Introduction
The pathogenesis of type 1 diabetes (T1D) involves
the activation of autoimmune T killer cells within the
pancreas-draining lymph nodes. Activated autoimmune T cells then
leave the regional lymphatics, enter the bloodstream and gradually
transmigrate from the bloodstream through the pancreatic
endothelium and into the islets of Langerhans where they destroy
insulin-producing β cells (1). The
dynamic interaction of T cell CD44 with its endothelial ligand (a
non-sulfated linear hyaluronan glycosaminoglycan) is essential for
accomplishing the firm adhesion of T cells to the pancreatic
endothelium and then for the transendothelial migration and
subsequent homing of the adherent T cells into the islets (2–4).
Our previous work and the studies of others have
suggested that the invasion-promoting membrane type-1 matrix
metalloproteinase (MT1-MMP) (5)
dynamically regulates the functionality of the cell
surface-associated signaling and adhesion receptor CD44 in cancer
cells and diabetogenic T cells (6–9). By
means of the regulatory proteolysis of CD44, MT1-MMP mediates the
transition from T cell adhesion to endothelial cells to T cell
transmigration. When combined, these cellular processes result in
the sustained homing of autoreactive T cells into the pancreatic
islets. As a result, the efficiency of T cell homing in the islets
is directly proportional to the severity of the diabetic disease.
The inhibition of MT1-MMP proteolysis of CD44 drastically reduced
the diabetogenic efficiency of T cells, immobilized T cells on the
endothelium, repressed the homing of diabetogenic T cells into the
pancreatic islets, reduced insulitis and mononuclear cell
infiltration and promoted the recovery of the insulin-producing β
cells in non-obese diabetic (NOD) mice with freshly developed T1D.
The importance of the MT1-MMP-CD44 axis in T1D has thus been
identified in a diabetes transfer model with NOD mice and in
freshly diabetic NOD mice (Savinov, 2005 #78) (9).
A highly potent MMP inhibitor,
3(S)-2,2-dimethyl-4[4-pyridin-4-yloxy-benzenesulfonyl]-thiomorpholine-3-carboxylic
acid hydroxamate (AG3340), has been used previously to efficiently
control T cell MT1-MMP activity (6,9). The
Ki values of AG3340 against MMP-2, MMP-3, MMP-13 and
MT1-MMP are ∼100, 300, 40 and 200 pM, respectively. Other
individual MMPs are significantly less sensitive to AG3340
inhibition (e.g. the Ki values for MMP-1 and MMP-7 are
10 and 55 nM, respectively). AG3340 was used as an oral
anti-angiogenic drug in phase I–III clinical trials in humans with
advanced non-small cell lung cancer and prostate cancer. The trials
were halted due to the drug’s lack of effectiveness in patients
with the late-stage disease (10).
To shed additional light on the physiological
significance of the MT1-MMP-CD44 axis in the homing of diabetogenic
T cells and also on the importance of the specific T cell
MT1-MMP-dependent targeting of CD44, the anti-diabetic potencies of
two broad-range non-hydroxamate MMP inhibitors
[2-(4-phenoxyphenylsulfonylmethyl)thiirane (SB-3CT) and
epigallocatechin-3-gallate (EGCG)] were tested using a transferred
diabetes model in NOD mice. SB-3CT and EGCG, however, do not
inhibit MT1-MMP efficiently. SB-3CT exhibits a dithiolate moiety
that chelates the active-site zinc. While SB-3CT is an effective
and selective MMP-2/MMP-9 gelatinase inhibitor, it either does not
inhibit or poorly inhibits other MMPs and the closely related
metalloprotease TACE (tumor necrosis factor α-converting enzyme)
(11,12). EGCG, a major catechin of green tea,
also exhibits inhibitory, albeit largely non-specific, effects on
MMPs (13–18). Due to their proven ability to
transfer diabetes to NOD mice effectively and rapidly (6,19,20),
highly diabetogenic, insulin-specific, CD8-positive,
Kd-restricted T cells of the TGNFC8 clone
(IS-CD8+ T cells) were used in the present study. The
results demonstrated that the MT1-MMP-targeting inhibitor AG3340,
but not SBC3T and EGCG (despite their potency against MMPs distinct
from MT1-MMP), exhibited a significant anti-diabetic action. The
specific effect of AG3340 demonstrates the importance of the
MT1-MMP-CD44 axis in diabetogenesis, thus making T cell MT1-MMP a
promising drug design target for T1D therapy.
