Introduction
Ovarian hyperstimulation procedures during in
vitro fertilization-embryo transfer (IVF-ET) cycles stimulate
the formation of multiple follicles and corpora lutea. However, the
quantity of endogenous progesterone produced by multiple corpora
lutea is not sufficient for the implantation and the continuation
of early pregnancy (1–3). There are several mechanisms leading
to luteal phase defect in IVF-ET cycles. The use of
gonadotropin-releasing hormone (GnRH) analogs in IVF-ET cycles
suppresses the production of endogenous gonadotropins. This, in
particular due to the suppression of luteinizing hormone (LH),
halts the activity of the corpus luteum (4). Furthermore, GnRH antagonists may also
shorten the luteal phase. Human chorionic gonadotropin (hCG) has a
negative feedback on the pituitary gland, and causes pituitary
suppression during the luteal phase (5). Thus, it is necessary to use exogenous
progesterone to support the luteal phase in IVF-ET cycles due to
the insufficient corpus luteum function (1–3,6). In
addition, the American Society for Reproductive Medicine (ASRM)
Position Statement recommends progesterone supplementation in IVF
cycles due to the higher pregnancy rates achieved by progesterone
administration compared with placebo or no treatment (1).
Among the routes of progesterone administration,
intravaginal (IV) and intramuscular (IM) routes are currently the
most commonly used (7,8). Although IM forms of progesterone
achieve higher serum progesterone levels, IV administration
provides higher local tissue levels in the endometrium (9). A previously published meta-analysis
demonstrated the benefit of IM over IV progesterone administration
for luteal phase support (LPS) on pregnancy outcomes (6). However, another meta-analysis
including additional randomized controlled studies showed that the
effects of IV and IM forms of progesterone on pregnancy end-points
were comparable (10). Notably,
the most effective route of progesterone administration in LPS
remains to be clarified.
The aim of the current study was to compare the
effects of IM and IV gel forms of progesterone used for LPS
following IVF-ET on clinical and on-going pregnancy rates.
Patients and methods
Patients
This retrospective single centre study was
undertaken in subjects who received IVF-ET treatment between
October 1999 to August 2009 in the Department of Obstetrics and
Gynaecology, Centre for Assisted Reproductive Techniques and
Infertility, Akdeniz University Hospital, Antalya, Turkey. Informed
consent was obtained from all patients. Medical charts of all
patients were reviewed, and data concerning the medical and
infertility-related history, pelvic examination, treatment
modalities and follow-up were recorded. The ethics committee of
Akdeniz University Hospital approved the study according to the
Good Clinical Practice guidelines of the International Conference
on Harmonization and national regulations (11).
Procedures
Controlled ovarian hyperstimulation (COH) was
applied according to the long protocol-GnRH analog or antagonist
protocol (12). Oocyte pick-up
(OPU) was performed 36 h after hCG injection. Starting from the
night of the OPU, patients received IV or IM progesterone and slow
oscillating transdermal oestrogen (Climara® Forte 7.8
mg; Schering German, Istanbul, Turkey) for LPS, until the 12th day
after ET when pregnancy tests were performed. LPS was provided by
the administration of 90 mg/day IV progesterone
(Crinone® 8% vaginal gel; Serono, Istanbul, Turkey) or
250 mg IM 17-α-hydroxyprogesterone caproate (17-HPC; Proluton
depot® ampules; Bayer Schering, Istanbul, Turkey) every
3 days. Serum β-hCG was analyzed on the 12th day after ET.
Endometrial thickness was measured on the day of hCG
administration.
Study outcomes
The primary outcome of the study was on-going
pregnancy rate. Secondary endpoints included β-hCG positivity,
biochemical pregnancy and clinical pregnancy rates. Biochemical
pregnancy was defined as the absence of clinical and sonographical
evidence of pregnancy despite positive β-hCG values (>10
mIU/ml). Clinical pregnancies were exclusive of biochemical and
ectopic pregnancies and required a detectable intrauterine
gestational sac on ultrasound examination. On-going pregnancies
were defined by the presence of intrauterine embryonic heart
activity, as determined by transvaginal ultrasonography.
