Introduction
Platelet volume indices (PVIs) are a group of
parameters which are inexpensive to measure and are derived from
routine blood counts. The mean platelet volume (MPV) and platelet
deviation width (PDW) are the best validated and prominent of these
and are attractive indices for research in clinical settings due to
their widespread availability to clinicians (1–3).
While data on the size (mean cell volume, MCV) and deviation width
(RDW) of red blood cells are universally used for the investigation
of anemia, the diagnostic value of PVIs in thrombocytopenia has not
been fully established. The MPV has been demonstrated to be
sensitive and specific in discriminating between thrombocytopenia
caused by idiopathic thrombocytopenia purpura (ITP) and by aplastic
anemia (4). The MPV may markedly
correlate with the presence or absence of bone marrow diseases in
thrombocytopenic patients (5). A
prospective evaluation has revealed that the mean MPV of normal
Thai individuals may be used as a cut-off value (7.9 fl) in
distinguishing hyperdestructive from hypoproductive
thrombocytopenia (6). However, it
has been reported that although MPV may be used as an initial
suggestion of bone marrow disease in thrombocytopenic patients, it
has limited sensitivity and specificity (7). It has also been suggested that MPV
and PDW are not useful for identifying the mechanism of
chemotherapy-induced thrombocytopenia in a population of adult
oncology patients (8).
Furthermore, there are few studies evaluating the diagnostic power
of PVI for discriminating thrombocytopenia caused by bone marrow
failure (BMF) from that caused by ITP in Chinese patients at
present. To explore the clinical usefulness of MPV and PDW in
predicting the presence of bone marrow disease in thrombocytopenic
patients, a retrospective evaluation of MPV and PDW in a standard
clinical setting of a tertiary teaching hospital was conducted.
Materials and methods
Subjects
Based on the clinical and laboratory information,
the thrombocytopenic patients were divided into two groups: those
with BMF (361 patients) and those with no evidence of BMF (213
patients). When platelet counts were <20×109/l, the
resultant PVI, in particular MPV, may exhibit significant
discrepancies (9). Considering
this fact, those patients with a platelet count
<20.0×109/l were excluded from further study. The
presence of bone marrow disease was determined with the aid of a
bone marrow examination in the 268 valid patients with BMF and the
124 with ITP. Two morphologists (Y.P. Hu and S.H. Zhuang) reported
on the bone marrow samples independently.
Instruments and quality control
EDTA anti-coagulated venous blood samples were
collected from patients attending the Department of Hematology at
the Jinhua Municipal Central Hospital (Zhejiang, China). The
present study was conducted in accordance with the Declaration of
Helsinki and with approval from the Ethics Committee of Jinhua
Municipal Central Hospital. Written informed consent was obtained
from all participants. Thrombocytopenia was defined as a platelet
count of <100×109/l. MPV was measured on a Beckman
Coulter (Beckman-Coulter, Miami, FL, USA) Gen-S automated analyzer.
Calibration was assessed daily with the commercial calibrant 5C
(Beckman Coulter) and monitored twice daily.
PVI measurement
Since no statistically significant changes have been
demonstrated to occur in platelet and platelet indices when blood
samples are stored at room temperature for 24 (5) and 48 h (10), all the whole blood counts were
assayed within 4 h of the sample collection, although not at a
fixed time-point. In addition, the pre-analytical variances, such
as the storage time after sample collection, were not considered
further in the present study.
Statistical analysis
All statistical evaluations were performed with the
SPSS 13.0 software package. P<0.05 was considered to indicate
statistically significant differences.
Results
Clinical characteristics and platelet
count
In total, 574 cases of thrombocytopenic patients
with platelet counts <100×109/l were retrieved from
the records dating from between March 2010 and March 2011.
According to the diagnoses, the 574 cases were divided into two
groups: ITP- (n=213) and BMF-associated thrombocytopenia (n=361). A
total of 89 of the 213 patients assigned to the ITP group and 93 of
the 361 patients assigned to the BMF group had platelet counts
<20×109/l. Since the resultant platelet indices, in
particular the MPV, are less reliable when the platelet count is
<20×109/l, these patients were excluded. However, it
was observed that the percentage of platelet counts
<20×109/l differed significantly (P<0.01) between
ITP (41.8%) and BMF (25.8%).