Materials and methods
General reagents
Reagents were from Sigma (St. Louis, MO, USA) unless
indicated otherwise. AG3340 was a gift of Dr Peter Baciu (Allergan,
Irvine, CA, USA). SB-3CT (an inhibitor of MMP-2 and MMP-9) and
α1-antitrypsin were purchased from Calbiochem (La Jolla, CA,
USA).
Mice and cells
NOD/LtJ mice were from the Jackson Laboratory (Bar
Harbor, ME, USA). IS-CD8+ cells (insulin-specific,
CD8-positive, Kd-restricted T cells of the TGNFC8 clone
from the NOD mouse pancreas) (20)
were maintained in Click’s medium supplemented with 5% fetal calf
serum, 2×105 M β-mercaptoethanol, 20 mM
penicillin-streptomycin, 3 mg/ml L-glutamine and 5 U/ml murine
interleukin-2. Every 3 weeks, the IS-CD8+ cells were
stimulated with irradiated NOD splenocytes (2000 Rad) loaded with
the L15YLVCGERG23 insulin B chain peptide (10 μg/ml) (19).
Induction of diabetes
Mice received AG3340 (1 mg/kg), SB-3CT or EGCG (10
or 100 mg/kg) or PBS IP. After 30 min, IS-CD8+ cells
(1×107) in PBS were injected IV into the irradiated (725
Rad, 24 h in advance) 5–8-week-old male recipient mice (5–6
animals/group). Afterwards, the mice received one injection of
their respective inhibitor every other day until they developed
diabetes (1–2 weeks). The onset of diabetes was monitored by
measuring urine and blood glucose levels with Diastix strips and a
glucose meter (Fisher Scientific, Hampton, NH, USA), respectively.
Mice with urine glucose levels ≥300 mg/dl for 3 consecutive days
were considered to be diabetic. The animal treatment protocols were
approved by the institutional Animal Care Committee.
Fluorescent labeling of
IS-CD8+ cells
IS-CD8+ cells (1×107/ml) were
labeled with a fluorescent didodecyl-tetramethylindocarbocyanine
perchlorate (DiI) dye. DiI-labeled cells (1×107) were
injected IV into irradiated (725 Rad, 24 h in advance) 5–8-week-old
mice (4–5 mice/group). The mice received AG3340 (1 mg/kg), SB-3CT
or EGCG (10 or 100 mg/kg) or PBS IP 30 min prior to the cell
injection. After 24 h, the pancreata were removed from euthanized
mice, fixed in 0.1 M periodate-lysine-paraformaldehyde phosphate
buffer, sucrose-saturated and freeze-molded in OCT compound (Sakura
Finetek, Torrance, CA, USA). Each pancreas was cryosectioned into 7
μm-sections separated by a 60 μm-interval. DiI-labeled cells were
counted by a blinded observer using a fluorescence microscope and
the cell positions relative to the islet boundary were recorded.
The DiI-cells localized within the islet boundary were considered
to be ‘inside’. The DiI-cells adjacent to an islet but outside of
the islet boundary were considered to be ‘at the entrance’ of the
islet (9,19).