Statistical methods
Demographic and clinical characteristics are
reported with descriptive analysis. Normality of data distribution
was assessed by the Shapiro-Wilk test. All continuous variables
were dichotomized by means of median values. Mann-Whitney U test,
Student’s t-test, and Chi-square tests were used for univariate
comparisons. Variables with a P<0.05 in univariate analysis were
included in the logistic regression analysis. A P-value of <0.05
was required to reject the null hypothesis. Effects on pregnancy
rates were reported by adjusted odds ratios (ORs) and 95%
confidence intervals (CIs). Post hoc power analysis revealed an OR
of 0.5 with a power of 100% and an overall two-sided type I error
of 5%, with inclusion of a total of 952 women in a two-sided Wald
test. All data management and analyses were performed using Stata
version 11 (Stata Corp., College Station, TX, USA) and Prism
version 5.0 for Mac OSX (GraphPad software Inc., La Jolla, CA,
USA).
Results
A total of 952 women in their first IVF-ET cycles
were included in the study. The IM 17-HPC group consisted of 632
women (66.4%) and the other 320 (33.6%) patients received IV
Crinone 8% gel. A total of 25 cycles were cancelled: 15 (2.4%)
cycles in 17-HPC group and 10 (3.1%) cycles in the Crinone group
(P=0.49). Thus, final analyses were performed in a total of 927
patients, including 617 patients in the 17-HPC group and 310 in the
Crinone group. The main clinical and demographic data of the
patients are shown in Table I.
 | Table ICharacteristics of IVF groups. |
Table I
Characteristics of IVF groups.
| Variables | IM 17-HPC
(n=632) | Crinone 8% gel
(n=320) | P-value |
|---|
| Age, years | | | 0.003 |
| Median | 32 | 31 | |
| Interquartile
range | 29–36 | 28–35 | |
| Duration of
infertility, years | | | 0.14 |
| Median | 7 | 6 | |
| Interquartile
range | 5–9 | 4–9 | |
| Etiology of
infertility, n (%) | | | 0.56 |
| Primary | 561 (88.8) | 288 (90.0) | |
| Secondary | 71 (11.2) | 32 (10.0) | |
| IVF indications, n
(%) | | | 0.06 |
| Male factor | 81 (12.8) | 35 (10.9) | |
| Tubal factor | 102 (16.1) | 46 (14.4) | |
| Ovulatory
dysfunction | 216 (34.2) | 137 (42.8) | |
| Endometriosis | 76 (12.0) | 31 (9.7) | |
| Male and female
factor | 13 (2.1) | 14 (4.4) | |
| Advanced maternal
age | 30 (4.8) | 10 (3.1) | |
| Unexplained | 93 (14.7) | 41 (12.8) | |
| Other | 21 (3.3) | 6 (1.9) | |
| Cancelled cycles, n
(%) | 15 (2.4) | 10 (3.1) | 0.49 |
| Basal FSH, IU/l | | | 0.01 |
| Median | 6.6 | 6.4 | |
| Interquartile
range | 5.4–8.0 | 4.2–7.8 | |
| Basal E2, pmol/l
(pg/ml) | | | <0.001 |
| Median | 305 (83.0) | 283 (77.0) | |
| Interquartile
range | 268–330
(73.0–90.0) | 202–340
(55.0–93.0) | |
| Duration of
gonadotropin stimulation, days | | | <0.001 |
| Median | 12.24 | 11.90 | |
| Standard
deviation | 1.08 | 1.45 | |
| Number of retrieved
oocytes | | | 0.03 |
| Median | 18 | 17 | |
| Interquartile
range | 15–21 | 13–21 | |
| Number of transferred
embryos | | | <0.001 |
| Median | 3 | 3 | |
| Interquartile
range | 3–3 | 2–3 | |
| E2 levelsa, pmol/l (pg/ml) | | | <0.001 |
| Median | 13928 (3794) | 12817 (3492) | |
| Interquartile
range | 11237–16988
(3061–4628) | 10336–15695
(2816–4276) | |
| Total dose of
gonadotropin, IU | | | <0.001 |
| Median | 3700 | 3413 | |
| Interquartile
range | 3000–4375 | 2750–4075 | |
| Endometrial
thickness, mm | | | <0.001 |
| Median | 8.3 | 7.6 | |
| Interquartile
range | 7.6–9.4 | 7.1–8.4 | |
The median age was 32 years (range, 20–46 years).
