Introduction
Currently, the majority of occupations in China
largely involve the use of hands. The incidence of hand injury
increases annually due to low-level consciousness for
self-protection. The fingertip skin defect is one of the most
common hand injuries that is often accompanied by tendon or bone
exposure and is usually treated with flaps. Common flap types
include the random-pattern abdominal flap, fascial pedicle dorsal
flap of the finger, advanced skin flap and cross-finger flap.
Previous studies focused on skin flap survival until the interest
shifted to sensory recovery in flaps. This change occurred with the
increase in patient demand for a high quality of life, as well as
rapid developments in microsurgery in recent years. According to
Bunnell, the founder of hand surgery, individuals with a loss of
sensation in their hands have difficulty lifting small objects and
holding them. At an annual meeting of American hand surgeons,
Moberg compared a hand without feeling to a hand without a purpose
(1). The loss of sensory function
in the hand affects the movement and perceptive functions of the
hand, thus leading to poor judgment and a tendency for injury. A
total of 23 patients with fingertip cutaneous deficiency (30
digits) were treated with random-pattern abdominal skin flap from
September 2010 to February 2011. Functional sensory recovery of the
flaps in these patients was observed, and four cases were subjected
to histological examination.
Materials and methods
General data
A total of 23 patients, 12 males and 11 females aged
between 18 and 50 years (mean age, 31 years), participated in this
study. All patients presented with a traumatic fingertip skin
defect accompanied by tendon or bone exposure, as well as complete,
partially incomplete or missing nail. This study was conducted in
accordance with the declaration of Helsinki and with approval from
the Ethics Committee of the Third Hospital of Hebei Medical
University. Written informed consent was obtained from each
participant prior to emergency surgery being performed. Among the
causes of injury were an incised wound in 8 patients, a dog bite in
1 patient and mangling in 14 patients. The injured fingers included
5 thumbs, 8 index fingers, 12 middle fingers, 2 ring fingers and 3
small fingers. The defect area contained finger pulp defects in 20
fingers, dorsal digital defects in 3 fingers, finger lateral
defects in 2 fingers, distal transection cut in 3 fingers and
distal degloving injury in 2 fingers. The dimensions of the defect
area ranged from 0.7×1.2 to 2.5×3 cm post-debridement, whereas
those of the skin flap incision ranged from 1×1.5 cm to 2.8×3.3
cm.
Surgical procedure
Under digital nerve block, the patients were
subjected to radical debridement by which foreign bodies and
necrotic tissues were removed without affecting the vitality of the
organization. Subsequently, the two arteria digitalis were ligated.
After the bleeding was stanched, the random-pattern abdominal flap
was constructed based on the shapes and sizes of the wounds.
Specific interventions were performed to relieve the affected limb.
The skin flap area was 15–20% larger than the actual wound area to
avoid excessive tension. The length-to-width ratio of the flap was
maintained at 2:1 or lower to ensure flap revascularization and to
allow accumulation of subdermal plexus as well as a certain amount
of fat on the flap. A normal flap color was observed prior to skin
flap and skin edge suture and the donor sites were directly closed.
The patient was positioned in a manner that allowed the torsion of
the skin flap pedicle to be prevented. The flap pedicle was divided
three weeks post-debridement during the second stage.
Follow-up content and detection
methods
General data
Each flap was examined for tactile, pain and
temperature sensation, as well as for two-point discrimination in
the second week and the first, third and sixth post-operative
months.
Detection of tactile, pain and
temperature sensation
The patients were instructed to close their eyes.
Detection of tactile sensation was performed by touching the skin
with a small bundle of cotton. Pain sensation detection was
performed by lightly puncturing the skin with a 2 ml syringe needle
and temperature sensation by touching the skin with two test tubes,
one of which was filled with cold water (0–10°C) and the other with
hot water (50–60°C).
Detection of two-point
discrimination
The patients were initially instructed to keep their
hands still and close their eyes. The detector (blunt compasses)
stimulated the flap from the proximal to the distal end, followed
by a gradual decrease in the distance of the detector feet. The
patients were instructed to immediately report any sensation felt
(one or two points). The procedure was repeated with decreased
distance until the patients were unable to distinguish the two
separate stimuli. The shortest distance was recorded.
