Introduction
Over the past decade, the treatment of acute lower
limb ischemia (ALLI) has become more complicated, largely due to
the decline in the number of patients presenting with embolism
owing to rheumatic vascular disease and atrial fibrillation and,
conversely, a sharp increase in elderly people with advanced
atherosclerosis presenting with thrombosis and a far more complex
disease pattern. Patients presenting with ALLI require persistently
high levels of medical care, yet show poor clinical outcomes with
high amputation and mortality rates, despite improvements having
been made in surgical techniques and perioperative patient care
(1). Prostaglandin E1
(PGE1) has been widely used in the treatment of
peripheral vascular disease (2)
due to its various pharmacological activities, including potent
vasodilation, inhibition of leukocyte adhesion and platelet
aggregation, and anti-inflammatory activity (3). However, Brass et al identified
that PGE1 failed to improve the outcome of lower limb
ischemia (4). Gabriel et al
reported that PGE1 worsens already impaired cellular
oxygen supply (5). Incorporating
PGE1 into lipid microspheres, as a lipid emulsion of
PGE1 (lipo-PGE1), provides a long duration of
action, few side-effects (2) and
greater variety of pharmacological effects compared with free
PGE1(6). Surgical
methods of treatment are considered to play a critical role in
improving the clinical outcomes of patients with ALLI (7). Hybrid procedures have been
demonstrated to provide an improved solution for the treatment of
severe peripheral vascular disease (8,9). The
present study was designed to assess the therapeutic effects of
lipo-PGE1 used in patients with ALLI as an adjuvant to
hybrid procedures.
Patients and methods
Patients
This study was performed according to the
Declaration of Helsinki and the protocol was approved by the ethics
committee of Zhengzhou University (Zhengzhou, China). The patients
were considered for enrolment in the study if they presented with
acute onset (<14 days) of ischemic symptoms of the legs and were
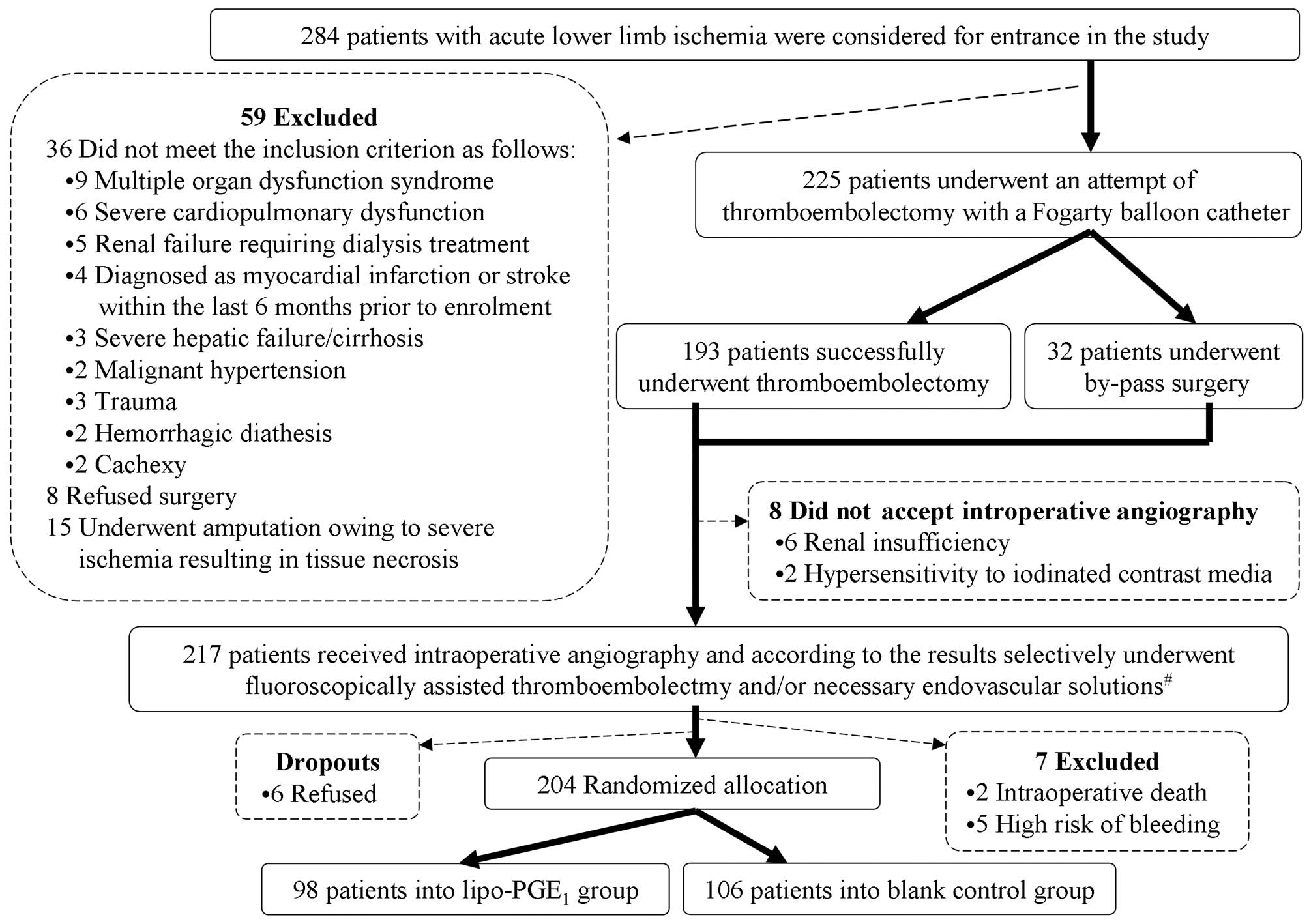
to be treated with hybrid procedures. There were 204 patients
(males, 115; females, 89) who met the criteria (Fig. 1).
All patients received a hypodermic injection of
2,500 units low-molecular-weight heparin calcium twice a day for
one week after surgery and also received relevant treatment for
concomitant diseases, including hypotensor drugs, hypoglycemic
agents and lipid-lowering medicines. Commonly used drugs for
concomitant clinical conditions were as follows: i) cardiovascular