The 17-HPC group was older (32 vs. 31 years of age, P=0.003); had
higher basal FSH (6.6 vs. 6.4 IU/l, P=0.01), basal estradiol (E2)
[305 pmol/l (83 pg/ml) vs. 283 pmol/l (77 pg/ml), P<0.001], and
serum E2 levels on the day of hCG administration [13,928 pmol/l
(3,794 pg/ml) vs. 12,817 pmol/l (3,492 pg/ml), P<0.001]; had a
longer duration of gonadotropin stimulation (12.24 vs. 11.90 days,
P<0.001), higher total dose of gonadotropins (3,700 vs. 3,413
IU, P<0.001) and thicker endometrial thickness (8.3 vs. 7.6 mm,
P<.001). The median numbers of retrieved oocytes and transferred
embryos were also higher in the 17-HPC group (P=0.03 and
P<0.001, respectively). The groups were comparable for the
remaining demographic characteristics including infertility
periods, infertility etiology and IVF indications. The most
frequent IVF-ET indication in the two groups was ovulatory
dysfunction (Table I).
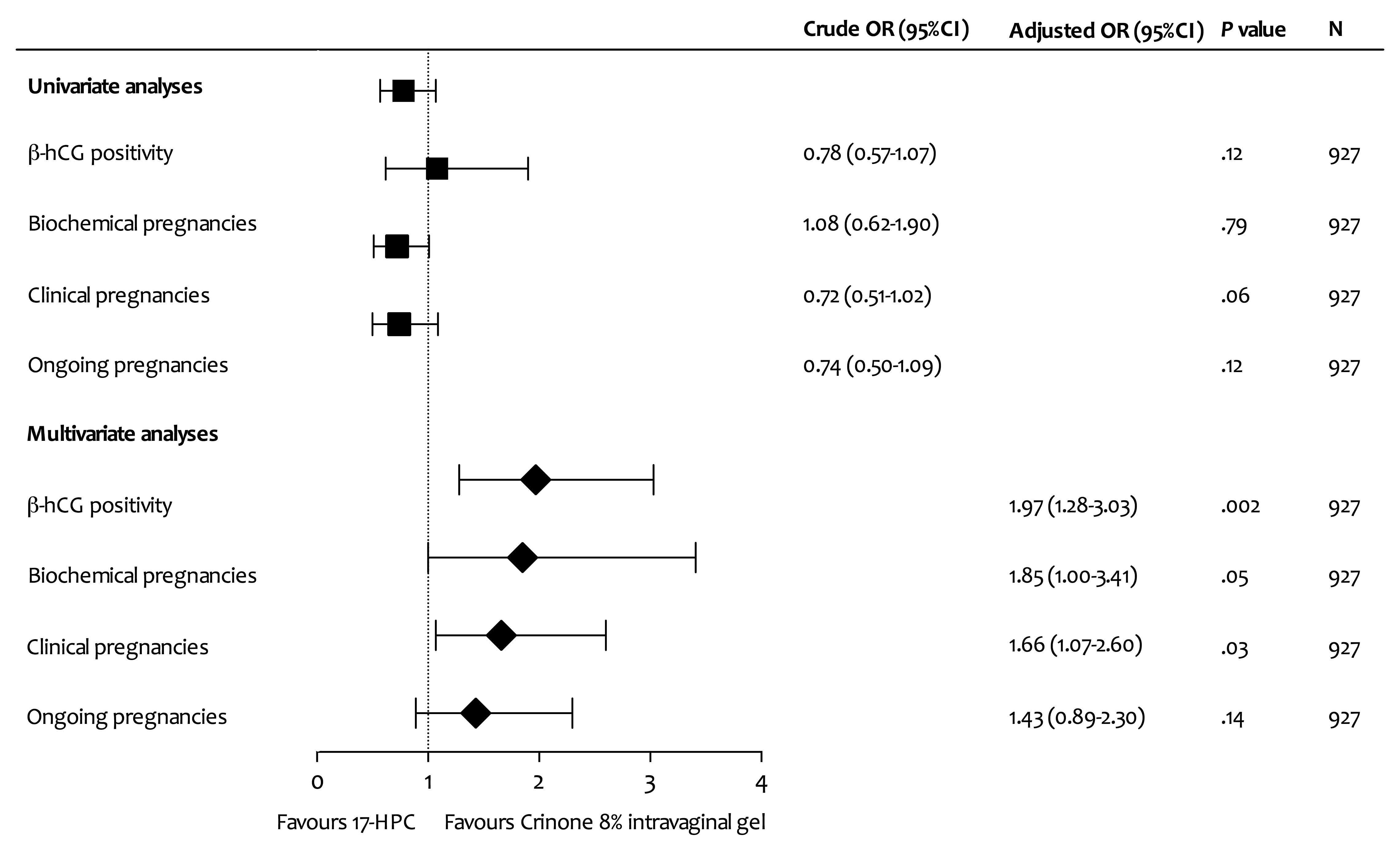
There were no statistically significant differences
between the Crinone and 17-HPC groups with respect to total β-hCG
positivity, biochemical pregnancy, clinical pregnancy and on-going
pregnancy rates in univariate analyses (Fig. 1). In multivariate regression
analysis, the use of Crinone was associated with higher β-hCG
positivity and clinical pregnancy rates (OR, 1.97; 95% CI,
1.28–3.03; P=0.002; and OR, 1.66; 95% CI, 1.07–2.60; P=0.03,
respectively). There was a trend toward higher biochemical
pregnancy rates favouring Crinone 8% IV gel (OR, 1.85; 95% CI,
1.00–3.41; P=0.05). However, no statistically significant
difference was found in terms of on-going pregnancy rates between
the groups (OR, 1.43; 95% CI, 0.89–2.30; P=0.14; Fig. 1).
Discussion
This analysis shows that Crinone vaginal gel is
associated with comparable pregnancy outcomes to IM progesterone,
17-HPC. To the best of our knowledge, this is the largest study
comparing these two forms of luteal phase progesterone support in
IVF.
Progesterones may be administered via the oral,
vaginal, or IM routes for LPS following IVF-ET. Despite it being an
easy-to-use form, oral progesterone support provides lower
implantation rates than other forms of progesterone (13,14).