Sensory function evaluation
The sensory function was evaluated according to the
British Medical Research Council classification: S4, normal
sensitivity; S3+, recovery of useful discriminatory sensitivity;
S3, recovery of complete tactile sensitivity without dysesthesia
and rough useful discrimination; S2, recovery of superficial
painful and incomplete tactile sensitivity with hyperesthesia
and/or dysesthesia; S1, recovery of deep painful sensitivity and
S0, no sensitivity.
Test method
Several flap tissues were collected during the flap
thinning of the four fingers. The tissues were fixed with 10%
formalin and then studied with hematoxylin and eosin, as well as
immunohistochemical stains under a light microscope.
Statistical analysis
The results of two-point discrimination in the third
and sixth post-operative months were presented as mean ± standard
deviation. Statistical analysis was conducted using the SPSS 11.0
software (SPSS Inc., Chicago, IL, USA) and the t-test was adopted
for comparison of the results between the two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical observation
A total of 30 flaps survived. The postoperative
follow-up period ranged from 2 weeks to 6 months. The appearance of
the finger and the flap, as well as the color, flexibility, texture
and sensory recovery were satisfactory (Table I). Only 4 finger flaps were
subjected to thinning surgery, due to a bloated flap. Sensory
recovery was rated S3+ and no significant donor area complications
occurred.
 | Table I.Sensory function recovery of 30
flaps. |
Table I.
Sensory function recovery of 30
flaps.
| Recovery of sensory
function
|
|---|
| Time | Tactile | Pain | Cold | Hot | Two-point
discrimination |
|---|
| 2 weeks | 4 | 2 | 0 | 0 | 0 |
| 1 month | 25 | 18 | 7 | 4 | 0 |
| 3 months | 30 | 30 | 28 | 23 | 30 |
| 6 months | 30 | 30 | 30 | 30 | 30 |
Morphological observations
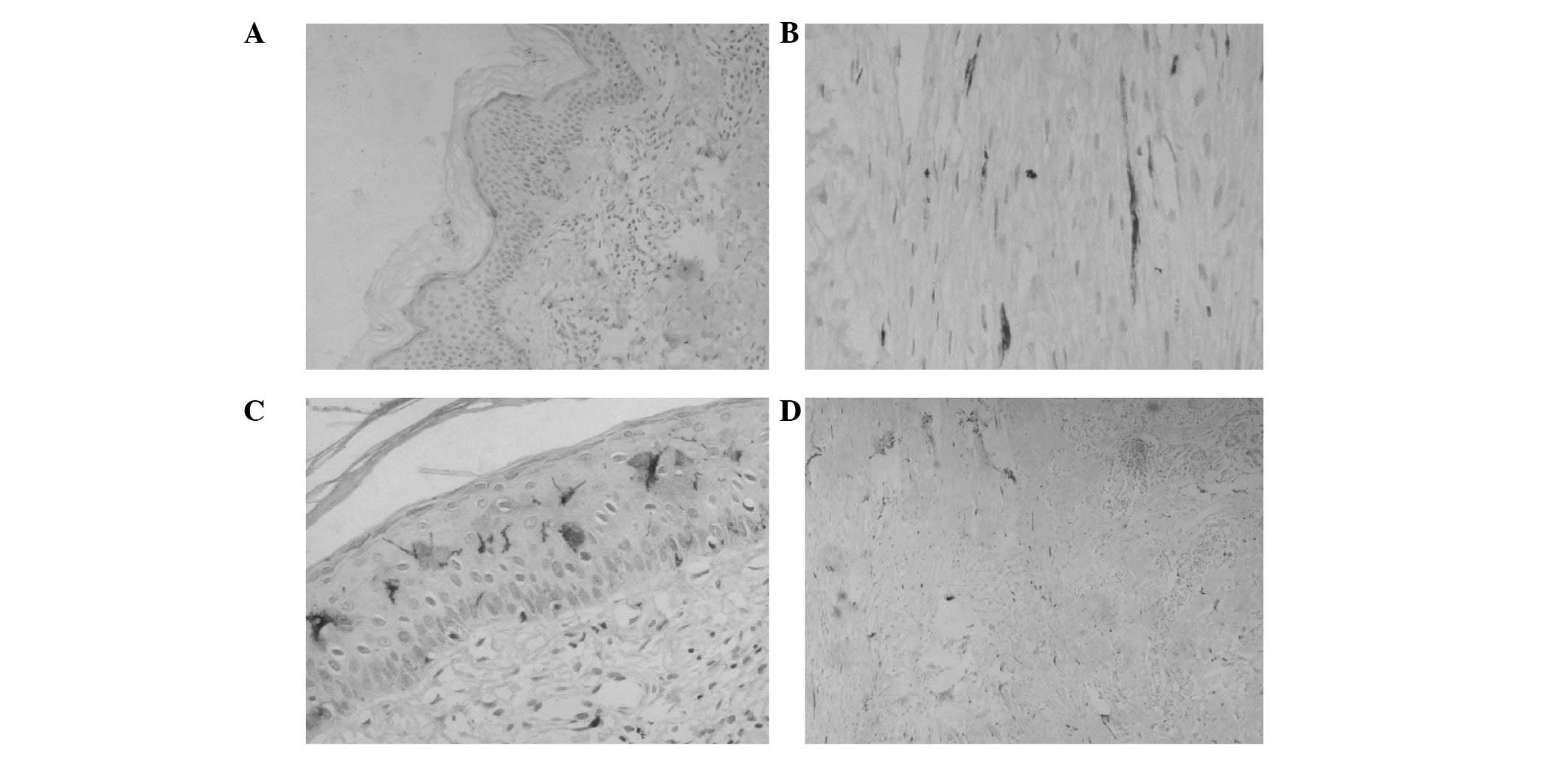
In the third post-operative month, the amount of
free nerve endings in the epidermal layer of the flap slice was
minimal (Fig. 1A) and the nerve
fiber in the plexiform dermal layer had a lower density (Fig. 1B). In the sixth post-operative
month, free nerve endings in the epidermal layer (Fig. 1C) and nerve fibers with a higher
density in the dermal layer were observed on the flap slice
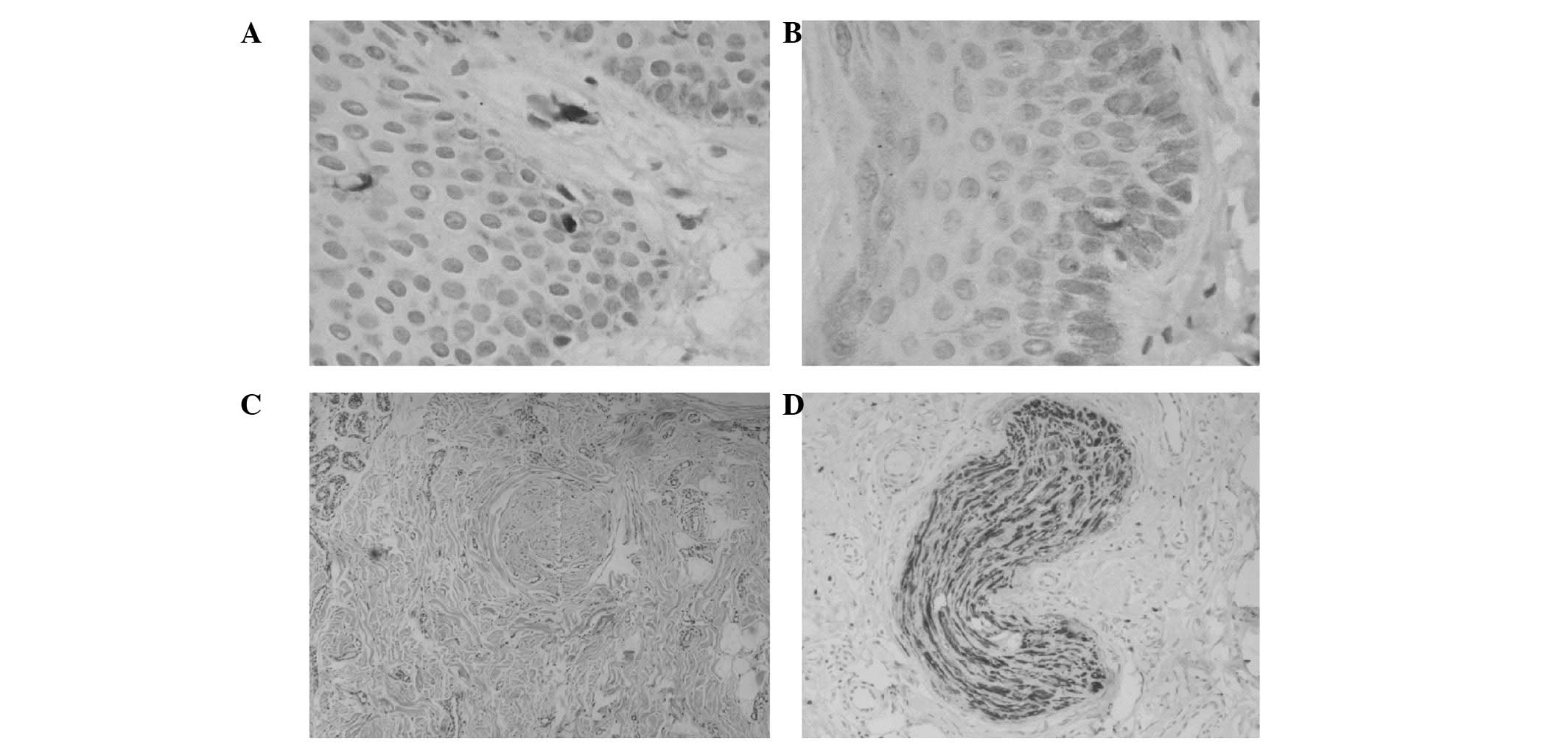
(Fig. 1D). A tactile corpuscle was
observed in the dermal papillary layer (Fig. 2A). A Merkel cell was observed in
the basal epidermal layer (Fig.
