Introduction
The epidemiology of gastric cardia adenocarcinoma
(GCA) is characterized by a regional distribution identical to that
of esophageal squamous cell carcinoma (SCC) (1). In Linzhou, northern China, the ratio
of the incidence rates for GCA and SCC is ∼1:3.2 (2). Approximately 60% of patients with
suspected SCC, who receive surgical treatment at Linzhou People's
Hospital, are confirmed as having preliminary SCC and the remaining
40% have GCA (3). Furthermore, it
is not a rare event in other high incidence areas for esophageal
cancer (4). An epidemiological
study has suggested that SCC and GCA share a similar pathogenesis
of malignant transformation (5).
The occurrence pattern of GCA is not similar to that of neoplasms
originating from the distal parts of the stomach. In recent years,
the incidence of gastric cancer has been declining (6), while that of GCA is tending to
increase (7). In reference to
variations in the epidemiology, etiology, pathophysiology and
clinical parameters between GCA and cancer in the distal part of
the stomach, we have proposed that GCA should be regarded as an
independent disease (8,9).
Like other forms of cancer, GCA is a multi-step and
progressive disease, which may require the involvement of the
alterations of multiple genes (10). The resulting imbalance of
homeostasis between tumor suppressor and oncogene is one of the key
elements in this chronic disease (11,12).
Although intestinal metaplasia (IM) is an inevitable step in the
histopathological model for GCA, which begins with normal mucosa
and progresses towards superficial cardia gastritis, atrophic
cardia gastritis and/or interstitial metaplasia, dysphasia and
finally to GCA (13), the
underlying molecular mechanisms for this multi-step process are not
clear. The present study aimed to examine the expression of
sulfuric, Das-1 and Ki67 proteins in GCA and IM adjacent to GCA to
enhance our understanding of the correlation between IM and
GCA.
Materials and methods
GCA patients and sample collection
Surgically resected GCA samples from 200 patients
(including 147 males and 53 females with a mean age of 59±9 years
and an age range of 46–79 years) were collected from Linzhou
People's Hospital, Linzhou Central Hospital and Esophageal Cancer
Hospital of Yaocun, Linzhou in 2010. This study was conducted in
accordance with the Declaration of Helsinki. This study was
conducted with approval from the Ethics Committee of The First
Affiliated Hospital of Henan University of Science and Technology.
Written informed consent was obtained from all participants. No
chemotherapy or radiotherapeutic regimens were undertaken for the
patients involved in this study. All the samples were fixed with
95% ethanol. In addition to GCA tissue blocks, 10–15 tissue blocks
adjacent to the GCA field were also dissected and then subjected to
paraffin-embedding. Serial sections of 5 μm were cut for
hematoxylin and eosin (H&E) staining, histochemistry and
immunohistochemistry (IHC).
Histochemical staining
High iron diamine-Alcian blue (HID/AB) staining was
performed according to the protocols established in our laboratory
previously (14). In brief,
deparaffinized sections were treated with high iron diamine for 24
h at room temperature followed by incubation with Alcian blue
solution for 20–30 min in a humified chamber. After washing with
distilled water, sections were treated with 0.5% neutral red
solution (Shanghai No. 3 Reagent Factory, Shanghai, China) for 1–2
min and then washed as above. The slides were sealed with neutral
gum. As for the avidin-biotin-peroxidase complex (ABC) method of
IHC, slides were deparaffinized with xylene and dehydrated with
serially graded ethanol followed by antigen retrieval by microwave
boiling for 10 min. After washing with phosphate-buffered saline
(PBS) three times for 5 min each, the slides were incubated with
0.5% H2O2 for 20 min to quench endogenous
peroxidase. Normal horse serum at a dilution of 1:50 was added to
each slide to block non-specific reactions and incubated for 20
min. Incubation with the primary mouse antibodies for Das-1 (Uscn
Life Science Inc., Wuhan, China) and Ki67 (Oncogene Research, San
Diego, CA, USA) was performed at 4°C overnight. The dilution of
Das-1 was 1:50. Following incubation with biotinylated rabbit
secondary antibody at a dilution of 1:200 for 45 mins and three
washes with PBS, ABC solution at a dilution 1:50:50 was applied to
each slide and incubated for 1 h. The positive results were
visualized with 3,3′-diaminobenzidine (DAB). Finally, slides were
mounted with neutral gum. During each batch of experiments, the
control slide (without the primary antibody) was used to ensure the
protocols were followed correctly.