It has unpleasant side-effects, including nausea, fluid retention,
sedation, drowsiness and other hypnotic effects as a result of
metabolites generated by the first-pass effect (15,16).
At present, oral progesterone is not a preferred method for LPS
following IVF-ET. IM progesterone in oil has higher rates of
clinical and on-going pregnancies compared with placebo or oral
forms of progesterone (17–19).
However, the IM progesterone-in-oil form has also various
side-effects, including serious inflammatory reactions, sterile
abscesses and significant patient discomfort. These side-effects
may last for long periods of time, even after the discontinuation
of the drug (20).
17-HPC, a slow-release, long-acting derivative of
progesterone, may hypothetically be regarded as a valid alternative
to the IM progesterone-in-oil form with an advantage of reduced
patient discomfort. It has been found to be comparable to the IM
progesterone-in-oil form with respect to clinical or on-going
pregnancy rates in a prospective randomized study (20). However, evidence regarding the
equivalent efficacy between the two formulations is limited.
The most commonly used vaginal regimens include 600
mg progesterone (200 mg three times a day) as an oil-in-capsule
formulation and daily 90 mg progesterone in a
polycarbophil-containing bioadhesive gel form (Crinone 8%). These
formulations have similar pregnancy outcomes and minor side-effects
in LPS (21–23).
Currently, standard progesterone administration
during LPS is provided by either the vaginal (as an oil-in-capsule
or bioadhesive gel form) or IM route (50 mg a day). Previously, a
number of studies sought to evaluate the optimal route of
progesterone administration during LPS (24–27).
Based on a meta-analysis of nine prospective randomized controlled
trials that included a total of 1,620 individuals, vaginal and IM
in-oil forms of progesterone applied during LPS are comparable in
terms of clinical pregnancy rate (34.2 vs. 36.3%; OR, 0.91; 95% CI,
0.74–1.13) and on-going pregnancy rate (25.3 vs. 26.5%; OR, 0.94;
95% CI, 0.71–1.26) (12). Overall,
this meta-analysis and most of the prospective trials included
support the results of univariate analysis in the current study.