Table I shows the
diagnoses for thrombocytopenic patients who had platelet counts
≥20.0×109/l. The clinical and laboratory characteristics
of the patients in the ITP and BMF groups are presented in Table II. Statistically significant
differences were observed in the MPV, PDW, platelet count and age
between the two groups of patients. Similar to the results of
previous studies, no significant differences were observed in the
gender distribution between the ITP and BMF groups.
 | Table IUnderlying diseases of the
thrombocytopenic patients with platelet count
≥20.0×109/l but <100×109/l. |
Table I
Underlying diseases of the
thrombocytopenic patients with platelet count
≥20.0×109/l but <100×109/l.
| Diagnosed cause of
thrombocytopenia | Number of patients
(%) |
|---|
| Bone marrow
failure | 268 |
| Acute lymphocytic
leukemia | 28 (10.4) |
| Acute myelogenous
leukemia | 118 (44.1) |
| Myelodysplasia
syndrome | 30 (11.2) |
| Aplastic
anemia | 24 (9.0) |
| Metastatic
malignancy | 11 (4.1) |
| CML blast
crisis | 17 (6.3) |
| Plasmocytic
dyscrasia | 34 (12.7) |
| Lymphoma | 6 (2.2) |
| Idiopathic purpura
thrombocytopenia | 124 |
| Total | 392 |
 | Table IIClinical and laboratory
characteristics in the groups of patients with
thrombocytopenia. |
Table II
Clinical and laboratory
characteristics in the groups of patients with
thrombocytopenia.
| Characteristic | ITP (n=124), mean ±
SD | BMF (n=268),mean ±
SD | P-value |
|---|
| Age (years) | 39.6±22.9 | 49.4±20.0 | 0.000 |
| Gender (M/F) | 65/59 | 135/133 | 0.706 |
| Platelets
(×109/l) | 46.5±21.4 | 53.7±22.9 | 0.003 |
| MPV (fl) | 10.3±1.8 | 9.0±1.8 | 0.000 |
| PDW (%) | 16.4±1.4 | 17.3±2.3 | 0.001 |
Correlation between PVI and platelet
count
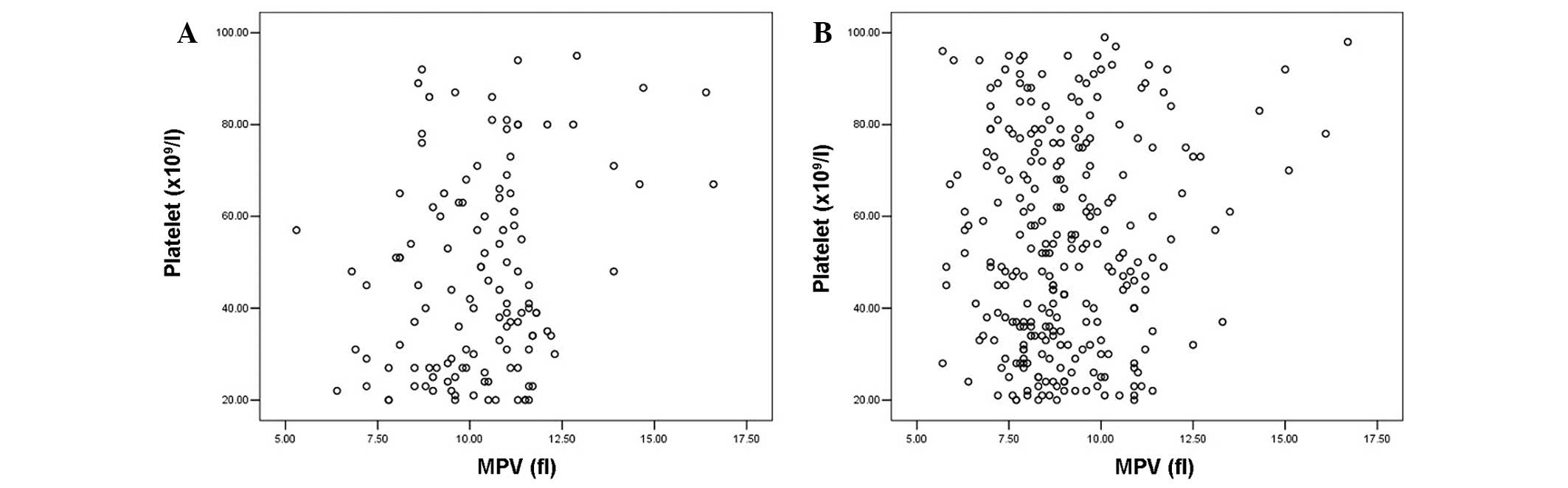
Fig. 1 shows the
scatter plots of platelet count against MPV for (A) patients with
ITP and (B) patients with BMF. Unlike the patients with BMF, a
positive correlation between the platelet count and MPV was
revealed in patients with ITP (r=0.248, P=0.005). Notably, a
positive correlation between the platelet count and PDW was also
demonstrated (Fig. 2) in the ITP
group (r=0.389, P=0.000) but not in the BMF group (r=−0.017,
P=0.778).