Cell biotinylation
IS-CD8+ cells were surface biotinylated
with sulfo-NHS-LC-biotin (Pierce, Rockford, IL, USA) (9), re-suspended in serum-free Click’s
medium supplemented with AG3340 (50 μM), SB-3CT (100 μM) or EGCG
(50, 100 and 500 μM) and allowed to adhere for 4 h to plastic
coated with 2% type I collagen. The cells were then lysed using 50
mM N-octyl-β-D-glucopyranoside (9). Biotin-labeled CD44 was captured on
streptavidine-agarose beads from both the cell lysate and medium
samples. The captured samples were examined by western blotting
with the CD44 antibody (clone IM7.8.1; BD Biosciences, Franklin
Lakes, NJ, USA).
MMP-2 activation
IS-CD8+ cells (1×106) in
serum-free Click’s medium were supplemented with purified MMP-2 (20
ng) and allowed to either adhere for 18 h to plastic coated with 2%
type I collagen or remain in solution. AG3340 (50 μM), SB-3CT (100
μM) or EGCG (50, 100 and 500 μM) were added to the cells. After 18
h, 30 μl samples of medium were withdrawn and analyzed by gelatin
zymography to identify the MMP-2 status.
Cleavage of α1-antitrypsin
α1-antitrypsin (250 ng) was co-incubated for 2 h at
37°C with p-aminophenylmercuric acetate-activated MMP-2 (7 ng)
(21,22). The reactions were stopped using 2%
SDS and analyzed using 10% gel electrophoresis followed by
Coomassie staining.
Results and discussion
MT1-MMP sheds CD44 in T cells
To demonstrate MT1-MMP proteolysis of T cell CD44,
IS-CD8+ T cells were surface biotinylated with
membrane-impermeable biotin. The labeled cells were then allowed to
either adhere to a gelatin-coated plastic or were kept in solution.
The cells were then lysed and biotin-labeled CD44 was captured from
the cell lysate and medium samples using streptavidine-agarose
beads. The captured samples were examined by western blotting with
the CD44 antibody to measure the level of the released, soluble
CD44 ectodomain and the residual, membrane-anchored, cellular CD44
in the medium and the cell lysates, respectively. In addition,
media samples were analyzed by gelatin zymography to determine the
activation status of MMP-2. The soluble MMP-2 proenzyme is known to
be directly activated by cellular MT1-MMP (21,23).
To inhibit cellular MT1-MMP and, as a result, to repress the
conversion of the MMP-2 proenzyme into the enzyme, the
IS-CD8+ T cells, where indicated, were supplemented with
AG3340, SB-3CT or EGCG (Fig.
1).
 | Figure 1.AG3340 inhibits MT1-MMP and the
shedding of CD44 in IS-CD8+ T cells. (Upper panel)
Gelatin zymography of MMP-2. To analyze the activation of MMP-2 by
cellular MT1-MMP, adherent (A) and non-adherent (NA)
IS-CD8+ cells were each incubated for 18 h in serum-free
medium. Purified MMP-2 (20 ng) was added to the cells. The
activation of MMP-2 was analyzed by gelatin zymography of the
medium aliquots to observe the conversion of the 68 kDa MMP-2
proenzyme into the 62 kDa MMP-2 mature enzyme. Where indicated,
AG3340, SB-3CT or EGCG were added to the cells for 18 h. (Middle
panel) Western blotting of CD44. IS-CD8+ cells were
surface-biotinylated and were then either allowed to adhere, in
serum-free medium, to plastic coated with type I collagen/gelatin
(adherent, A) or remained in suspension (non-adherent, NA). Where
indicated, AG3340, SB-3CT or EGCG were added to the cells. Cell
lysate and medium samples were captured with streptavidin-agarose
beads. CD44 was analyzed in the captured sample aliquots (50 mg
total protein each) by western blotting with an antibody to the
CD44 ectodomain. (Bottom panel) MMP-2 is inhibited by low
concentrations of SB-3CT. α1-Antitrypsin was incubated with MMP-2.
The digest samples were analyzed by reducing SDS-gel
electrophoresis. Where indicated, SB-3CT was added to the samples.