2B). The morphological integrity of the Pacinian corpuscle was
observed in the deep dermal layer (Fig. 2C and D).
Statistical significance
The two-point discrimination data in the third and
sixth post-operative months were 16.13±2.57 and 9.67±2.35 mm,
respectively. A significant difference was observed between the two
groups (P<0.05; Table II). The
two-point discrimination recovery in the third post-operative month
was improved compared with that in the sixth month.
 | Table II.Two-point discrimination recovery of
30 flaps. |
Table II.
Two-point discrimination recovery of
30 flaps.
| Time (months) | Two-point
discrimination (mm) |
|---|
| 3 | 16.13±2.57 |
| 6 | 9.67±2.35 |
Discussion
Various senses of the skin, attributed to the
sensory nerve endings and receptors, receive in vivo and
in vitro stimulation, which are converted into certain
action potentials along the nerve fiber. Different regions of the
body have different skin sensations since the receptors in these
parts and the density of the nerve fibers are different (2). The receptors contain free nerve
endings and Merkel cells, as well tactile, Pacinian, Ruffini and
Krause corpuscles. The free nerve endings detect pain sensation.
Tactile corpuscles are for sensing tactile sensations, Pacinian
corpuscles for pressure sensations and Krause corpuscles for
temperature sensations. The static two-point discrimination test
determined the density and function of the Merkel cell-axon
complex. The regeneration of receptors is required for sensory
recovery.
Previous studies evaluated the functional sensory
recovery through bud anastomosis by chemotaxis. The bud regenerated
and grew into a degenerative endoneurial tube and established
contact with the sensory end organs (3–5).
Manek et al(6) suggested
that the nerve from the surrounding skin also grew along the
degenerative endoneurial tube.
In this study, the flap slice in the third
post-operative month showed minimal free nerve endings in the
epidermal layer (Fig. 1A) and low
density nerve fibers in the dermal layer (Fig. 1B), which indicate pain sensation
recovery. The tactile sensation was also recovered; however, no
tactile corpuscle was observed in the dermal papillary layer.
Several studies (7) indicated that
normal skin without tactile corpuscles may still experience tactile
sensation, during which the free nerve endings primarily induce the
sensation as opposed to the tactile corpuscle. The flap slice in
the sixth post-operative month presented free nerve endings in the
epidermal layer (Fig. 1C), as well
as numerous nerve fibers with higher density in the dermal layer
(Fig. 1D). This indicates an
improved pain sensation recovery. Tactile corpuscles were observed
in the dermal papillary layer (Fig.
2A). Morphological integrity of Pacinian corpuscles was
observed in the deep dermal layer (Fig. 2C and D). A Merkel cell was observed
in the basal epidermal layer (Fig.
2B). These results indicate that a significant recovery was
achieved for tactile and pressure sensations, as well as the
two-point discrimination of the flap, which corresponds to clinical
function determination. No significant Krause corpuscles were
observed in the slice due to the staining methods. This study
failed to morphologically demonstrate the temperature sensation
recovery.
