).
VEGF promotes endothelial cell proliferation, migration and
survival in support of tumor angiogenesis. In addition, VEGF is a
potent stimulator of vessel permeability, having originally been
recognized for its function as a vascular permeability factor
(5,6). Due to its fundamental role in tumor
angiogenesis, VEGF serves as a logical target for antiangiogenic
cancer therapy. Breast cancer is the most common neoplasm in women
(7). Tumor angiogenesis is
essential for the growth and spread of breast cancer cells. There
are at least 6 different angiogenic growth factors associated with
tumor angiogenesis in breast cancer. The major mediator of tumor
angiogenesis is VEGF (8). VEGF
expression is increased in a number of tumor types, including
breast cancer (9). Overexpression
of VEGF is associated with a poor prognosis for patients with
breast cancer (10). In addition
to its prognostic role, VEGF is also a validated target in the
treatment of this disease. Recently, various antiangiogenic agents
have shown efficacy in the treatment of breast cancer (11,12).
FP3 (also known as KH902 or KH903) is an engineered
protein which contains the extracellular domain 2 of VEGF receptor
1 (Flt-1) and extracellular domains 3 and 4 of VEGF receptor 2
(Flk-1, KDR) fused to the Fc region of human immunoglobulin G1
(11,13). Previous studies revealed that FP3
has promise as a local antiangiogenic treatment for human choroidal
neovascularization (CNV)-associated age-associated macular
degeneration (AMD) (11,14–16).
In subsequent studies, it has been demonstrated that FP3 has an
inhibitory effect on the VEGF-mediated proliferation and migration
of human umbilical vein endothelial cells and VEGF-mediated vessel
sprouting of the rat aortic ring in vitro(13). Previous studies also revealed that
FP3 has antitumor effects and anti-angiogenic effects in a
non-small cell lung cancer cell line (A549) (13) and patient-derived tumor tissue
xenograft models of gastric cancer (17), as well as in colon carcinoma with
lymphatic and hepatic metastases in nude mice (18).
It is unknown whether FP3 has an antitumor effect in
breast cancer and what the mechanism behind the potential effect of
FP3 would be. For this purpose, the present study was designed to
evaluate the potential antitumor effects of FP3 in a breast cancer
cell line subcutaneous xenograft model in nude mice and assess the
antiangiogenenic effects of FP3 in this model.
Materials and methods
Materials
The MDA-MB-231 human breast cancer cells were
purchased from American Type Culture Collection (ATCC, Manassas,
VA, USA). RPMI-1640 medium, fetal bovine serum (FBS), penicillin
and streptomycin were purchased from Gibco (Grand Island, NY, USA).
Methanol, acetic acid, crystal violet and hydrogen peroxide were
purchased from Sigma (St. Louis, MO, USA). The antibody against
platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31; rat
monoclonal, clone MEC 13.3) was purchased from BD Pharmingen (San
Diego, CA, USA). Fluorescent (Cy3-conjugated) secondary antibody
(goat anti-rat) was purchased from Jackson ImmunoResearch (West
Grove, PA, USA). Bevacizumab (Avastin) was purchased from Roche,
Inc. (Roche, Nutley, NJ, USA). FP3 was provided as a gift by
Kanghong Biotechnology Inc. (Kanghong, Chengdu, China).
Animals
Female BALB/c nude mice (4–6 weeks old) purchased
from Slaccas Laboratory Animal Co. (Shanghai, China) were housed in
a barrier facility and acclimated to 12-h light-dark cycles for ≥3
days prior to use. The use of experimental animals adhered to the
‘Principles of Laboratory Animal Care’ (NIH publication #85-23,
revised in 1985). All experiments were approved by the
Institutional Animal Care and Use Committee of Zhejiang University
(approval ID: SYXK(ZHE)2005-0072).
Cell culture
MDA-MB-231, a breast cancer cell line, was
maintained in RPMI-1640 medium supplemented with 10% FBS, 200 IU/ml
penicillin and 200 μg/ml streptomycin. The culture medium
was replaced every other day. After reaching confluence, the cells
were subcultured.