AG3340,
3(S)-2,2-dimethyl-4[4-pyridin-4-yloxy-benzenesulfonyl]-thiomorpholine-3-carboxylic
acid hydroxamate; MT1-MMP, membrane type-1 matrix
metalloproteinase; MMP-2, matrix metalloproteinase-2; SB-3CT,
2-(4-phenoxyphenylsulfonylmethyl)thiirane; EGCG,
epigallocatechin-3-gallate. |
Consistent with previous observations (6,9),
endogenous MT1-MMP was latent in non-adherent T cells, while the
adhesion of T cells induced the activation of MT1-MMP. MT1-MMP
activation resulted in the subsequent activation of exogenous MMP-2
and the cleavage of T cell CD44. By contrast, non-adherent T cells
did not activate MMP-2 and shed cell CD44 inefficiently. AG3340
fully blocked the activation of MMP-2 and shedding of CD44 in
adherent IS-CD8+ T cells. SB-3CT (an inefficient
inhibitor of MT1-MMP) had no significant effect on either MMP-2
activation or CD44 shedding, while only an exceedingly high (500
mM) concentration of EGCG demonstrated a partial inhibition of
MMP-2 activation without any significant effect on CD44
proteolysis.
SB-3CT was highly potent at inhibiting MMP-2
proteolysis of α1-antitrypsin (a sensitive and readily available
protein substrate of MMPs) (24–26).
In the absence of SB-3CT, MMP-2 proteolysis led to conversion of
the 61 kDa α1-antitrypsin serpin into the 55 kDa degradation
fragment that represented the N-terminal portion of the
α1-antitrypsin molecule. In turn, a nanomolar range of
concentrations of SB-3CT totally blocked the cleavage of
α1-antitrypsin in vitro (Fig.
1).
AG3340 inhibits the intra-islet homing of
IS-CD8+ cells in NOD mice
To determine the anti-diabetic potential of the
SB-3CT and EGCG non-MT1-MMP inhibitors relative to that of AG3340,
NOD mice received an IP injection of the indicated concentrations
of SB-3CT, EGCG or AG3340. DiI-labeled IS-CD8+ cells
were then injected IV into the NOD mice. After 24 h, labeled
IS-CD8+ cells were counted at the periphery and inside
the islets (Fig. 2). In the
absence of AG3340, T cells efficiently transmigrated into the
islets. By contrast, in the presence of AG3340 T cells were
detected at the islet entrance. A few cells were found inside the
islets. SB-3CT and EGCG, which were used at a much higher
concentration than AG3340, did not affect the homing of
IS-CD8+ cells into the pancreatic islet (Fig. 3).
 | Figure 2.AG3340 inhibits the intra-islet homing
of IS-CD8+ T cells. NOD mice were treated with AG3340,
SB-3CT or EGCG by injection. In 30 min, this injection was followed
by the injection of DiI-labeled IS-CD8+ T cells. After