drugs (benazepril, perindopril, valsartan, telmisartan, losartan,
extended-release nifedipine, amlodipine besylate, levamlodipine
besylate, extended-release felodipine, isosorbide mononitrate,
nitroglycerin, indapamide, chlorthalidone and metoprolol tartrate);
ii) hypoglycemic drugs (insulin, glimepiride, gliclazide,
sitagliptin, metformin and acarbose); iii) statins (atorvastatin,
simvastatin and rosuvastatin); and iv) antineoplastic drugs
(carboplatin, rituximab, vincristine, floxuridine and etoposide).
The dosage and category of individual drugs was adjusted according
to the suggestions of the consultants. Patients were instructed to
take 2.5 mg/day warfarin from the third day after surgery; thus the
overlapping time between low-molecular-weight heparin calcium
administration and warfarin administration was 3–4 days. The blood
coagulation function was detected every three days after having
taken warfarin for three days, and the dosage of warfarin was
adjusted according to the international normalized ratio
(INR)results, until the INR indicated a therapeutic effect
(2–3). When patients took warfarin, aspirin
and thienopyridines were not administered.
Interventions
The 204 patients were randomly divided into a
lipo-PGE1 group and blank control group (Table I). Following surgery, the
lipo-PGE1 group received a daily 6 h intravenous
infusion of lipo-PGE1 (Kaishi, Beijing Tide
Pharmaceutical Co., Ltd., Beijing, China) at a dose of 20
μg/day for 12–14 consecutive days. Throughout the study,
treatment with other vasoactive drugs, including buflomedil,
fasudil and iloprost were not permitted. All patients were followed
up for 6 months for occurrence of major clinical events. During the
study period, the combined incidence of perioperative (30 days)
mortality (POM) and major adverse limb events (MALE) was defined as
the primary endpoint. The secondary efficacy endpoint was defined
as the occurrence of a major adverse cardiovascular event (MACE),
which consisted of myocardial infarction, stroke or mortality from
any cause (10). During the
follow-up period, the concentration of hemoglobin A1C of diabetic
patients was tested every three months if the patient had stable
glycemic control; otherwise it was tested once a month. The target
level for low-density lipoprotein (LDL)-cholesterol was <100
mg/dl for the majority of patients and was expected to be <70
mg/dl or a reduction of 30–40% from the baseline for patients
having diabetes and/or cardiovascular disease. The level of
LDL-cholesterol of patients taking statins was measured once a
month.