In vitro MDA-MB-231 cell clonogenic
assay
MDA-MB-231 cells were cultured in RPMI-1640 with 10%
FBS. Cells in the exponential growth phase were trypsinized, washed
and counted. The cells were seeded in triplicate at a density of
2,500 cells per 100-mm dish containing 10 ml complete medium. Cells
were treated 2 h after seeding with FP3 (20, 50 or 100
μg/ml) or bevacizumab (50 μg/ml). The control plates
received medium only. After 10 days of incubation at 37°C, the
medium was drained and colonies were rinsed, fixed with a mixture
of methanol and acetic acid (10:1) and stained with 1% crystal
violet. The colonies containing >50 cells were counted.
Mice tumor xenografts
For inoculation, ∼1.0×107 MDA-MB-231
cells in 0.2 ml serum-free RPMI-1640 were injected subcutaneously
into the right flank of 32 female athymic nude mice. The mice
developed visible tumors within 3 weeks of the inoculation. The
tumors were allowed to grow to >60 mm3 prior to
imaging. Subsequently, the mice were divided into 4 treatment
groups containing 8 mice each and the treatments were initiated. An
8-mouse group was used as an implantation and normal saline
(NS)-treated negative control.
Mouse drug treatments and tumor growth
regression assay
Following the tumor cell implantation and within 2
weeks of inoculation, the mice received 200 μl vehicle (NS),
FP3 (2, 6 or 18 mg/kg) or bevacizumab (Avastin; 6 mg/kg)
intravenously. The mice were treated with the drugs from 21 days
after implantation at the indicated doses twice per week (n=8 for
each dose) for 3 weeks. The animals were then sacrificed and the
tumors were measured ex vivo with calipers (tumor volume =
length × width2 /2).
Immunohistochemical staining
Formalin-fixed, paraffin-embedded 5-μm thick
tumor sections were analyzed by immunohistochemical analysis
according to the previously described method (18). The sections were baked,
deparaffinized in xylene and rehydrated. Antigen retrieval was
performed with citrate buffer (pH 6.0) in a microwave at 98°C for 5
min. Following a PBS wash, endogenous peroxidases were quenched in
3% hydrogen peroxide for 20 min. The slides were then blocked with
1% normal goat serum for 20 min at room temperature and incubated
with rat anti-mouse PECAM-1 (CD31) polyclonal antibody at a 1:100
dilution. The slides were incubated overnight at 4°C. The following
day, the slides were washed several times with PBS and the
specimens were incubated for 1 h at room temperature with
fluorescent (Cy3-conjugated) secondary antibody (goat anti-rat)
diluted (1:200) in PBS. Specimens were rinsed again with PBS and
mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA).
The tissue sections were examined and digitally photographed using
a Zeiss Axiophot fluorescence microscope (Carl Zeiss, Thornwood,
NY, USA) equipped with single, dual and triple fluorescence filters
and a low-light, externally cooled, three-chip charge-coupled
device (CCD) camera (480×640 pixel RGB-color images, CoolCam;
SciMeasure Analytical Systems, Atlanta, GA, USA) and saved as TIFF
files.
Statistical analysis
Data are presented as the mean ± SEM and were
analyzed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
Differences among the means of the groups were determined using
one-way ANOVA. P<0.05 was considered to indicate a statistically
significant difference.
Results
FP3 significantly inhibits MDA-MB-231
cell proliferation in vitro and blocks tumor growth in vivo
The antiproliferative effect of FP3 on MDA-MB-231
cells in vitro was evaluated. The results demonstrated that
FP3 directly inhibited the tumor cells. FP3 reduced MDA-MB-231 cell
colony formation by >77%. (Fig.
1)
To begin to evaluate FP3 as an anticancer
therapeutic agent for breast cancer and to compare it with other
effective agents targeting the VEGF pathway, its ability to block
the growth of a breast cancer cell line, MDA-MB-231, in a mouse
subcutaneous tumor model was evaluated. Following implantation,
tumor cells were allowed to grow for 3 weeks and formed large
retroperitoneal tumors >60 mm3. Injections of FP3 (2,
6 and 18 mg/kg body weight), bevacizumab (6 mg/kg body weight) or
NS were then administered intravenously, biweekly for 3 weeks,
after which the animals were sacrificed and the tumors excised and
measured. FP3 significantly inhibited the growth of the tumor
xenografts (Fig. 2).