24 h, the cryo-sections of the pancreata were examined using a
fluorescence microscope. The DiI-labeled cells were ascribed their
position, either at the entrance of the islet or inside the
pancreatic islets, and counted. At least 100 islets per mouse (4–5
mice/group) were examined. The islets are easily recognized by
their morphological characteristics including lower fluorescence
and a compact, dense, structure. Representative images of the
pancreatic islets from NOD mice that received an injection of
DiI-labeled cells are shown. AG3340,
3(S)-2,2-dimethyl-4[4-pyridin-4-yloxy-benzenesulfonyl]-thiomorpholine-3-carboxylic
acid hydroxamate; SB-3CT,
2-(4-phenoxyphenylsulfonylmethyl)thiirane; EGCG,
epigallocatechin-3-gallate; NOD, non-obese diabetic; DiI,
didodecyl-tetramethylindocarbocyanine perchlorate. |
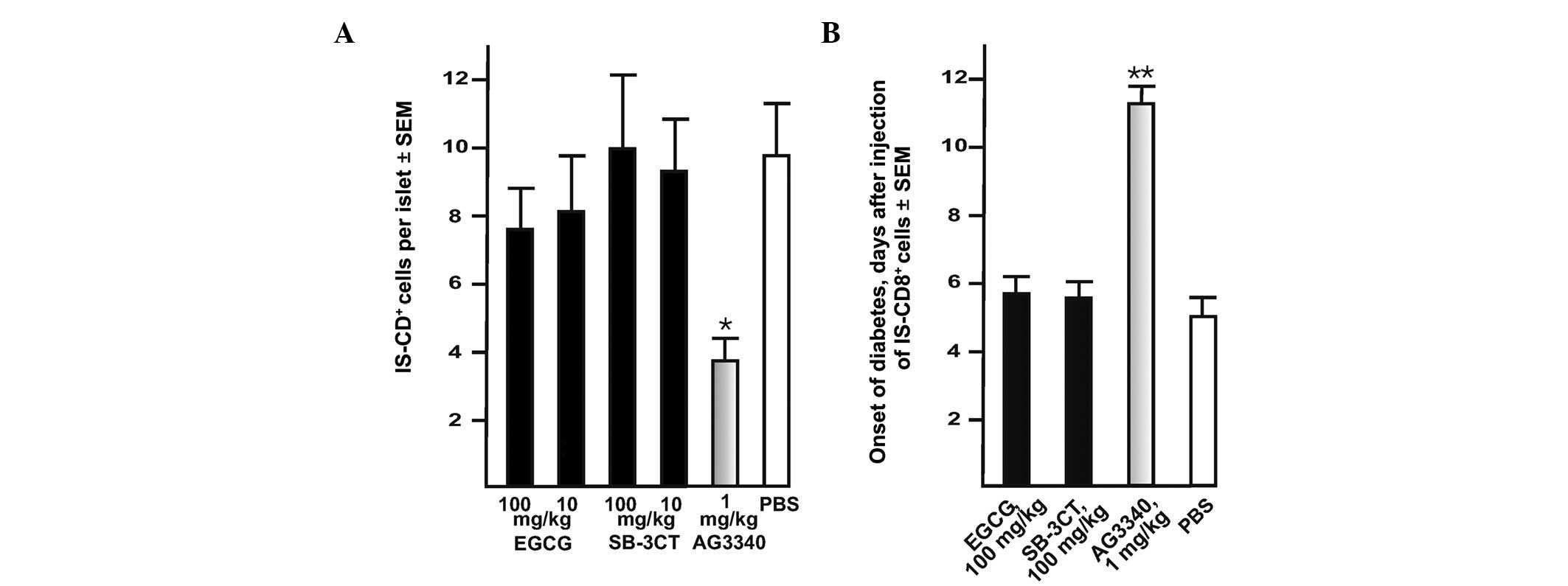 | Figure 3.AG3340 inhibits transendothelial
migration of IS-CD8+ T cells and delays the onset of
transferred diabetes in NOD mice. (A) AG3340 inhibits the
transmigration of IS-CD8+ cells into the pancreatic
islets. Mice received AG3340, SB-3CT, EGCG or PBS 30 min prior to
the injection of the cells. IS-CD8+ cells were labeled
with DiI and then injected in NOD mice. In 24 h, the labeled cells
with their intra-islet location were counted in the cryostat
sections of the entire pancreas. (B) AG3340 delays the onset of
adoptively transferred diabetes in NOD mice. IS-CD8+
cells were injected in NOD mice. Mice received AG3340, SB-3CT,EGCG
or PBS by one injection every other day until they developed
diabetes (approximately 1–2 weeks). The onset of diabetes was
monitored daily by measuring urine glucose levels with Diastix
reagent strips. Mice with urine glucose levels of ≥300 mg/dl for 3
consecutive days were considered diabetic. *P=0.02,
**P=0.015 by Fisher’s test. AG3340,
3(S)-2,2-dimethyl-4[4-pyridin-4-yloxy-benzenesulfonyl]-thiomorpholine-3-carboxylic
acid hydroxamate; NOD, non-obese diabetic; SB-3CT,
2-(4-phenoxyphenylsulfonylmethyl)thiirane; EGCG,
epigallocatechin-3-gallate; DiI,
didodecyl-tetramethylindocarbocyanine perchlorate. |
MT1-MMP inhibitor delays development of
transferred diabetes in NOD mice
To corroborate the results further,
IS-CD8+ cells were injected in NOD mice. Prior to the
cell injection (30 min), the mice received either the inhibitors or
PBS (control) IP. The inhibitor injections continued every other
day until the mice developed diabetes. AG3340 at a concentration as
low as 1 mg/kg delayed the onset of diabetes approximately 2-fold
compared with the control (Fig.