Predictive efficiency of MPV and PDW
The functions of MPV and PDW for the diagnosis of
BMF were evaluated in thrombocytopenic patients in the present
study. It was revealed that the level of MPV did not predict the
risk of BMF for the thrombocytopenic patients. Table III shows the correlation between
MPV and its negative-predictive value for BMF. An MPV of ≥11.0 fl
has a negative-predictive value of 59.0% and may be applied in
20.7% of patients.
 | Table IIIComparison of the negative-predictive
value of the MPV for bone marrow diseases at various MPV
values. |
Table III
Comparison of the negative-predictive
value of the MPV for bone marrow diseases at various MPV
values.
| MPV threshold
(fl) | Number of
patients | Number of patients
without bone marrow disease | % of all patients
within this group | Negative-predictive
value given MPV (%) | Odds ratio | 95% CI |
|---|
| ≥11.0 | 81 | 48 | 20.7 | 59.3 | 0.222 | 0.133–0.371 |
| ≥10.5 | 112 | 61 | 28.6 | 54.5 | 0.243 | 0.152–0.387 |
| ≥10.0 | 139 | 73 | 35.5 | 52.5 | 0.228 | 0.145–0.359 |
| ≥9.5 | 180 | 87 | 45.9 | 48.3 | 0.226 | 0.143–0.358 |
| ≥9.0 | 213 | 96 | 54.3 | 45.1 | 0.226 | 0.139–0.367 |
| ≥8.5 | 263 | 108 | 67.1 | 41.1 | 0.203 | 0.114–0.362 |
| ≥8.0 | 306 | 114 | 78.1 | 37.3 | 0.222 | 0.110–0.446 |
| ≥7.5 | 343 | 117 | 87.5 | 34.1 | 0.322 | 0.140–0.739 |
A low MPV is associated with a higher risk of BMF in
thrombocytopenic patients. Table
IV shows the correlation between the MPV and the
positive-predictive value for BMF at various cut-off values of MPV.
An MPV of <8.0 fl has the highest positive-predictive value for
BMF (88.4%) and may be applied in 21.9% of patients with low
platelet count.
 | Table IVComparison of the positive-predictive
value of the MPV for bone marrow diseases at various MPV
values. |
Table IV
Comparison of the positive-predictive
value of the MPV for bone marrow diseases at various MPV
values.
| MPV threshold
(fl) | Number of
patients | Number of patients
with bone marrow disease | % of all patients
within this group | Positive-predictive
value given MPV (%) | Odds ratio | 95% CI |
|---|
| <11.0 | 311 | 235 | 79.3 | 75.6 | 4.498 | 2.692–7.514 |
| <10.5 | 280 | 217 | 71.4 | 77.5 | 4.120 | 2.586–6.564 |
| <10.0 | 253 | 202 | 64.5 | 79.8 | 4.381 | 2.785–6.891 |
| <9.5 | 212 | 175 | 54.1 | 82.5 | 4.425 | 2.794–7.006 |
| <9.0 | 179 | 151 | 45.7 | 84.4 | 4.425 | 2.724–7.189 |
| <8.5 | 129 | 113 | 32.9 | 87.6 | 4.921 | 2.760–8.774 |
| <8.0 | 86 | 76 | 21.9 | 88.4 | 4.513 | 2.243–9.077 |
| <7.5 | 49 | 42 | 12.5 | 85.7 | 3.106 | 1.353–7.129 |
The correlation between PDW and its predictive
efficiency for BMF in thrombocytopenic patients in the present
study is summarized in Tables V
and VI. A PDW of ≥19.0% has the
highest positive-predictive value for BMF in thrombocytopenic
patients in the present study but may only be applied in 5.4% of
the study patients. A PDW of ≥17.5% has a positive-predictive value
of 83.9% and may be applied to 36.5% of these patients. By
contrast, a PDW of <16.0% has the highest negative-predictive
value (58.9%) for BMF and may be applied to 18.6% of
thrombocytopenic patients.
