Introduction
Cerebral venous sinus thrombosis (CVST) is a rare
cerebrovascular disease in the clinic, and it usually affects young
males. Its clinical manifestations are complex and lack
specificity, its causes are perplexing (1), its incidence rate accounts for
0.5–2.0% of strokes (2) and it is
easily missed or misdiagnosed by clinicians. As its mortality rate
is 30–50% according to early reports (3), it is considered a rare and high-risk
cerebrovascular disease. With the development of imaging
technology, the early diagnosis and treatment of CVST is gradually
becoming possible. According to previous studies, its mortality
rate has dropped to 9.4% (4,5).
Computed tomography (CT) is widely used for the
early imaging of patients with CVST. Conventional CT scanning has a
low sensitivity for CVST diagnosis, which is possibly associated
with anatomical variations of the venous sinus. The main direct
sign of acute CVST on a conventional CT image is that a cortical
venous sinus or dural sinus presents a high density (6–8), and
the indirect signs are low density lesions or cerebral hemorrhage
in the trans-arterial innervation zone of the brain parenchyma. In
addition, enhanced CT is able to show venous sinus filling and
defects and is capable of showing the classic ‘empty δ sign’.
In the various phases of CVST, magnetic resonance
imaging (MRI) is generally more sensitive than CT. If the existence
of thrombosis in any one venous sinus is detected by MRI
examination, CVST may be diagnosed (9). The main early signs of CVST in simple
MRI scans include flow shadow disappearance and signal intensity
changes in the venous sinus. In the first week of incidence of
venous thrombosis, the T1-weighted image presents the same signal
intensity as brain tissue and the T2-weighted image presents a
lower signal intensity due to the increase in deoxygenated
hemoglobin content. In the second week, metahemoglobin is present
in the venous thrombus and the T1- and T2-weighted images present
high intensity signals. In the chronic phase, with the evolution of
the thrombus and the paramagnetic products of deoxygenated
hemoglobin and metahemoglobin, the gradient echo- and magnetic
susceptibility-weighted images present low signals (10–13).
In T2-weighted images having low intensity signals, it is extremely
difficult to identify normal flow shadows, and it may be necessary
to use enhanced MRI or magnetic resonance venography (MRV) to
assist diagnosis. The secondary signs observable by MRI include
brain swelling, edema and/or hemorrhage (6). In an MRV examination, the direct
signs of CVST are high flow signal loss or fuzzy edges of a
normally-developed venous sinus or irregular lower blood flow
signals. The former indicates complete obstruction and the latter
indicates thrombosis underfilling or recanalization thrombosis
following complete obstruction of the venous sinus. The indirect
signs are superficial and deep venous dilation of the brain, venous
stasis and collateral circulation formation (14,15).
For CVST, digital subtraction angiography (DSA)
reveals non-development of the venous sinus, development delay or
slowing of the vein structure accompanied by venous dilation of the
cortex, scalp or face and venous inverse flow (10). Also, DSA is able to show certain
veins which are not visible by CT or MRI, particularly cortical
veins and certain deep vein structures. Hypoplasia or atresia of
cerebral veins or venous sinus may make it not possible for MRV or
CTV to be definitely diagnosed, but the venous phase in brain
angiography may be clearly shown (6).
Reasonable selection of the detection methods for
identifying the early characteristics of CVST may be crucial in the
early diagnosis and treatment of CVST. In the current study, a
retrospective analysis of the clinical and imaging data of 62
patients with CVST diagnosed by MRI and/or DSA was conducted.