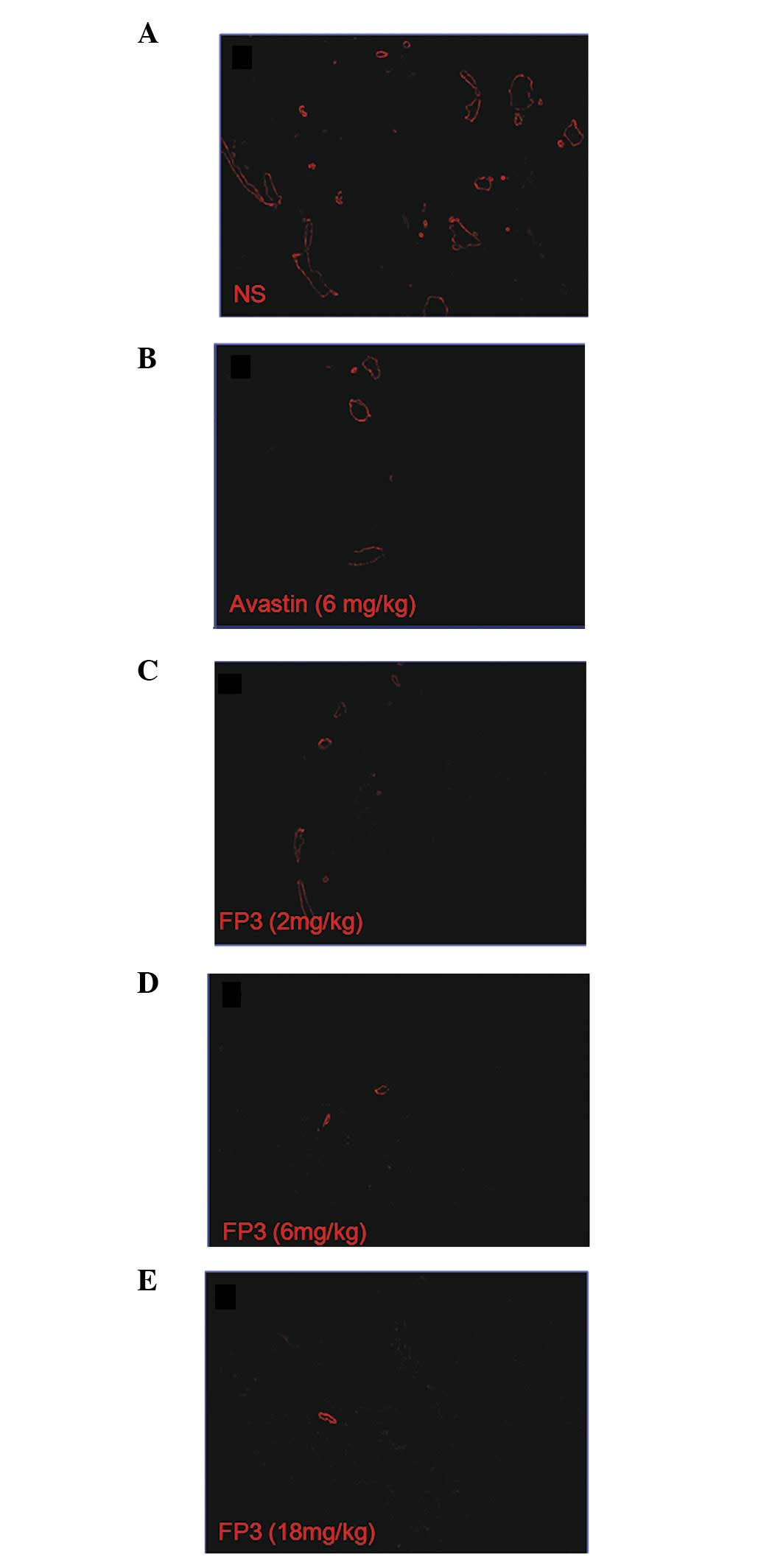
FP3 results in decreased vasculature of
tumors
To evaluate the effects of FP3 on tumor-associated
angiogenesis, the tumor xenografts were sectioned and
immune-stained with an anti-body to PECAM-1 so that the vasculature
was visualized. This analysis revealed that the higher doses of FP3
almost completely blocked tumor-associated angiogenesis, with the
stunted tumors being largely avascular (Fig. 3D and E). The lowest dose of FP3 (2
mg/kg) was not as effective at inhibiting tumor growth compared to
a higher dose (6 or 18 mg/kg). However, the lowest dose of FP3 (2
mg/kg) appeared to be as effective as the higher doses (6 or 18
mg/kg) at blocking tumor-associated angiogenesis (Fig. 3C–E). In contrast to the FP3-treated
tumors, the control tumors in the vehicle-treated mice were not
only much larger but also had a high vascular density (Fig. 3A).