Criteria for HID/AB and IHC analyses
The criterion for diagnosis using HID/AB has been
established previously (15). The
sulfuric mucous was detected as black staining while the sialic
mucus appeared as blue staining in the cytoplasm and/or outside the
cells under a microscope. For positive Das-1 staining, the
cytoplasm presented brown staining and there was no staining in the
nuclei. In accordance with Mirza et al(16), the expression of Das-1 was
considered positive if a substantial number of cells and >1
gland were stained. If only an occasional goblet cell was stained,
the sample was considered negative. With regard to the criterion
for Ki67 staining, brown staining was observed exclusively in the
nuclei of cells. Ki67 staining was classified according to the
protocols reported by Yi (17): +
represents <25% positively-stained cells, ++ indicates 26–50%
positive cells, +++ indicates 51–75% positive cells and ++++
indicates >76% positively-stained cells in ten randomly selected
fields under a microscope with ×400 magnification.
Statistical analysis
The κ consistency test was employed to evaluate the
significance of statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Detection rate of IM lesions adjacent to
GCA
The detection rate of IM lesions in resected tissues
adjacent to GCA was 65/200 (32.5%).
Ki67 protein expression in GCA and
matched IM lesions
Ki67 expression was identified in GCA and in the
adjacent IM of the same patient (Fig.
1 and Table I).
 | Table ICo-expression of Das-1, Ki67 and
sulfuric proteins in GCA and matched adjacent IM lesions from the
same patient. |
Table I
Co-expression of Das-1, Ki67 and
sulfuric proteins in GCA and matched adjacent IM lesions from the
same patient.
| | GCA
|
|---|
| | Das-1 protein
| | Ki67 protein
| | Sulfuric protein
| |
|---|
| | Positive n (%) | Negative n (%) | Total | Positive n (%) | Negative n (%) | Total | Positive n (%) | Negative n (%) | Total |
|---|
| IM (adjacent to
GCA) | Positive | 22 (48.75) | 3 (3.75) | 25 | 20 (30.77) | 2 (3.08) | 22 | 19 (29.23) | 19 (29.23) | 38 |
| Negative | 9 (5.00) | 31 (42.50) | 40 | 19 (29.23) | 24 (36.92) | 43 | 10 (15.38) | 17 (26.15) | 27 |
Das-1 protein expression in GCA and
matched IM lesions
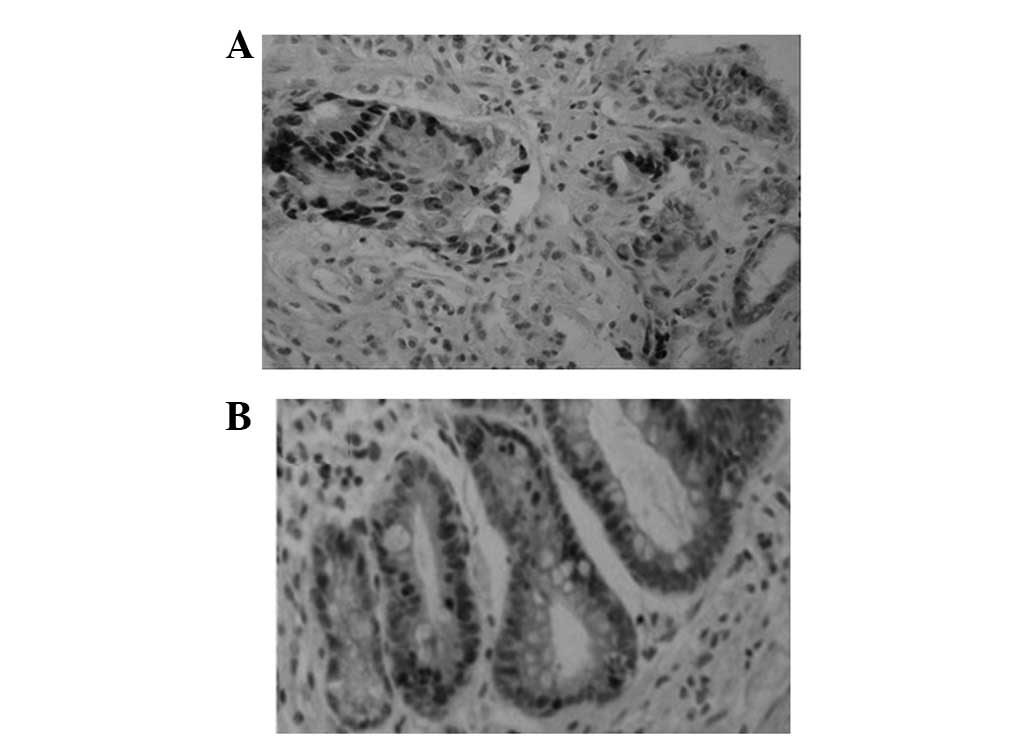
Similar to the expression pattern of Ki67, the Das-1
protein was identified in GCA and the adjacent IM of the same
patient (Fig. 2 and Table I).