Materials and methods
General data
A total of 62 patients who were hospitalized at
Tiantan Hospital affiliated to Capital Medical University between
January 2002 and July 2007 were involved in the study. There were
26 male and 36 female cases. Their ages ranged from 15 to 60 years
and the average age was 30.6±16.5 years. They were admitted in the
acute or subacute phase. For disease course, 15 cases were within 1
week, 36 cases were between 1 week and 1 month and 11 cases were
>1 month from onset. For possible disease causes, 12.90% (8/62)
cases were due to pregnancy, 16.13% (10/62) cases were due to
delivery or abortion, 4.84% (3/62) were due to oral administration
of contraceptives, 12.90% (8/62) were due to cerebral facial
infection and for the remaining cases, the causes were unclear. For
clinical manifestations, 56 cases (90.32%) presented headache; 16
cases (25.81%) presented choked papilla, 13 cases (20.97%)
presented limited neural function defect, 11 cases (17.74%)
presented epileptic seizure and 5 cases (8.06%) presented
consciousness disorder. In addition, there were 50 cases with
lumbar puncture manometry >180 mm H2O (1 mm
H2O = 0.0098 kPa), accounting for 80.65% of the
patients. This study was conducted in accordance with the
declaration of Helsinki. This study was conducted with approval
from the Ethics Committee of Capital Medical University. Written
informed consent was obtained from all participants.
Imaging examination
i) CT scanning: a Prospeed spiral CT systemic
scanner (General Electric Company, Fairfield, CT, USA) was used to
conduct 122 neurocranial CT examinations for 62 patients
successively. Of these, 32 cases received one CT examination, 30
cases received two CT examinations and 10 cases received three CT
examinationsat the Beijing Taintan Hospital. In the week after
onset, 46 cases received the first CT examination. Between 1 week
and 1 month after onset, 16 cases received the first CT
examination. The second and third examinations were conducted up to
1.5 years after onset. ii) MRI examination: a Model 1.5-T
superconducting machine (GE Company) was used to conduct enhanced
scanning with conventional T1 and T2 weighted sequences and
intravenous injection of a contrast agent. A total of 56 cases
received MRI and MRV examinations. iii) DSA examination: a Model
DSA-2000A machine (Toshiba Corporation, Tokyo, Japan) was used to
conduct aortic arch angiography and cerebral angiography. A total
of 32 cases received DSA examination. In this group, MRI, MRV and
DSA examinations were synchronously conducted for 21 cases.