The central route involves the regeneration of the
nerve from the central flap area towards the edge (8). Clinical studies identified that the
nerve-anastomosed flap demonstrated stronger capacities in scope
and sensory recovery time since the growth of the proximal nerve
along the nerve endoneurial tube induced skin flap sensory
recovery. This phenomenon is known as the nerve contact guidance
theory (9). Chang et
al(10) used six different
methods to reconstruct skin flap sensory function and the results
of their study supported the above argument. The nerve-anastomosed
flap was the flap with nerve implantation and sensory recovery
through the central route. Daniel et al(11) found that the Tinel’s sign in the
flap of nerve implantation exceeded the nerve anastomosis in the
second post-operative month, which indicates nerve regeneration.
The majority of flap areas demonstrated sensory recovery in the
fifth post-operative month, which indicates that nerve anastomosis
restored nerve continuity and created the conditions for flap
sensory recovery. Gao (12)
indicated that in nerve anastomosis of the flap, the regenerative
nerve grows along the nerve distribution of the flap and arranges
the nerve endings or sensory corpuscles. Li et al(13) also demonstrated that the flap with
neural implantation obtains nerve reinnervation through the central
route. However, studies (14) have
shown that nerve anastomosis is only beneficial for the recovery of
two-point discrimination and not for other sensory restoration.
These studies hypothesized that neural anastomosis was only
beneficial for Merkel cell-axonal complexes.
The peripheral route shows the opposite effect to
the central route. In the peripheral route, the nerve regenerates
at the base and edge of the flap and is concentrated in the central
region. The traditional free flap, which is the flap without nerve
anastomosis, receives reinnervation through the peripheral
route.
Turko et al(15) examined a group of free flaps with
denervation by extracting flap slices and observing their
regeneration under an electron microscope. Sprouting axons
regenerated from the periphery and base of the flap and grew into
the flap through chemotaxis. Waris et al(16) conducted a study based on
cholinesterase determination in the skin graft, whereby the skin
graft obtained sensory recovery by peripheral nerve regeneration.
By immunohistochemical analysis, Manek et al(6) observed in their animal experimental
study that graft basal nerve regeneration succeeded peripheral
tissue regeneration and that sensory fiber regeneration occurred
first. Liu et al(17)
concluded that the sensory nerve endings regenerate and the
direction of nerve regeneration is from the peripheral to the
central area.
In this present study, up to 30 flaps survived, with
four of them positive for tactile sensation in the proximal area of
the flap in the second post-operative week, while two flaps
exhibited a partially restored pain sensation. This phenomenon was
caused by the higher nerve density in the normal tissues near the
flap that grew into the proximal area of the flap within a short
period of time and formed free nerve endings in the epidermal
layers of the flap, which are responsible for pain sensation.
Another reason may be that in the early period of nerve
regeneration, free nerve endings or residual tactile corpuscles
without complete degeneration were responsible for tactile
sensation instead of the tactile corpuscle. Denervation of the
tactile corpuscle was shown to induce short-term nerve-ending
degeneration without significantly changing the overall morphology
(18), during which the residual
tactile corpuscle played a certain role. In the first
post-operative month, the majority of the flaps restored tactile
sensation; however, a number of those in the central area failed to
restore tactile sensation. More than half of the flaps restored
pain sensation. The peripheral area of the 4 flaps restored cold
and hot temperature sensation, whereas that of 3 flaps restored
only cold sensation. Each flap with hot sensation recovery also
restored cold sensation. These observations suggest that the cold
sensation recovery of the flap was faster than the hot
sensation.
In the third post-operative month, the central area
of all flaps restored tactile and pain sensation, a number of which
exhibited hyperalgesia. Only 2 flaps failed to restore temperature
sensation. The two-point discrimination of 30 flaps demonstrated
varying degrees of recovery. The majority of the flaps in the
proximal and peripheral areas were more sensitive compared with
those in the central area. In the sixth post-operative month, pain,
tactile and temperature sensation, as well as the two-point
discrimination of all flaps, were restored with a flap sensation
function score of S3+, during which no statistically significant
difference was indicated in sensation between the proximal or
peripheral area and the center of the flap.
In this study, the phase recovery of tactile and
pain sensations was faster in the second post-operative week up to
the first post-operative month, whereas that of cold and hot
sensations, as well as the two-point discrimination, was faster in
the first up to the third post-operative month. The sensory
recovery time of the flap was closely associated with the thickness
of the flap (16,19,20).
In our study, the sensory recovery time of the flap with thinning
surgery was longer than that without surgery. Therefore, the
abdominal free flap restored the sensation through the nerve in the
peripheral and basal tissues, which grew onto the flap, known as
the peripheral route. The direction of nerve regeneration was from
proximal to distal, as well as from peripheral to central areas.