Sulfuric mucous protein in GCA and
matched IM lesions
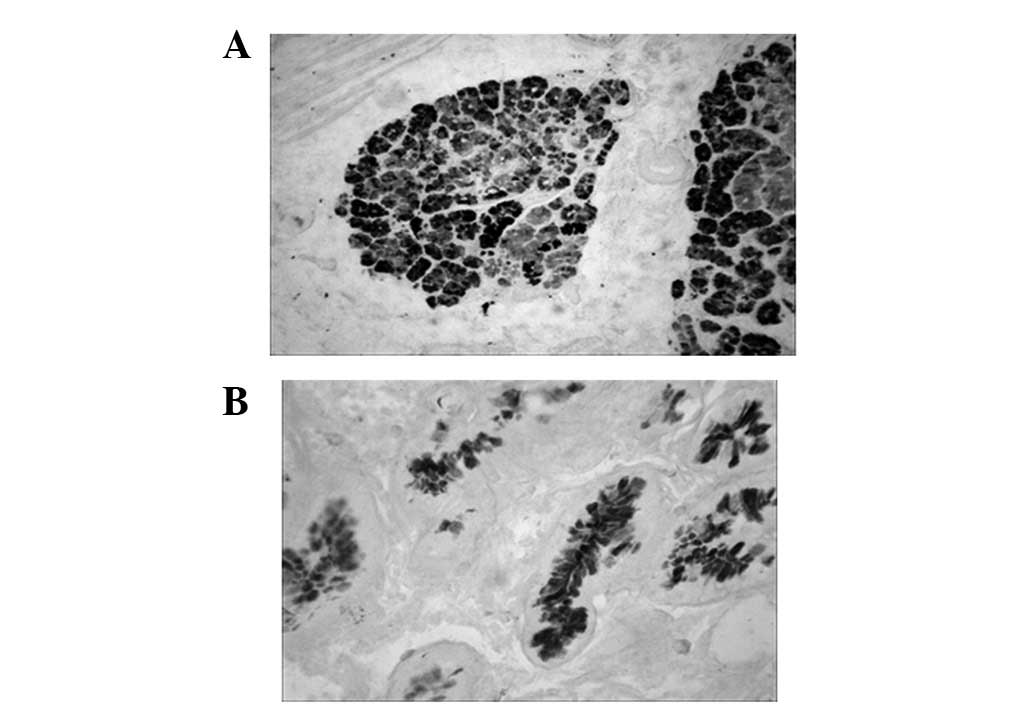
The sulfuric mucous protein was stained as
black-brown in GCA following HID/AB staining. In the same patient,
the sulfuric mucous protein did not show a pronounced co-expression
tendency in GCA and surrounding IM tissues (Fig. 3 and Table I).
Discussion
The current study demonstrates the presence of Das-1
protein in GCA and the surrounding IM lesions. This finding implies
that these two morphologically different lesions are homologous in
terms of molecular alterations. GCA may originate from IM. Das-1
protein may contribute to the transformation from IM to GCA in this
multi-stage process. Another finding of the present study was the
co-expression of the Ki67 protein in GCA and the corresponding IM,
which further supports our above hypothesis.