Results
CT examination
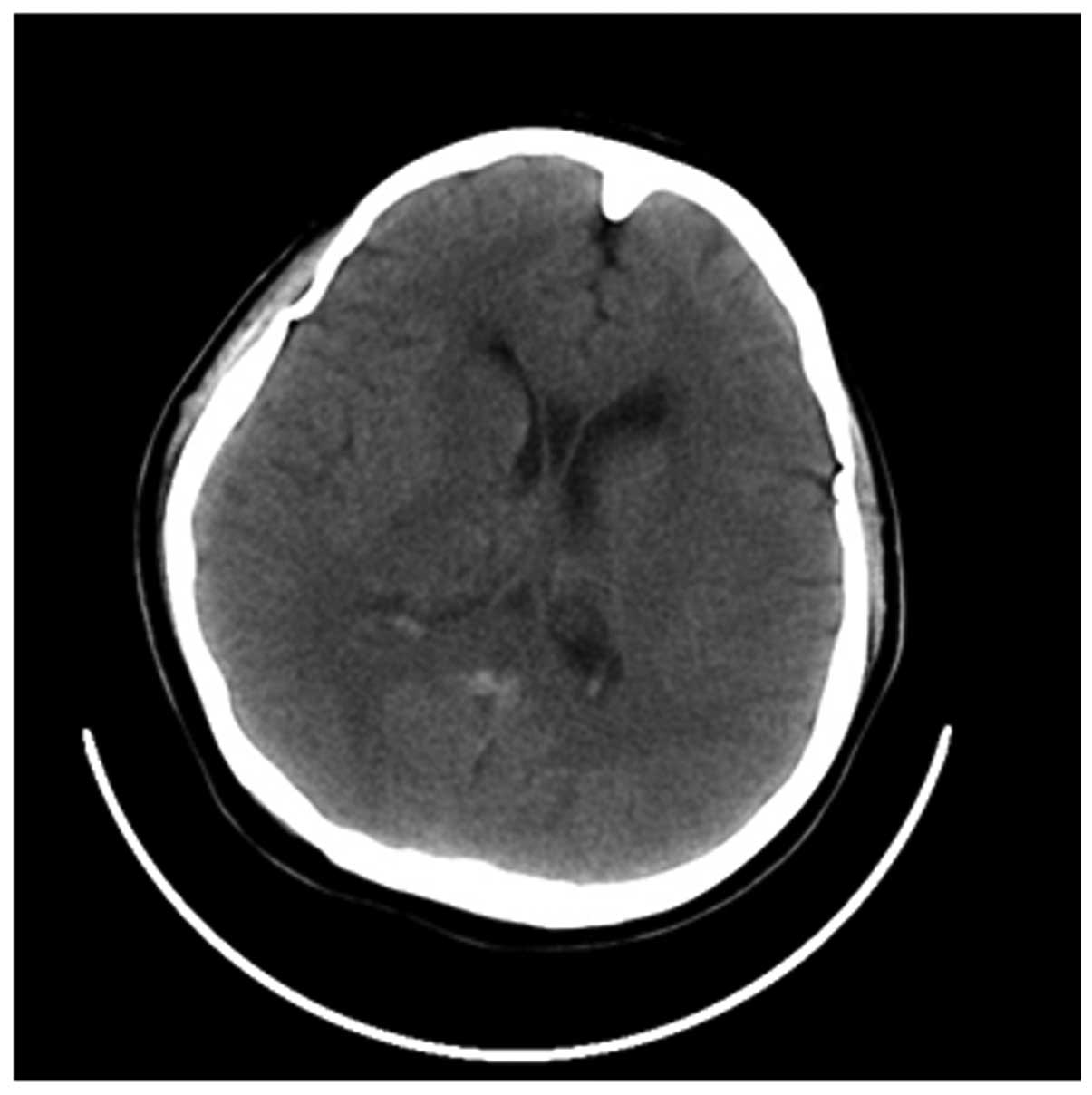
For the 62 cases receiving the first CT examination,
13 cases presented the direct signs of CVST and the positive rate
was 20.97%, while 15 cases presented indirect signs and the
positive rate was 24.19%. Among the 46 cases receiving the first CT
examination within 1 week after onset, 22 cases presented direct
and/or indirect signs of CVST and the positive rate was 47.83%.
Among the 16 cases receiving the first CT examination within 1 week
to 1 month after onset, 6 cases presented direct and/or indirect
signs of CVST and the positive rate was 37.50% (Fig. l).
MRI examination
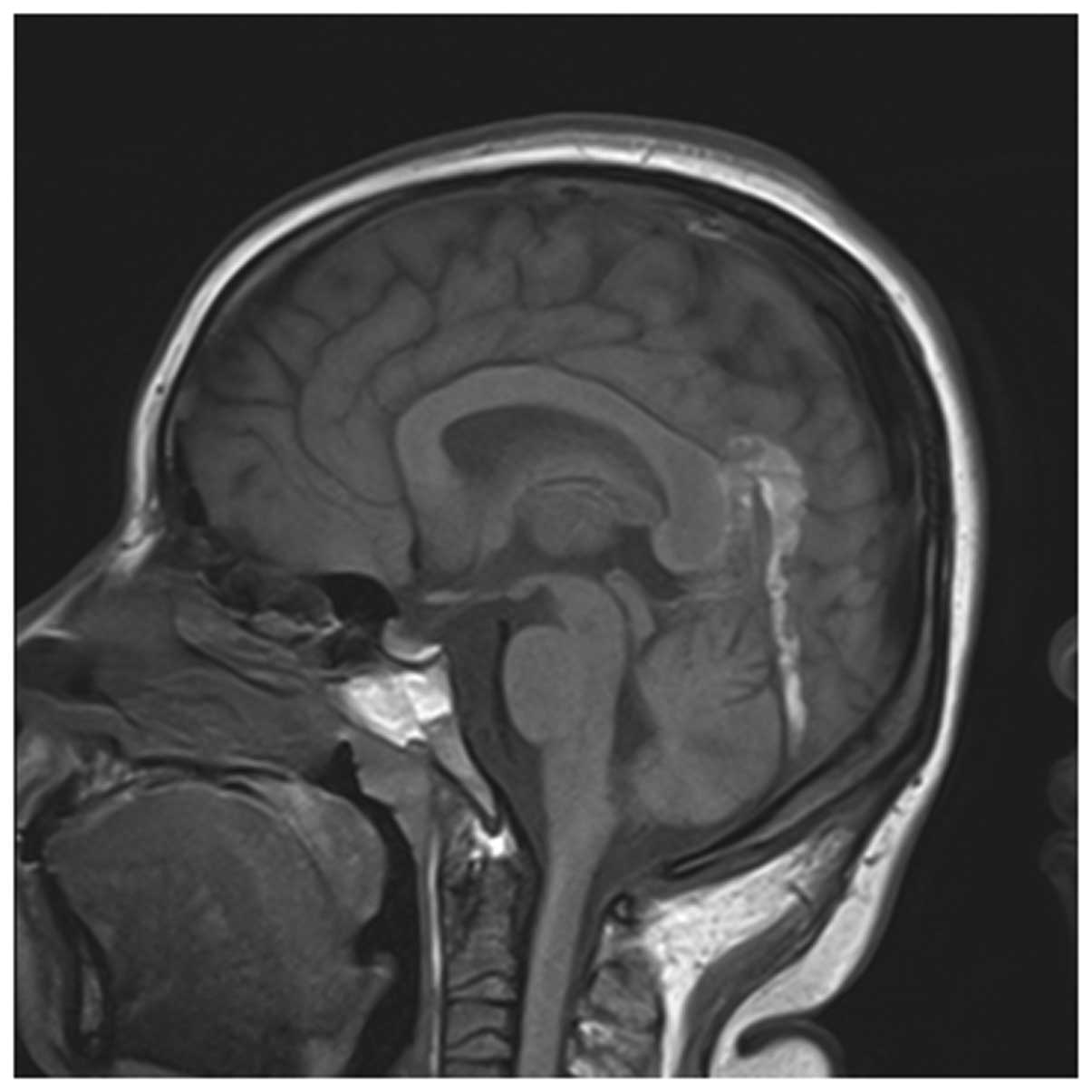
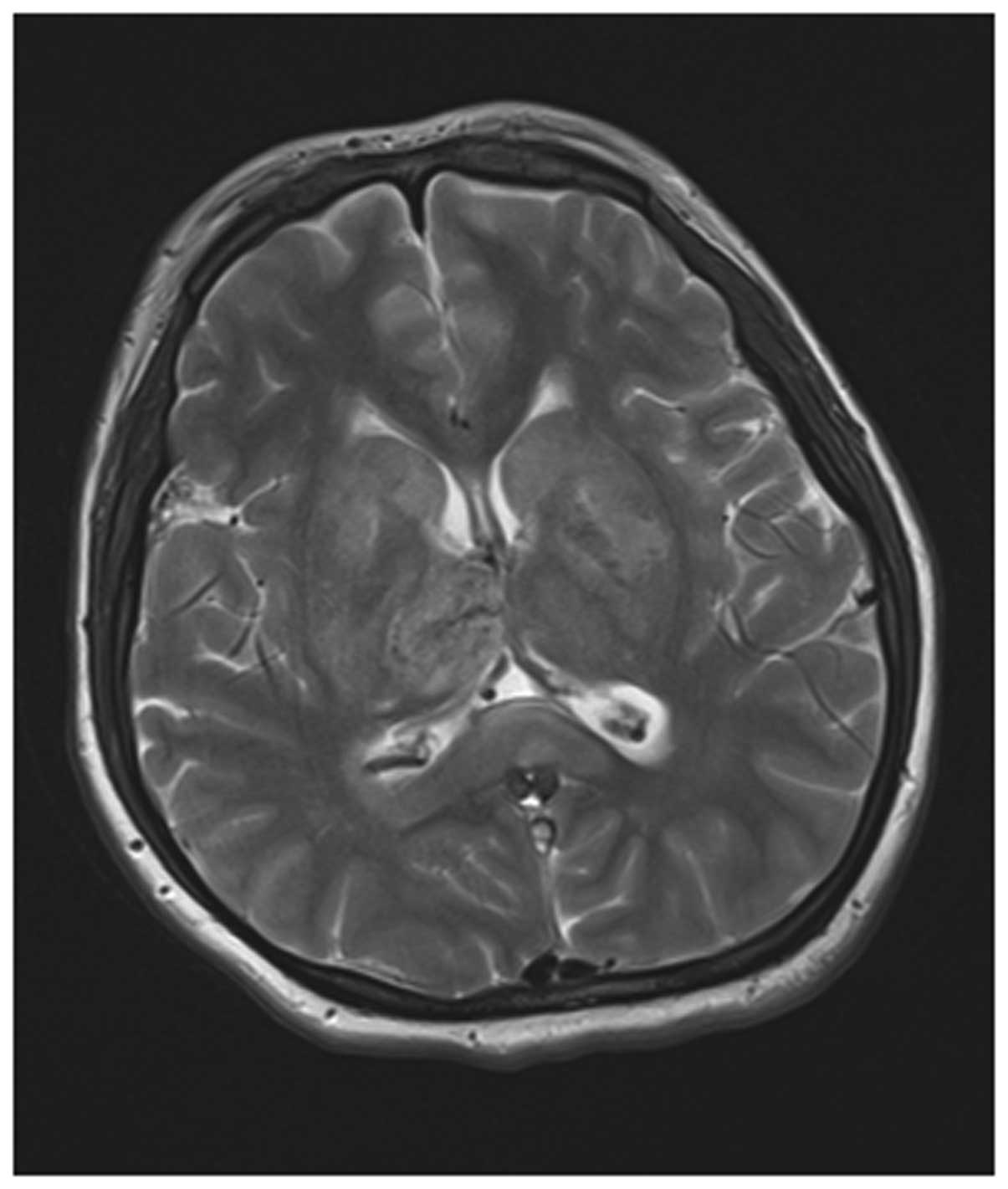
Among the 56 cases receiving both MRI and MRV
examinations, 54 cases presented adverse development or
non-development of the venous sinus at lesion sites and the
positive rate was 96.43%. Their MRI manifestations presented
punctiform and sheet-like hemorrhagic cerebral infarction and
extensive brain edema while partial cases presented cerebral
ventricle dilation. According to the staging method of Isensee
et al(4), 30 cases were in
the acute phase, 18 cases were in the subacute phase and 6 cases