3). By contrast, there was no delay of the transferred diabetes
onset in mice which received SB-3CT and EGCG, which are potent
inhibitors of MMPs other than MT1-MMP.
As has been shown previously in the context of a
type 2 diabetes rat model, MMP-2, MMP-12 and MT1-MMP are
upregulated in diabetic males and high-fat-fed female Zucker
diabetic fatty rats as compared with their non-diabetic lean
counterparts (27). PD166793
[(S)-2-(4′-bromo-biphenyl-4-sulfonylamino)-3-methyl butyric acid; a
broad-range inhibitor with EC50 values of 6100, 47, 12,
7200, 7900, 8 and 240 nM against MMP-1, MMP-2, MMP-3, MMP-7, MMP-9,
MMP-13 and MT1-MMP, respectively] (28,29)
preserved β cell mass, presumably, by affecting the turnover of
certain extracellular matrix molecules in the islets. Despite the
fact that the mechanisms of the protective effects and relative
importance of the individual targets of the MMP inhibitors in T1D
and in type 2 diabetes are not completely understood, it is clear
that in a transfer diabetes model in NOD mice only AG3340, the
antagonist of MT1-MMP, delivered clinically relevant effects. Due
to the wide-range specificity of the MMP inhibitors, only a
simultaneous assessment of AG3340, SB-3CT and EGCG permitted us to
conclude that T cell MT1-MMP is predominant in T1D. Based on these
data, it is likely that the combined effect of the individual MMPs,
including MMP-2 and MMP-9, which are distinct from MT1-MMP and
efficiently inhibited by SB-3CT, is less important. We conclude
that MT1-MMP antagonists would be efficient in delaying T1D
transfer into NOD mice. These results demonstrate the functional
importance of the MT1-MMP-CD44 axis in mediating the efficiency of
transendothelial migration and the homing of diabetogenic T cells
into the pancreatic islets (30).
These current findings, particularly when combined
with our prior results (6,9), provide a working hypothesis for the
novel, anti-diabetic, application of the sharply focused, specific
inhibitors of MT1-MMP. The data suggest that the inhibition of T
cell MT1-MMP is a step forward in the design of novel and effective
therapies for T1D. It is now likely that the pharmacological
inhibition of MT1-MMP by specific antagonists will diminish the
homing of T killer cells into the islets. Consequently, is possible
that this favorable event would stimulate the regeneration of
insulin-producing β cells in the islets (9), leading to a more positive outcome for
T1D patients (31–33).
Abbreviations:
|
AG3340
|
3(S)-2,2-dimethyl-4[4-pyridin-4-yloxybenzenesulfonyl]-thiomorpholine-3-carboxylic
acid hydroxamate
|
|
DiI
|
didodecyl-tetramethylindocarbocyanine
perchlorate
|
|
EGCG
|
epigallocatechin-3-gallate
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
MT1-MMP
|
membrane type-1 matrix
metalloproteinase
|
|
NOD
|
non-obese diabetic
|
|
SB-3CT
|
-(4-phenoxyphenylsulfonylmethyl)thiirane
|
|
T1D
|
type 1 diabetes
|
Acknowledgements
This study was supported by National
Institutes of Health grants CA83017, CA77470 and RR020843 (Strongin
AY) and JDRF grant 262008-276 (Savinov AY).