Das-1 (formerly known as 7E12H12) was developed
against a 40-kDa colonic epithelial protein (18). It specifically recognizes a
>200-kDa colon epithelial protein that complexes with a 40-kDa
protein and acts as a chaperone to bring the 40-kDa protein to the
colonic epithelial surface. Its role in cell proliferation,
differentiation and carcinogenesis is unclear. Experimental data
show that high Das-1 protein expression may be used to identify
risk factors for the occurrence of cancer of cardia intestinal
tissue.
The colonic type of IM, which contains sulfuric
mucous protein, has more malignant features and has increased
malignant potential. Morphologically, colonic IM is immature and/
or dedifferentiated with respect to histological structure and cell
appearance. This type of IM is considered a manifestation of a
typical cardia mucosa. The presence of a large quantity of sulfuric
mucous protein in the glands is often indicative of aberrant gene
expression. In IM lesions, a variety of tumor-associated antigens,
including the Ras p21 oncogene product, are increasingly
identified. In terms of genetic features, aneuploidal cells and the
DNA content in the colonic type of IM increase with IM progression.
Increasing evidence indicates that the colonic type of IM shares
more biological characteristics with neoplastic cells than other
type of IM (19). The present
study also revealed that the detection rate of the colonic type of
IM adjacent to GCA was increased compared with that in normal
cardia mucosa. The sulfuric mucous protein, however, did not show
the consistency as strikingly as Das-1 and Ki67 proteins, which
demonstrate pronounced co-expression. The possible causes for the
different expression patterns of sulfuric mucous protein may be as
follows: i) IM containing sulfuric mucous protein, although readily
prone to be transformed, has to undertake a multi-step process and
clone selection prior to gaining malignant properties; ii) the
number of samples involved in this study is limited and, therefore,
a larger study is required; and iii) the correlation between the
colonic type of IM and GCA requires in-depth investigation.
Currently, the mechanisms of carcinogenesis in the
IM mucosa are not clear. The epithelial cells in IM and the enzymes
within are able to take up lipids and lipid-soluble substances, in
a similar manner to the intestinal epithelium; however, the
epithelium of IM does not possess infrastructure-like chyle vessels
for transporting these substances. These retained substances inside
IM cells are highly carcinogenic and may contribute to the
transformation (20). Previously,
it was hypothesized that genes of stem cells in the proliferation
center within a mucosal gland are mutated following stimulation,
and the depression of colonic types of gene leads to the
differentiation of stem cells towards colonic-type cells. Two types
of IM, complete IM and incomplete IM, are formulated based on the
mature or immature morphology of metaplasia. The lesions are likely
to get worse if differentiation is aggravated and ultimately form a
neoplasm (21).
In conclusion, these results not only increased our
insight into the correlation between IM and GCA using
histochemistry and IHC, but also provide a basis for the
elucidation of the tumor-genesis mechanisms of GCA and the
identification of highly-specific and highly-sensitive biomarkers
for detection and early diagnosis. In combination with clinical
practice, further studies are required to determine the importance
of histochemical staining in the classification of GCA, to examine
the correlation between grades of malignancy, lymphatic metastasis
and various types of IM and to investigate the response sensitivity
following radiotherapy and chemotherapy.
Acknowledgements
This study was supported by the
National Outstanding Young Scientist Award of China (No. 30025016)
and the National Natural Science Foundation of China (No.
30670956).
References
|
1.
|
Abrams JA, Sharaiha RZ, Gonsalves L,
Lightdale CJ and Neugut AI: Dating the rise of esophageal
adenocarcinoma: analysis of Connecticut Tumor Registry data,
1940–2007. Cancer Epidemiol Biomarkers Prev. 20:183–186.