were in the chronic phase. According to MRI, the lesions were
distributed as follows: 22 cases occurred at the superior sagittal
sinus, 15 cases occurred at the lateral sinus, 13 cases occurred at
the sigmoid sinus, 7 cases occurred at the straight sinus and 2
cases occurred at the internal jugular vein. Five of these cases
had lesions at both the superior sagittal and lateral sinuses
(Figs. 2 and 3).
DSA examination
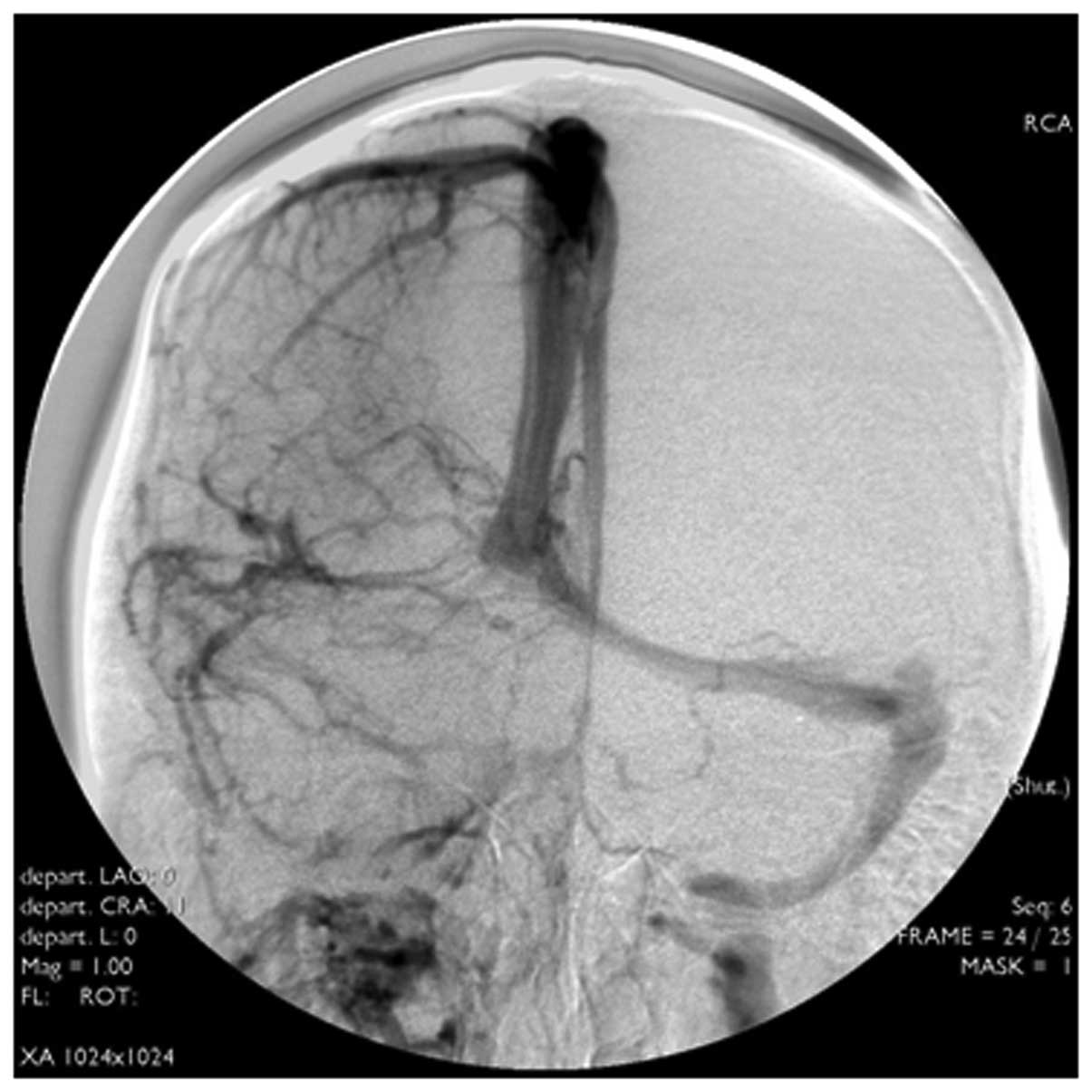
For the 32 cases receiving DSA examination, they
were diagnosed with CVST. Their typical DSA manifestations were
that contrast filling at the lesion sites was discontinuous, venous
development was interrupted or delayed for over 5–10 sec and
cortical superficial veins were dilated. Also, the backflow of
partial collateral branches was established and filling was
defective. With regard to the primary manifestations at lesion
sites, 12 cases presented superior sagittal sinus thromboses, 3
cases presented sigmoid sinus thromboses, 4 cases presented lateral
sinus thromboses and 2 cases presented straight sinus thromboses.
In addition, 5 cases presented both superior sagittal sinus and
lateral sinus thromboses, 4 cases presented both superior sagittal
sinus and sigmoid sinus thromboses and 2 cases presented sigmoid
sinus and lateral sinus thromboses (Fig. 4).
MRI, MRV and DSA examinations
MRI, MRV and DSA examinations were synchronously
conducted for 21 cases. Of the 20 cases that were positive in both
MRI and MRV examinations, the DSA examination of 19 cases was also
positive and the coincidence rate of the two was 95.00%. In
addition, DSA examination presented as positive one case that MRI
and MRV examinations presented as negative.
Treatment and prognosis
All 62 cases received dehydration treatment. Heparin
or low-molecular weight heparin treatment was administered in 38
cases, local thrombolysis with urokinase was conducted for 18
cases, mechanical thrombectomy was conducted for 4 cases and stent
implantation was conducted for 2 cases. As a result, the symptoms
were relieved. A total of 30 cases nearly healed, 25 cases were in
the process of healing, 6 cases had left hospital and 1 case
succumbed. For the healed and improved patients, no cases recurred
in the follow-up period (5–12 months) and the improvement and
healing rate was 88.71%.
Discussion
The cerebral venous sinus is the main channel of
cerebral venous blood backflow. CVST causes cerebral venous blood
backflow disorder and induces elevation of the intracranial blood
pressure to generate the corresponding clinical symptoms and signs.