References
|
1.
|
Mathis D, Vence L and Benoist C: beta-Cell
death during progression to diabetes. Nature. 414:792–798. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Butcher EC and Picker LJ: Lymphocyte
homing and homeostasis. Science. 272:60–66. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Nandi A, Estess P and Siegelman M:
Bimolecular complex between rolling and firm adhesion receptors
required for cell arrest; CD44 association with VLA-4 in T cell
extravasation. Immunity. 20:455–465. 2004. View Article : Google Scholar
|
|
4.
|
Weber C: Novel mechanistic concepts for
the control of leukocyte transmigration: specialization of
integrins, chemokines, and junctional molecules. J Mol Med (Berl).
81:4–19. 2003.
|
|
5.
|
Seiki M: Membrane-type 1 matrix
metalloproteinase: a key enzyme for tumor invasion. Cancer Lett.
194:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Savinov AY, Rozanov DV, Golubkov VS, Wong
FS and Strongin AY: Inhibition of membrane type-1 matrix
metalloproteinase by cancer drugs interferes with the homing of
diabetogenic T cells into the pancreas. J Biol Chem.
280:27755–27758. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Suenaga N, Mori H, Itoh Y and Seiki M:
CD44 binding through the hemopexin-like domain is critical for its
shedding by membrane-type 1 matrix metalloproteinase. Oncogene.
24:859–868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kajita M, Itoh Y, Chiba T, et al:
Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes
cell migration. J Cell Biol. 153:893–904. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Savinov AY, Rozanov DV and Strongin AY:
Mechanistic insights into targeting T cell membrane proteinase to
promote islet beta-cell rejuvenation in type 1 diabetes. FASEB J.
20:1793–1801. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cappuzzo F, Bartolini S and Crinó L:
Emerging drugs for non-small cell lung cancer. Expert Opin Emerg
Drugs. 8:179–192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ikejiri M, Bernardo MM, Bonfil RD, et al:
Potent mechanism-based inhibitors for matrix metalloproteinases. J
Biol Chem. 280:33992–34002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Rosenblum G, Meroueh SO, Kleifeld O, et
al: Structural basis for potent slow binding inhibition of human
matrix metalloproteinase-2 (MMP-2). J Biol Chem. 278:27009–27015.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Annabi B, Lachambre MP, Bousquet-Gagnon N,
Page M, Gingras D and Beliveau R: Green tea polyphenol
(−)-epigallocatechin 3-gallate inhibits MMP-2 secretion and
MT1-MMP-driven migration in glioblastoma cells. Biochim Biophys
Acta. 1542:209–220. 2002.
|
|
14.
|
Cheng XW, Kuzuya M, Kanda S, et al:
Epigallocatechin-3-gallate binding to MMP-2 inhibits gelatinolytic
activity without influencing the attachment to extracellular matrix
proteins but enhances MMP-2 binding to TIMP-2. Arch Biochem
Biophys. 415:126–132. 2003. View Article : Google Scholar
|
|
15.
|
Cheng XW, Kuzuya M, Nakamura K, et al:
Mechanisms of the inhibitory effect of epigallocatechin-3-gallate
on cultured human vascular smooth muscle cell invasion.