2011.PubMed/NCBI
|
|
2.
|
Guanrei Y and Sunglian Q: Incidence rate
of adenocarcinoma of the gastric cardia, and endoscopic
classification of early cardial carcinoma in Henan Province, the
People's Republic of China. Endoscopy. 19:7–10. 1987.PubMed/NCBI
|
|
3.
|
Wang LD, Gao WJ, Yang WC, et al: Prelimary
analysis of the statistics on 3933 cases with esophageal cancer and
gastric cardia cancer from the subjects in the People's Hospital of
Linzhou in 9 years. J Henan Med Univ. 32:9–11. 1997.(In
Chinese).
|
|
4.
|
Tinmouth J, Green J, Ko YJ, et al: A
population-based analysis of esophageal and gastric cardia
adenocarcinomas in Ontario, Canada: incidence, risk factors, and
regional variation. J Gastrointest Surg. 15:782–790. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wang LD: Mechanisms of human esophageal
and gastric cardia multistage carcimegenesis. Zhonghua Zhong Liu
Fang Zhi Za Zhi. 13:321–324. 2006.(In Chinese).
|
|
6.
|
Coupland VH, Allum W, Blazeby JM, et al:
Incidence and survival of oesophageal and gastric cancer in England
between 1998 and 2007, a population-based study. BMC Cancer.
12:112012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Fan YJ, Song X, Li JL, et al: Esophageal
and gastric cardia cancers on 4238 Chinese patients residing in
municipal and rural regions: a histopathological comparison during
24-year period. World J Surg. 32:1980–1988. 2008.PubMed/NCBI
|
|
8.
|
Gertler R, Stein HJ, Loos M, Langer R,
Friess H and Feith M: How to classify adenocarcinomas of the
esophagogastric junction: as esophageal or gastric cancer? Am J
Surg Pathol. 35:1512–1522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yamashita H, Katai H, Morita S, Saka M,
Taniguchi H and Fukagawa T: Optimal extent of lymph node dissection
for Siewert type II esophagogastric junction carcinoma. Ann Surg.
254:274–280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sharma P, Weston AP, Morales T, Topalovski
M, Mayo MS and Sampliner RE: Relative risk of dysplasia for
patients with intestinal metaplasia in the distal oesophagus and in
the gastric cardia. Gut. 46:9–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sugimura T: Multistep carcinogenesis: a
1992 perspective. Science. 258:603–607. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Weinberg RA: How cancer arises. Sci Am.
275:62–70. 1996. View Article : Google Scholar
|
|
13.
|
Zhou Q and Wang LD: The studies on the
biological characteristics of gastric cardia carcinoma. World J
Gastroenterol. 6:636–637. 1998.(In Chinese).
|
|
14.
|
Chen H, Wang LD, Fan ZM, Gao SG, Guo HQ
and Guo M: The comparison study of the three histochemical staining
methods in gastric cardia intestinal metaplasia staining. Henan Med
Res. 12:10–13. 2003.(In Chinese).
|
|
15.
|
Gao SG, Wang LD, Fan ZM, et al:
Histochemical studies on intestinal metaplasia adjacent to gastric
cardia adenocarcinoma in subjects at high-incidence area in Henan,
north China. World J Gastroenterol. 11:4634–4637. 2005.PubMed/NCBI
|
|
16.
|
Mirza ZK, Das KK, Slate J, et al: Gastric
intestinal metaplasia as detected by a monoclonal antibody is
highly associated with gastric adenocarcinoma. Gut. 52:807–812.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yi XP: The advances in application
researching on estimating prognosis of carcinoma with
immunohistochemical method. Cancer Res Prev Treat. 23:382–385.
1996.(In Chinese).
|
|
18.
|
Das KM, Sakamaki S, Vecchi M and Diamond
B: The production and characterization of monoclonal antibodies to
a human colonic antigen associated with ulcerative colitis:
cellular localization of the antigen by using the monoclonal
antibody. J Immunol. 139:77–84. 1987.
|
|
19.
|
Zullo A, Hassan C, Romiti A, et al:
Follow-up of intestinal meta-plasia in the stomach: When, how and
why. World J Gastrointest Oncol. 4:30–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Correa P: Is gastric cancer preventable?
Gut. 53:1217–1219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tang LJ: Review of research on gastric
intestinal metaplasia. J Prac Oncol. 10:77–79. 1996.(In
Chinese).
|