 | Table VComparison of the positive-predictive
value of PDW for bone marrow disease at various PDW values. |
Table V
Comparison of the positive-predictive
value of PDW for bone marrow disease at various PDW values.
| PDW threshold
(%) | Number of
patients | Number of patients
with bone marrow disease | % of all patients
within this group | Positive-predictive
value given PDW (%) | Odds ratio | 95% CI |
|---|
| ≥19.0 | 21 | 18 | 5.4 | 85.7 | 2.904 | 0.839–10.049 |
| ≥18.5 | 43 | 35 | 11.0 | 81.4 | 2.178 | 0.979–4.846 |
| ≥18.0 | 87 | 71 | 22.2 | 81.6 | 2.433 | 1.347–4.393 |
| ≥17.5 | 143 | 120 | 36.5 | 83.9 | 3.561 | 2.132–5.946 |
| ≥17.0 | 214 | 173 | 54.6 | 80.8 | 3.687 | 2.350–5.782 |
| ≥16.5 | 273 | 216 | 69.6 | 79.1 | 4.883 | 3.066–7.775 |
| ≥16.0 | 319 | 238 | 81.4 | 74.6 | 4.212 | 2.479–7.155 |
 | Table VIComparison of the negative-predictive
values of PDW for bone marrow disease at various PDW values. |
Table VI
Comparison of the negative-predictive
values of PDW for bone marrow disease at various PDW values.
| PDW threshold
(%) | Number of
patients | Number of patients
without bone marrow disease | % of all patients
within this group | Negative-predictive
value given PDW (%) | Odds ratio | 95% CI |
|---|
| <19.0 | 371 | 121 | 94.6 | 32.6 | 0.344 | 0.100–1.192 |
| <18.5 | 349 | 116 | 89 | 33.2 | 0.459 | 0.206–1.021 |
| <18.0 | 305 | 108 | 77.8 | 35.4 | 0.411 | 0.228–0.742 |
| <17.5 | 249 | 101 | 63.5 | 40.6 | 0.281 | 0.168–0.469 |
| <17.0 | 178 | 83 | 45.4 | 46.6 | 0.271 | 0.173–0.425 |
| <16.5 | 119 | 67 | 30.4 | 56.3 | 0.205 | 0.129–0.326 |
| <16.0 | 73 | 43 | 18.6 | 58.9 | 0.237 | 0.140–0.403 |
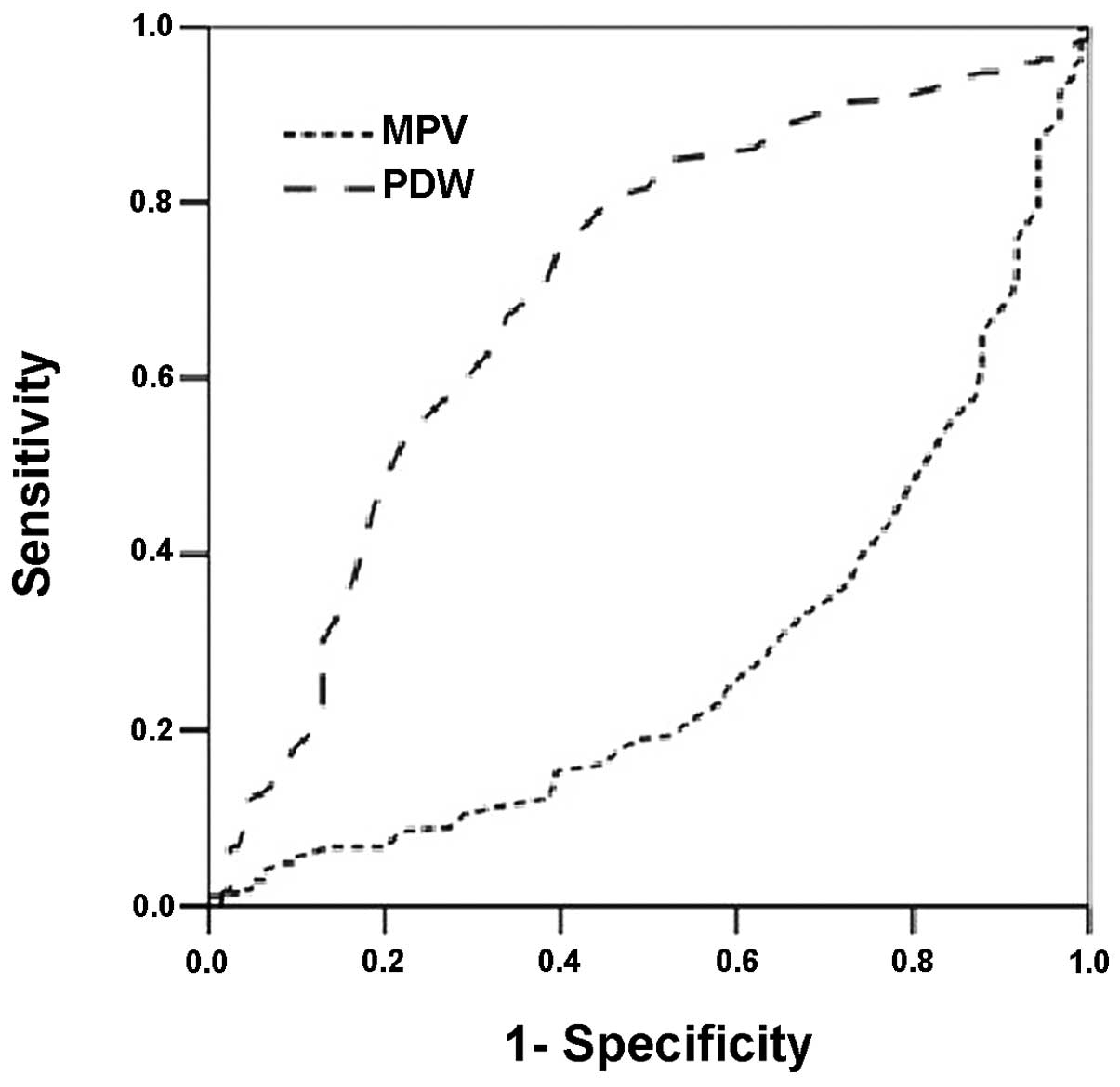
The areas under the curve (AUCs) of MPV and PDW in