The only prospective study comparing 17-HPC with Crinone
demonstrated superior efficacy with 17-HPC (28). Discrepancies between the results of
our study and previous studies may be attributed to the method of
dealing with confounding factors. Differences in randomization
strategies used in prospective studies and multivariate regression
modelling, if any, in retrospective analyses are the main cause of
heterogeneity in the results of various studies.
We conclude that LPS with IV progesterone gel in
comparison with IM 17-HPC appears to be associated with better
clinical pregnancy rates in IVF-ET cycles. However, this benefit is
clinically irrelevant in terms of on-going pregnancy outcomes.
References
|
1.
|
Practice Committee of American Society for
Reproductive Medicine in collaboration with Society for
Reproductive Endocrinology and Infertility: Progesterone
supplementation during the luteal phase and in early pregnancy in
the treatment of infertility: an educational bulletin. Fertil
Steril. 90(Suppl): S150–S153. 2008. View Article : Google Scholar
|
|
2.
|
Fatemi HM, Popovic-Todorovic B,
Papanikolaou E, Donoso P and Devroey P: An update of luteal phase
support in stimulated IVF cycles. Hum Reprod Update. 13:581–590.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Penzias AS: Luteal phase support. Fertil
Steril. 77:318–323. 2002. View Article : Google Scholar
|
|
4.
|
Smitz J, Erard P, Camus M, et al:
Pituitary gonadotrophin secretory capacity during the luteal phase
in superovulation using GnRH-agonists and HMG in a desensitization
or flare-up protocol. Hum Reprod. 7:1225–1229. 1992.PubMed/NCBI
|
|
5.
|
Albano C, Grimbizis G, Smitz J, et al: The
luteal phase of nonsupplemented cycles after ovarian superovulation
with human menopausal gonadotropin and the gonadotropin-releasing
hormone antagonist Cetrorelix. Fertil Steril. 70:357–359. 1998.
View Article : Google Scholar
|
|
6.
|
Daya S and Gunby JL: Luteal phase support
in assisted reproduction cycles. Cochrane Database Syst Rev.
3:CD0048302004.
|
|
7.
|
Levine H: Luteal support in IVF using the
novel vaginal progesterone gel Crinone 8%: results of an open-label
trial in 1184 women from 16 US centers. Fertil Steril. 74:836–837.
2000.PubMed/NCBI
|
|
8.
|
Penzias AS and Alper MM: Luteal support
with vaginal micronized progesterone gel in assisted reproduction.
Reprod Biomed Online. 6:287–295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Miles RA, Paulson RJ, Lobo RA, Press MF,
Dahmoush L and Sauer MV: Pharmacokinetics and endometrial tissue
levels of progesterone after administration by intramuscular and
vaginal routes: a comparative study. Fertil Steril. 62:485–490.
1994.
|
|
10.
|
Zarutskie PW and Phillips JA: A
meta-analysis of the route of administration of luteal phase
support in assisted reproductive technology: vaginal versus
intramuscular progesterone. Fertil Steril. 92:163–169. 2009.
View Article : Google Scholar
|
|
11.
|
ICH Topic E 6 (R1) Guideline for Good
Clinical Practice. ICH Harmonised Tripartite Guideline. Version of
July 1996 including post step errata of July 2002. European
Medicines Agency; London, UK: 2002, Available from: http://www.emea.europa.eu/pdfs/human/ich/013595en.pdfuri.
Accessed April 3, 2013.
|
|
12.
|
Arslan M, Bocca S, Mirkin S, Barroso G,
Stadtmauer L and Oehninger S: Controlled ovarian hyperstimulation
protocols for in vitro fertilization: two decades of experience
after the birth of Elizabeth Carr. Fertil Steril. 84:555–569. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Friedler S, Raziel A, Schachter M,
Strassburger D, Bukovsky I and Ron-El R: Luteal support with
micronized progesterone following in-vitro fertilization using a
down-regulation protocol with gonadotropin-releasing hormone
agonist: a comparative study between vaginal and oral
administration. Hum Reprod. 14:1944–1948. 1999. View Article : Google Scholar
|
|
14.
|
Pouly JL, Bassil S, Frydman R, et al:
Luteal support after in-vitro fertilization. Crinone 8%, a
sustained release vaginal progesterone gel versus Utrogestan, an
oral micronized progesterone. Hum Reprod. 11:2085–2089. 1996.
|
|
15.
|
Arafat ES, Hargrove JT, Maxson WS,
Desiderio DM, Wentz AC and Andersen RN: Sedative and hypnotic
effects of oral administration of micronized progesterone may be
mediated through its metabolites. Am J Obstet Gynecol.