The various sensory recovery sequences were as follows: the first
recovery was tactile and pain sensation; the second recovery was
cold sensation; the third recovery was hot sensation and the final
recovery was two-point discrimination.
References
|
1.
|
Polatkan S, Orhun E, Polatkan O, Nuzumlali
E and Bayri O: Evaluation of the improvement of sensibility after
primary median nerve repair at the wrist. Microsurg. 18:192–196.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Zur KB, Genden EM and Urken ML: Sensory
topography of the oral cavity and the impact of free flap
reconstruction: a preliminary study. Head Neck. 26:884–889. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chen SZ and Jian SJ: Neural implants for
reconstruction of skin flap: an experimental study and clinical
application. Chin J Plastic Surg. 7:162–164. 1991.
|
|
4.
|
Chen B, Chen SZ, Li YJ and Li XY:
Experimental study on free endings and sensory corpuscles
degenerative process. Chin J Trauma. 17:108–109. 2001.
|
|
5.
|
Li XY, Li HY and Chen SZ: Electron
microscopic observation of the denervated flap reinnervation after
implantation of sensory nerve. Zhonghua Yi Xue Za Zhi. 74:624–625.
1994.(In Chinese).
|
|
6.
|
Manek S, Terenghi G, Shurey C, Nishikawa
H, Green CJ and Polak JM: Neovascularisation precedes neural
changes in the rat groin skin flap following denervation: an
immunohistochemical study. Br J Plast Surg. 46:481993. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Li XY, Chen SZ, Li YJ, Cheng B, Chen H and
Qu H: Degeneration and regeneration of free nerve endings in
denervated monkeys after implantation. Chin J Microsurg.
21:2841998.(In Chinese).
|
|
8.
|
Lin HC and Dong GY: Flap Surgery. Shanghai
Scientific & Technical Publishers; Shang Hai: pp. 2322006
|
|
9.
|
Ide C, Tohyama K, Yokota R, Nitatori T and
Onodera S: Schwann cell basal lamina and nerve regeneration. Brain
Res. 286:61–75. 1983. View Article : Google Scholar
|
|
10.
|
Chang KN, DeArmond SJ and Buncke H Jr:
Sensory reinnervation in microsurgical reconstruction of the heel.
Plast Reconstr Surg. 78:652–664. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Daniel RK, Terzis J and Midgley RD:
Restoration of sensation to anesthetic hand by a free neurovascular
flap from the foot. Plast Reconstr Surg. 57:275–280. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Gao YH: Experimental study on the sensory
nerve regeneration of free skin flap with anastomosis or without
anastomosis of nerves. Zhonghua Zheng Xing Shao Shang Wai Ke Za
Zhi. 6:217–218. 1990.PubMed/NCBI
|
|
13.
|
Li XY, Li HY and Chen SZ: CB-HRP
anterograde tracing observation of nerve regeneration after
implantation of skin flap of fiber distribution. Chin J Microsurg.
17:2121994.
|
|
14.
|
Lu W and Yu GZ: Observation and evaluation
in free flap sensory recovery. Chin J Microsurg. 16:1771993.(In
Chinese).
|
|
15.
|
Turko E, Jurecka W, Sikos G and
Piza-Katzer H: Sensory recovery in myocutaneous, noninnervated free
flaps: a morphologic, immunhistochemical, and electron microscopic
study. Plast Reconstr Surg. 92:238–247. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Waris T, Rechardt L and Kyosola K:
Reinnervation of human skin grafts: a histochemical study. Plast
Reconstr Surg. 72:439–447. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Liu F and Chang ZL: Sensory nerve
regeneration after implantation of free skin flap: an animal
experimental study. China J Modern Med. 12:28–31. 2002.
|
|
18.
|
Li XY, Chen SZ, Li YJ, Chen B, Chen H and
Qu H: Nerve implanted into monkey’s denervated finger tactile
corpuscles of degeneration and regeneration of the electron
microscopic observation. Chin J Reparative Reconstructive Surg.
13:193–198. 1999.
|
|
19.
|
Hastiongs H II: Dual innervated index to
thumb cross finger or island flap reconstruction. Microsurgery.
8:168–172. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Maquieira NO: An innervated full-thickness
skin graft to restore sensibility to fingertip and heel. Plast
Reconstr Surg. 53:568–575. 1974. View Article : Google Scholar : PubMed/NCBI
|