receiver operating characteristic (ROC) analysis were 0.281 and
0.700, respectively, for the diagnosis of BMF in thrombocytopenic
patients in the present cohort (Fig.
3).
Discussion
Thrombocytopenia is a common clinical manifestation
of many diseases and has numerous causes, including decreased bone
marrow production, increased spleen sequestration and accelerated
destruction of platelets (11).
One of the major causes of increased platelet destruction is immune
thrombocytopenia, in which autoantibodies bind to platelet
antigens, causing their premature destruction by the
reticular-endothelial system, particularly the spleen (12). BMF, including hematological
malignancy, tumor infiltration and aplastic anemia, is another
common cause of thrombocytopenia and its diagnosis requires
confirmation by hematological morphology, bone marrow examination,
immunophenotyping and karyotyping which are familiar only to
hematologists.
Rapid diagnostic blood tests are used routinely in
clinical practice to assess the risk of specific underlying
diseases in groups of patients presenting a defined set of symptoms
or test results.
Although platelet parameters, such as MPV and PDW,
have been available for some time, their clinical usefulness has
been elusive (13), in particular
due to the delay between blood collection and analysis.
The results of the present study revealed that
patients with BMF have higher platelet counts, lower MPV and higher
PDW than patients with ITP. The reduced production of platelets in
patients with ITP involves more platelets being consumed by the
reticular-endothelial system and this higher turnover is in turn
reflected by the lower platelet counts and higher MPV (14,15).