Discussion
Tumor vessels are considered to be dynamic in terms
of the formation of new vessels or angiogenesis. Tumors acquire
their vasculature by endothelial cell sprouting, co-option of
pre-existing vessels, intussusceptive microvascular growth,
postnatal vasculogenesis, glomeruloid angiogenesis or vasculogenic
mimicry. It should be emphasized that in the majority of cases,
these mechanisms are interlinked, participating simultaneously in
physiological and pathological angiogenesis (11). VEGF promotes certain or all of
these processes, rendering VEGF a rational target for
antiangiogenic drug development (19). Since anti-VEGF approaches act by
blocking tumor-associated angiogenesis, which appears to be widely
required by numerous different types of tumors, these approaches
may prove to be generally useful against a wide variety of cancer
types (20).
VEGF expression is increased in a number of tumor
types, including breast cancer (9). VEGF-A is highly expressed in numerous
tumors of the lung, brain and gastrointestinal and urogenital
tracts, as well as in situ and invasive breast cancer
(21). There is a positive
correlation between VEGF levels and poor clinical outcomes,
including patient survival (10).
Anti-VEGF treatment inhibits the growth of human breast tumor
xenografts in animals (22).
FP3 is a humanized fusion protein which combines
ligand binding elements taken from the extracellular domains of
VEGF receptors 1 and 2 and the Fc portion of IgG1 and is designed
to bind to all forms of VEGF-A (13). In order to further substantiate the
antitumor and anti-angiogenesis effects of FP3 in breast cancer, an
MDA-MB-231 subcutaneous xenograft model in nude mice was used. The
results of the study showed that, in the MDA-MB-231 human breast
cancer xenograft model, FP3 effectively inhibited tumor growth
(Fig. 2). Treatment with FP3 also
resulted in stunted and almost completely avascular tumors
(Fig. 3). The antitumor activity
of FP3 is most likely mediated by the inhibition of angiogenesis
since the microvessel density values in FP3-treated tumors were
significantly decreased. The fact that FP3 resembled the
well-defined angiogesis inhibitor bevacizumab with regard to tumor
growth and microvessel density measurements, is an additional
indication of the antiangiogenic activity of FP3.
Whether FP3 has a direct cell-killing or inhibitory
effect in MDA-MB-231 cells in vitro was investigated using a
clonogenic assay. FP3 was identified to have a direct cell-killing
effect on MDA-MB-231 cells (Fig.
1). These results indicated that the inhibitory effect of FP3
on the MDA-MB-231 tumor xenograft model growth may partially result
from the inhibition of the tumor cells.
The results of the present study reveal that FP3 has
an excellent antitumor effect against breast cancer xenografts and
indicate that it may have potential as an effective antiangiogenic
agent in the treatment of breast cancer.
Acknowledgements
This study was supported by the State
Key Basic Research and Development Program of China (973 Program,
Grant No. 2009CB521704), National High-tech Research &
Development Program of China (863 Program, Grant No. 2006AA02A245),
National Natural Science Foundation of China (Grant No. 81000894),
Zhejiang Provincial Natural Science Foundation of China (Grant No.
Y12H160045), Zhejiang Provincial Science and Technology Projects
(Grant No. 2009C13021, 2011C23087), Zhejiang Provincial Medical and
Healthy Science and Technology Projects (Grant No. 2011KYB137),
Science Research Fund of Taizhou (Grant No. A102KY09), Science
Research Fund of Shaoxing (Grant No. 2011D10013) and Science
Research Fund of Zhuji (Grant No. 2011CC7874).
References
|
1
|
Folkman J: Fundamental concepts of the
angiogenic process. Curr Mol Med. 3:643–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrara N: Vascular endothelial growth
factor: basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Senger DR, Galli SJ, Dvorak AM, Perruzzi
CA, Harvey VS and Dvorak HF: Tumor cells secrete a vascular
permeability factor that promotes accumulation of ascites fluid.