As the lesion sites, thromboses, elevation rates and extents of
intracranial blood pressure and mechanical tolerances of the
patients differ, the clinical manifestations are complex and
diverse. Furthermore, CVST induces brain edema, congestion and
hemorrhagic cerebral infarction in the drainage area. The lesions
appear early in the course of the disease, the lesion range is wide
and the lesions are not confined to the arterial innervation area.
Therefore, the joint action of these effects results in the very
complex and diverse clinical manifestations of CVST and severe
symptoms and may even cause coma and mortality.
CT is the most popular and common craniocerebral
examination technique. In CT, the direct signs of CVST include the
‘band sign’, the ‘empty δ sign’ and intravenous high density
shadows, indicating thrombosis (16). The indirect signs include
hemorrhagic cerebral infarction, extensive brain edema and
irregular perimeters. In the early stages, the ventricle may reduce
due to edema. In the advanced stages, interstitial fluid is drained
into the ventricle due to an osmotic concentration increase in the
ventricular wall caused by a dilated and tortuous drainage vein,
which causes the ventricular enlargement. Among the patients in
this group, the positive rate of CT direct signs was 20.97% and the
positive rate of indirect signs was 24.19%, which was in line with
the results of Renowden (14).
Although the direct and indirect signs of CT have significant
diagnostic values, the rate of positives is low; the band sign is
observed in only 20–30% of cases and the empty δ sign in 16–46%
(17). Therefore, we consider that
a negative CT examination negative cannot exclude a diagnosis of
CVST. For suspected cases, it is necessary to conduct MRI or DSA
examination for further confirmation.
MRI is able to better reflect the pathophysiological
evolution process of CVST. In addition, MRV may better reflect the
blood flow state of the venous sinus, which is not influenced by
thrombus signal time change, and more clearly reveal local edema
and hemorrhage of the brain parenchyma (18,19).
Therefore, the combination of MRI and MRV is able to provide a CVST
diagnosis sensitivity reaching 90% or more (20). For the cases in the current study,
the positive rate of a combination of MRI and MRV examinations was
96.43% and the coincidence rate of MRI combined with MRV and DSA
examinations was 95.00%, indicating that MRI combined with MRV
examination may be very useful in the early diagnosis of CVST.
Considering that MRI combined with MRV examination is simple,
accurate, noninvasive and reproducible and more directly and
objectively reflects the thrombus site and state of blood flow and
enables dynamic observation of the thrombus evolution process by
multiple-angle and multiple-sequence imaging, we consider that a
combination of MRI and MRV examination is the preferred method of
diagnosing CVST. However, MRV has also some shortcomings. For
smaller thrombi, images are unclear and signals are easily missed
during imaging to cause false positives. Therefore, it is necessary
to conduct DSA examination for a definite diagnosis in cases of
venous dysplasia.
DSA makes it possible to judge whether there is a
blood backflow disorder by dynamic observation of the cycle time of
cerebral blood flow in the arterial, parenchymal, venous and venous
sinus phase and thus diagnoses cerebral venous sinus disease. At
present, DSA is regarded as the gold standard for diagnosing CVST
(21). DSA more clearly reveals
CVST, stenosis and other lesions, which is useful when conducting
contact thrombolysis and mechanical thrombectomy in the venous
sinus, stent implantation in the treatment of venous sinus stenosis
and other interventional and disease condition monitoring
treatments. However, DSA also has its limitations. For example, it
does not show the thrombus itself and it has a traumatic effect and
involves a certain amount of radiation. Also, certain individuals
are allergic to the iodine agent and it has the risk of
complications during surgery. In addition, it requires a higher
technical competency and may only be conducted in a qualified
hospital.
In summary, as CT, MRI combined with MRV and DSA
examinations have their respective advantages and disadvantages and
MRI has an excellent correspondence with DSA with regard to
positive detection rate and focus distribution, our approach will
be to firstly conduct CT screening for highly suspected cases
according to the disease history in clinical work and then adopt
the corresponding examination strategies early according to
comprehensive considerations, including the disease condition of
the patient, relevant hospital conditions, willingness of the
patient and treatment measures. We consider that MRI combined with
MRV examination is the preferred means of diagnosing CVST, while
DSA examination may reduce missed diagnoses and the misdiagnosis
rate. For cases requiring interventional treatment and hospitals
with interventional treatment qualification, DSA may act as the
preferred examination means.