PDW is measured using an automated blood analyzer based on a
logarithmic transformation of the platelet count. A higher PDW in
patients with BMF is consistent with a significant dysplasia of
hematopoiesis in the bone marrow.
MPV has been evaluated as a diagnostic tool in
different conditions with thrombocytopenia with contradictory
results. It has been demonstrated that MPV has sufficient
sensitivity and specificity to discriminate aplastic anemia
(4), bone marrow disease (5), hypoproductive thrombocytopenia
(6) and bone marrow metastasis of
solid tumors (16) from
immune-associated thrombocytopenia. Furthermore, it has been
reported that MPV and PDW may be safely relied on for a positive
diagnosis of ITP. MPV and PDW were superior to the platelet large
cell ratio (17). MPV also has
clinical benefits for the diagnosis and/or prognosis of
Crimean-Congo hemorrhagic fever (18), inherited macrothrombocytopenias
(19) and cardiovascular and
pulmonary embolism (20).
However, it has been reported that although MPV may
be used as an initial suggestion of bone marrow disease in
thrombocytopenic patients, it has limited sensitivity and
specificity (7). It has also been
suggested that MPV and PDW are not useful for identifying the
mechanism of chemotherapy-induced thrombocytopenia in a population
of adult oncology patients (8).
Similarly, MPV cannot be used to identify the etiology of
thrombocytopenia in the babies of preeclamptic mothers (21). The results of the present study
revealed that, although there are significant differences in the
MPV and PDW between BMF and ITP, the two parameters do not have
enough sensitivity and specificity to predict the presence of bone
marrow disease in thrombocytopenic patients.
In the preliminary phase of the present study, the
incidence of platelet counts <20×109/l was observed
to be higher in the present series of thrombocytopenic patients
(182/574, 31.7%) than that reported by Bowles et al (58/473,
12.3%) (5). The percentage of
thrombocytopenic patients with platelet counts
<20×109/l was higher in patients with ITP than in the
patients with BMF (89/213, 41.8% vs. 93/361, 25.8%; P<0.01).
Methodologically speaking, the validity of MPV and PDW is
negatively affected by a platelet count <20×109/l.
Although Bowles et al reported that the MPV retained a
diagnostic predictive value at platelet counts ≤20×109/l
(5), the thrombocytopenic patients
with platelet counts <20×109/l were not included in
the present study for further analysis. It is unclear whether
patients with platelet counts <20×109/l were included
in calculating the diagnostic predictive efficiency of MPV and PDW
in other reports and this may be one of the reasons for the
discrepancies between various authors. Considering the relatively
high prevalence of thrombocytopenia with platelet counts
<20×109/l, further studies should be carried out
which include patients with platelet counts <20×109/l
to elucidate the possible impact on the overall predictive
efficiency of MPV and PDW for the diagnosis of BMF in
thrombocytopenic patients.
As noted by Leader et al, the majority of
these data on the diagnostic predictive efficiency of MPV and PDW
in thrombocytopenic patients are from retrospective studies, some
of which had small study populations and confounding factors
influencing platelet volume. Additionally, the cut-off values
derived from these retrospective studies have not been validated
prospectively (20). In future,
improved research designs and standardized PVI measurements may
significantly increase the diagnostic predictive power of MPV and
PDW in the differential diagnosis of thrombocytopenia.
In conclusion, significant differences were observed
in platelet count, MPV, PDW and patient age between patients with
ITP and patients with BMF. However, MPV and PDW do not have enough
predictive efficiency for the diagnosis of BMF in thrombocytopenic
patients.
References
|
1
|
Giles C: The platelet count and mean
platelet volume. Br J Haematol. 48:31–37. 1981.
|
|
2
|
Reardon DM, Hutchinson D, Preston FE and
Trowbridge EA: The routine measurement of platelet volume: a
comparison of aperture-impedance and flow cytometric systems. Clin
Lab Haematol. 7:251–257. 1985.PubMed/NCBI
|
|
3
|
Trowbridge EA, Reardon DM, Hutchinson D
and Pickering C: The routine measurement of platelet volume: a
comparison of light-scattering and aperture-impedance technologies.
Clin Phys Physiol Meas. 6:221–238. 1985.PubMed/NCBI
|
|
4
|
Kaito K, Otsubo H, Usui N, et al: Platelet
size deviation width, platelet large cell ratio, and mean platelet
volume have sufficient sensitivity and specificity in the diagnosis
of immune thrombocytopenia. Br J Haematol. 128:698–702.