Science. 219:983–985. 1983. View Article : Google Scholar
|
|
6
|
Shibuya M: Structure and function of
VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct
Funct. 26:25–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
8
|
Cook KM and Figg WD: Angiogenesis
inhibitors: current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown LF, Berse B, Jackman RW, Tognazzi K,
Guidi AJ, Dvorak HF, Senger DR, Connolly JL and Schnitt SJ:
Expression of vascular permeability factor (vascular endothelial
growth factor) and its receptors in breast cancer. Hum Pathol.
26:86–91. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Linderholm B, Grankvist K, Wilking N,
Johansson M, Tavelin B and Henriksson R: Correlation of vascular
endothelial growth factor content with recurrences, survival, and
first relapse site in primary node-positive breast carcinoma after
adjuvant treatment. J Clin Oncol. 18:1423–1431. 2000.
|
|
11
|
Teng LS, Jin KT, He KF, Wang HH, Cao J and
Yu DC: Advances in combination of antiangiogenic agents targeting
VEGF-binding and conventional chemotherapy and radiation for cancer
treatment. J Chin Med Assoc. 73:281–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teng LS, Jin KT, He KF, Zhang J, Wang HH
and Cao J: Clinical applications of VEGF-trap (aflibercept) in
cancer treatment. J Chin Med Assoc. 73:449–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin K, He K, Teng F, Li G, Wang H, Han N,
Xu Z, Cao J, Wu J, Yu D and Teng L: FP3: a novel VEGF blocker with
antiangiogenic effects in vitro and antitumour effects in vivo.
Clin Transl Oncol. 13:878–884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang M, Zhang J, Yan M, Li H, Yang C and
Yu D: Recombinant anti-vascular endothelial growth factor fusion
protein efficiently suppresses choridal neovascularization in
monkeys. Mol Vis. 14:37–49. 2008.
|
|
15
|
Zhang M, Yu D, Yang C, Xia Q, Li W, Liu B
and Li H: The pharmacology study of a new recombinant human VEGF
receptor-fc fusion protein on experimental choroidal
neovascularization. Pharm Res. 26:204–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang M, Zhang J, Yan M, Luo D, Zhu W,
Kaiser PK and Yu DC; KH902 Phase 1 Study Group: A phase 1 study of
KH902, a vascular endothelial growth factor receptor decoy, for
exudative age-related macular degeneration. Ophthalmology.
118:672–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin K, He K, Han N, Li G, Wang H, Xu Z,
Jiang H, Zhang J and Teng L: Establishment of a PDTT xenograft
model of gastric carcinoma and its application in personalized
therapeutic regimen selection. Hepatogastroenterology.
58:1814–1822. 2011.PubMed/NCBI
|
|
18
|
Jin K, Li G, Cui B, Zhang J, Lan H, Han N,
Xie B, Cao F, He K, Wang H, Xu Z, Teng L and Zhu T: Assessment of a
novel VEGF targeted agent using patient-derived tumor tissue
xenograft models of colon carcinoma with lymphatic and hepatic
metastases. PLoS One. 6:e283842011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Frischer JS, New T, Kim ES, Serur
A, Lee A, Kadenhe-Chiwishe A, Pollyea DA, Yokoi A, Holash J,
Yancopoulos GD, Kandel JJ and Yamashiro DJ: TNP-470 promotes
initial vascular sprouting in xenograft tumors. Mol Cancer Ther.
3:335–343. 2004.PubMed/NCBI
|
|
20
|
Holash J, Davis S, Papadopoulos N, Croll
SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe
E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ,
Yancopoulos GD and Rudge JS: VEGF-Trap: a VEGF blocker with potent
antitumor effects. Proc Natl Acad Sci USA. 99:11393–11398. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roskoski R Jr: Vascular endothelial growth
factor (VEGF) signaling in tumor progression. Crit Rev Oncol
Hematol. 62:179–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borgström P, Gold DP, Hillan KJ and
Ferrara N: Importance of VEGF for breast cancer angiogenesis in
vivo: implications from intravital microscopy of combination
treatments with an anti-VEGF neutralizing monoclonal antibody and
doxorubicin. Anticancer Res. 19:4203–4214. 1999.PubMed/NCBI
|