References
|
1
|
Bousser MG and Ferro JM: Cerebral venous
thrombosis: an update. Lancet Neurol. 6:162–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stam J: Thrombosis of the cerebral veins
and sinuses. N Engl J Med. 352:1791–1798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Bruijn SF and Stam J: Randomized,
placebo-controlled trial of anticoagulant treatment with
low-molecular-weight heparin for cerebral sinus thrombosis. Stroke.
30:484–488. 1999.PubMed/NCBI
|
|
4
|
lsensee C, Reul J and Thron A: Magnetic
resonance imaging of thrombosed dural sinuses. Stroke. 25:29–34.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dentali F, Crowther M and Ageno W:
Thrombophilic abnormalities, oral contraceptives, and risk of
cerebral vein thrombosis: a meta-analysis. Blood. 107:2766–2773.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsai FY, Nguyen B, Lin WC, et al:
Endovascular procedures for cerebrovenous disorders. Acta Neurochir
Suppl. 101:83–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leach JL, Fortuna RB, Jones BV and
Gaskill-Shipley MF: Imaging of cerebral venous thrombosis: current
techniques, spectrum of findings, and diagnostic pitfalls.
Radiographics. 26(Suppl 1): S19–S41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Linn J, Ertl-Wagner B, Seelos KC, et al:
Diagnostic value of multidetector-row CT angiography in the
evaluation of thrombosis of the cerebral venous sinuses. AJNR Am J
Neuroradiol. 28:946–952. 2007.PubMed/NCBI
|
|
9
|
Khandelwal N, Agarwal A, Kochhar R, et al:
Comparison of CT venography with MR venography in cerebral
sinovenous thrombosis. AJR Am J Roentgenol. 187:1637–1643. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai FY, Kostanian V, Rivera M, Lee KW,
Chen CC and Nguyen TH: Cerebral venous congestion as indication for
thrombolytic treatment. Cardiovasc Intervent Radiol. 30:675–687.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Damak M, Crassard I, Wolff V and Bousser
MG: Isolated lateral sinus thrombosis: a series of 62 patients.
Stroke. 40:476–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sagduyu A, Sirin H, Mulayim S, et al:
Cerebral cortical and deep venous thrombosis without sinus
thrombosis: clinical MRI correlates. Acta Neurol Scand.
114:254–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van den Bergh WM, van der Schaaf I and van
Gijn J: The spectrum of presentations of venous infarction caused
by deep cerebral vein thrombosis. Neurology. 65:192–196. 2005.
|
|
14
|
Renowden S: Cerebral venous sinus
thrombosis. Eur Radiol. 14:215–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Connor SE and Jarosz JM: Magnetic
resonance imaging of cerebral venous sinus thrombosis. Clin radiol.
57:449–461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ford K and Sarwar M: Computed tomography
of dural sinus thrombosis. AJNR Am J Neuroradiol. 2:539–543.
1981.PubMed/NCBI
|
|
17
|
Smith R and Hourihan MD: Investigating
suspected cerebral venous thrombosis. BMJ. 334:794–795. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Forbes KP, Pipe JG and Heiserman JE:
Evidence for cytotoxic edema in the pathogenesis of cerebral venous
infarction. AJNR Am J Neuroradiol. 22:450–455. 2001.PubMed/NCBI
|
|
19
|
Mullins ME, Grant PE, Wang B, Gonzalez RG
and Schaefer PW: Parenchymal abnormalities associated with cerebral
venous sinus thrombosis: assessment with diffusion-weighted MR
imaging. AJNR Am J Neuroradiol. 25:1666–1675. 2004.PubMed/NCBI
|
|
20
|
Sajjad Z: MRI and MRV in cerebral venous
thrombosis. J Pak Med Assoc. 56:523–526. 2006.PubMed/NCBI
|
|
21
|
Patel SG, Collie DA, Wardlaw JM, et al:
Outcome, observer reliability, and patient preferences if CTA, MRA,
or Doppler ultrasound were used, individually or together, instead
of digital subtraction angiography before carotid endarterectomy. J
Neurol Neurosurg Psychiatry. 73:21–28. 2002. View Article : Google Scholar
|