159:1203–1209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Maxon W and Hargrove JT: Bioavailability
of oral micronized progesterone. Fertil Steril. 44:622–626.
1985.
|
|
17.
|
Artini PG, Volpe A, Angioni S, Galassi MC,
Battaglia C and Genazzani AR: A comparative randomized study of
three different progesterone support of the luteal phase following
IVF/ET programme. J Endocrinol Invest. 18:51–56. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Abate A, Perino M, Abate FG, Brigandì A,
Costabile L and Manti F: Intramuscular versus vaginal
administration of progesterone for luteal phase support after in
vitro fertilization and embryo transfer: a comparative study. Clin
Exp Obstet Gynecol. 26:203–206. 1999.PubMed/NCBI
|
|
19.
|
Abate A, Brigandi A, Abate FG, Manti F,
Unfer V and Perino M: Luteal phase support with 17
alpha-hydroxyprogesterone versus unsupported cycles in in vitro
fertilization: a comparative randomized study. Gynecol Obstet
Invest. 48:78–80. 1999. View Article : Google Scholar
|
|
20.
|
Costabile L, Gerli S, Manna C, Rossetti D,
Di Renzo GC and Unfer V: A prospective randomized study comparing
intramuscular progesterone and 17α-hydroxyprogesterone caproate in
patients undergoing in vitro fertilization-embryo transfer cycles.
Fertil Steril. 76:394–396. 2001.
|
|
21.
|
Ludwig M, Schwartz P, Babahan B, et al:
Luteal phase support using either Crinone 8% or Utrogest: results
of a prospective randomized study. Eur J Obstet Gynecol Reprod
Biol. 103:48–52. 2002.
|
|
22.
|
Ng EH, Miao B, Cheung W and Ho PC: A
randomized comparison of side effects and patient inconvenience of
two vaginal progesterone formulations used for luteal support in in
vitro fertilization cycles. Eur J Obstet Gynecol Reprod Biol.
111:50–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kimzey LM, Gumowski J, Merriam GR, Grimes
GJ Jr and Nelson LM: Absorption of micronized progesterone from a
nonqualifying vaginal cream. Fertil Steril. 56:995–996.
1991.PubMed/NCBI
|
|
24.
|
Propst AM, Hill JA, Ginsburg ES, Hurwitz
S, Politch J and Yanushpolsky EH: A randomized study comparing
Crinone 8% and intramuscular progesterone supplementation in in
vitro fertilization-embryo transfer cycles. Fertil Steril.
76:1144–1149. 2001.
|
|
25.
|
Perino M, Brigandi FG, Abate FG, Costabile
L, Balzano E and Abate A: Intramuscular versus vaginal progesterone
in assisted reproduction: a comparative study. Clin Exp Obstet
Gynecol. 24:228–231. 1997.PubMed/NCBI
|
|
26.
|
Smitz J, Devroey P, Faguer B, Bourgain C,
Camus M and Van Steirteghem AC: A prospective randomized comparison
of intramuscular or intravaginal natural progesterone as a luteal
phase and early pregnancy supplement. Hum Reprod. 7:168–175.
1992.
|
|
27.
|
Damario MA, Goudas VT, Session DR, Hamit
DG and Dumesic DA: Crinone 8% vaginal progesterone results in lower
implantation efficiency after IVF-ET. Fertil Steril. 72:830–836.
1999.
|
|
28.
|
Unfer V, Casini ML, Costabile L, Gerli S,
Baldini D and Di Renzo GC: 17α-hydroxyprogesterone caproate versus
intravaginal progesterone in IVF-embryo transfer cycles: a
prospective randomized study. Reprod Biomed Online. 9:17–21.
2004.
|