2005.PubMed/NCBI
|
|
5
|
Bowles KM, Cooke LJ, Richards EM and
Baglin TP: Platelet size has diagnostic predictive value in
patients with thrombocytopenia. Clin Lab Haematol. 27:370–373.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Numbenjapon T, Mahapo N, Pornvipavee R, et
al: A prospective evaluation of normal mean platelet volume in
discriminating hyperdestructive thrombocytopenia from
hypoproductive thrombocytopenia. Int J Lab Hematol. 30:408–414.
2008.
|
|
7
|
Chandra H, Chandra S, Rawat A and Verma
SK: Role of mean platelet volume as discriminating guide for bone
marrow disease in patients with thrombocytopenia. Int J Lab
Hematol. 32:498–505. 2010.PubMed/NCBI
|
|
8
|
ten Berg MJ, Huisman A, van den Bemt PM,
den Breeijen H, Egberts TC and van Solinge WW: Discriminative value
of platelet size indices for the identification of the mechanism of
chemotherapy-induced thrombocytopenia. Biomarkers. 16:51–57.
2011.PubMed/NCBI
|
|
9
|
Diquattro M, Gagliano F, Calabrò GM, et
al: Relationships between platelet counts, platelet volumes and
reticulated platelets in patients with ITP: evidence for
significant platelet count inaccuracies with conventional
instrument methods. Int J Lab Hematol. 31:199–206. 2009.
|
|
10
|
Vogelaar SA, Posthuma D, Boomsma D and
Kluft C: Blood sample stability at room temperature for counting
red and white blood cells and platelets. Vascul Pharmacol.
39:123–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Handin RI: Bleeding and thrombosis.
Harrison’s Principles of Internal Medicine. Harrison TS, Fauci AS,
Braunwald E, Isselbacher KJ, et al: 14th edition. McGraw-Hill; New
York: pp. 84–90. 1998
|
|
12
|
Woods VL Jr, Kurata Y, Montgomery RR, et
al: Autoantibodies against platelet glycoprotein Ib in patients
with chronic immune thrombocytopenic purpura. Blood. 64:156–160.
1984.PubMed/NCBI
|
|
13
|
Jackson SR and Carter JM: Platelet volume:
laboratory measurement and clinical application. Blood Rev.
7:104–113. 1993.PubMed/NCBI
|
|
14
|
Blanchette V and Carcao M: Approach to the
investigation and management of immune thrombocytopenic purpura in
children. Semin Hematol. 37:299–314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed S, Siddiqui AK, Shahid RK, Kimpo M,
Sison CP and Hoffman MA: Prognostic variables in newly diagnosed
childhood immune thrombocytopenia. Am J Hematol. 77:358–362. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aksoy S, Kilickap S, Hayran M, et al:
Platelet size has diagnostic predictive value for bone marrow
metastasis in patients with solid tumors. Int J Lab Hematol.
30:214–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ntaios G, Papadopoulos A, Chatzinikolaou
A, et al: Increased values of mean platelet volume and platelet
size deviation width may provide a safe positive diagnosis of
idiopathic thrombocytopenic purpura. Acta Haematol. 119:173–177.
2008.PubMed/NCBI
|
|
18
|
Ekiz F, Gürbüz Y, Basar O, et al: Mean
platelet volume in the diagnosis and prognosis of Crimean-Congo
hemorrhagic fever. Clin Appl Thromb Hemost. 3:March 19–2012.(Epub
ahead of print).
|
|
19
|
Noris P, Klersy C, Zecca M, et al:
Platelet size distinguishes between inherited
macrothrombocytopenias and immune thrombocytopenia. J Thromb
Haemost. 7:2131–2136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leader A, Pereg D and Lishner M: Are
platelet volume indices of clinical use? A multidisciplinary
review. Ann Med. 3:March 13–2012.(Epub ahead of print).
|
|
21
|
Akcan AB, Oygucu SE, Ozel D and Oygür N:
Mean platelet volumes in babies of preeclamptic mothers. Blood
Coagul Fibrinolysis. 22:285–287. 2011.PubMed/NCBI
|