Arterioscler Thromb Vasc Biol. 25:1864–1870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Dell’Aica I, Donà M, Sartor L, Pezzato E
and Garbisa S: (−)Epigallocatechin-3-gallate directly inhibits
MT1-MMP activity, leading to accumulation of nonactivated MMP-2 at
the cell surface. Lab Invest. 82:1685–1693. 2002.
|
|
17.
|
Demeule M, Brossard M, Pagé M, Gingras D
and Béliveau R: Matrix metalloproteinase inhibition by green tea
catechins. Biochim Biophys Acta. 1478:51–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yamakawa S, Asai T, Uchida T, Matsukawa M,
Akizawa T and Oku N: (−)-Epigallocatechin gallate inhibits
membrane-type 1 matrix metalloproteinase, MT1-MMP, and tumor
angiogenesis. Cancer Lett. 210:47–55. 2004.
|
|
19.
|
Savinov AY, Wong FS, Stonebraker AC and
Chervonsky AV: Presentation of antigen by endothelial cells and
chemoattraction are required for homing of insulin-specific
CD8+ T cells. J Exp Med. 197:643–656. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wong FS, Visintin I, Wen L, Flavell RA and
Janeway CA Jr: CD8 T cell clones from young nonobese diabetic (NOD)
islets can transfer rapid onset of diabetes in NOD mice in the
absence of CD4 cells. J Exp Med. 183:67–76. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Strongin AY, Collier I, Bannikov G, Marmer
BL, Grant GA and Goldberg GI: Mechanism of cell surface activation
of 72-kDa type IV collagenase. Isolation of the activated form of
the membrane metalloprotease. J Biol Chem. 270:5331–5338. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Strongin AY, Marmer BL, Grant GA and
Goldberg GI: Plasma membrane-dependent activation of the 72-kDa
type IV collagenase is prevented by complex formation with TIMP-2.
J Biol Chem. 268:14033–14039. 1993.PubMed/NCBI
|
|
23.
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Li W, Savinov AY, Rozanov DV, et al:
Matrix metalloproteinase-26 is associated with estrogen-dependent
malignancies and targets alpha1-antitrypsin serpin. Cancer Res.
64:8657–8665. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Mast AE, Enghild JJ, Nagase H, Suzuki K,
Pizzo SV and Salvesen G: Kinetics and physiologic relevance of the
inactivation of alpha 1-proteinase inhibitor, alpha
1-antichymotrypsin, and antithrombin III by matrix
metalloproteinases-1 (tissue collagenase), -2 (72-kDa
gelatinase/type IV collagenase), and -3 (stromelysin). J Biol Chem.
266:15810–15816. 1991.
|
|
26.
|
Strongin AY: Mislocalization and
unconventional functions of cellular MMPs in cancer. Cancer
Metastasis Rev. 25:87–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zhou YP, Madjidi A, Wilson ME, et al:
Matrix metalloproteinases contribute to insulin insufficiency in
Zucker diabetic fatty rats. Diabetes. 54:2612–2619. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
O’Brien PM, Ortwine DF, Pavlovsky AG, et
al: Structure-activity relationships and pharmacokinetic analysis
for a series of potent, systemically available biphenylsulfonamide
matrix metalloproteinase inhibitors. J Med Chem. 43:156–166.
2000.
|
|
29.
|
Peterson JT, Hallak H, Johnson L, et al:
Matrix metalloproteinase inhibition attenuates left ventricular
remodeling and dysfunction in a rat model of progressive heart
failure. Circulation. 103:2303–2309. 2001. View Article : Google Scholar
|
|
30.
|
Savinov AY and Strongin AY: Matrix
metalloproteinases, T cell homing and beta-cell mass in type 1
diabetes. Vitam Horm. 80:541–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Chong AS, Shen J, Tao J, et al: Reversal
of diabetes in non-obese diabetic mice without spleen cell-derived
beta cell regeneration. Science. 311:1774–1775. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Suri A, Calderon B, Esparza TJ, Frederick
K, Bittner P and Unanue ER: Immunological reversal of autoimmune
diabetes without hematopoietic replacement of beta cells. Science.
311:1778–1780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Nishio J, Gaglia JL, Turvey SE, Campbell
C, Benoist C and Mathis D: Islet recovery and reversal of murine
type 1 diabetes in the absence of any infused spleen cell
contribution. Science. 311:1775–1778. 2006. View Article : Google Scholar : PubMed/NCBI
|

