 | Table IClinical baseline characteristics of
the two groups. |
Table I
Clinical baseline characteristics of
the two groups.
| Parameter | Control (n=106) | Lipo-PGE1
(n=98) |
|---|
| Age (mean ± SD,
years) | 64.6±9.4 | 66.1±8.9 |
| Gender
(male/female) | 60/46 | 55/43 |
| Risk factors | | |
| Hypertension | 69.8 | 71.4 |
| Coronary artery
disease | 26.4 | 24.5 |
| Diabetes mellitus
2 | 35.0 | 37.8 |
| Hemoglobin A1C
(mean ± SD) | 7.7±1.0 | 7.9±1.1 |
| Re-evaluation
(mean ± SD) | 6.7±0.5 | 6.6±0.6 |
| Hyperlipidemia | 58.5 | 63.3 |
| LDL-cholesterol
(mean ± SD, mg/dl) | 126±20 | 123±24 |
| Re-evaluation
(mean ± SD, mg/dl) | 85±18 | 89±23 |
| Cerebrovascular
disease | 25.5 | 23.5 |
| Atrial
fibrillation/arrhythmias | 51.9 | 53.1 |
| Renal disease | 14.2 | 16.3 |
| Chronic peripheral
arterial disease | 21.7 | 18.4 |
| Previous
revascularization lower limb | 17.9 | 15.3 |
| Carcinoma | 16.0 | 14.3 |
| Smoking (>15
cigarettes/day for >15 years) | 39.6 | 41.8 |
| Still smoking | 32.1 | 31.6 |
| Vessels involved | | |
| Common/external
iliac artery | 20.8 | 21.4 |
| Common/superficial
femoral artery | 71.7 | 74.5 |
|
Popliteal/infrapopliteal artery | 7.5 | 4.1 |
| Clinical category of
acute ischemia | | |
| Class I-IIa | 33.0 | 35.7 |
| Class IIb-III | 67.0 | 64.3 |
| Thromboembolectomy
(success cases/n) | 84.9 | 86.7 |
| Bypass | 15.1 | 13.3 |
| Adjunctive
intervention (%) | | |
| Fluoroscopically
assisted thromboembolectomy | 20.8 | 21.4 |
| Balloon
angioplasty | 13.2 | 11.2 |
| Stenting | 9.4 | 8.2 |
|
Endarterectomy | 11.3 | 10.2 |
| Hospital stay (mean
± SD, days) | 15.2±3.4 | 14.8±3.1 |
Statistical analysis
The unpaired t-test and Chi-square test or Fisher’s
exact test were employed for comparison of the baseline results of
the two groups, as appropriate. The clinical outcomes (POM,
combined POM and MALE and all clinical events) of the two groups
were evaluated with the log-rank test. Multivariable analyses were
performed with a Cox proportional hazard regression model to
compare the outcomes for experimental treatment, class of ischemia
according to SVS-ISCVS-TASC criteria (1,11)
(class I-IIa vs. IIb-III) and surgical method (thromboembolectomy
vs. bypass). Proportional hazard assumptions were tested for all
the covariates. All statistical tests were two-sided and a value of
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS software
(version 12.0, SPSS, Inc., Chicago, IL, USA) on the basis of the
intention-to-treat analysis.
Results
In the control and lipo-PGE1 group, 8.5
and 6.1% of patients were lost to follow-up, respectively. Due to
the inconvenience of measuring blood coagulation or poor
compliance, 42.6% patients received aspirin and clopidogrel
(antiplatelet therapy) instead of warfarin during the follow-up
period, and only 8 patients (5 patients in the control group and 3
patients in the lipo-PGE1 group) stopped taking oral
anticoagulants and anti-platelet drugs 1–2 months after being
discharged from hospital. The treatment for concomitant diseases
were similar for the two groups (statins, 71.7 and 74.5%;
anti-hypertensive drugs, 56.6 and 58.2%; warfarin, 45.3 and 46.9%;
and antiplatelet therapies, 41.5 and 43.9% in the control and
lipo-PGE1 groups, respectively). The
lipo-PGE1-treated and control groups did not differ with
respect to baseline characteristics (Table I). The comparative clinical
outcomes of the two groups are shown in Table II. The combined incidence of POM
and MALE (primary end-point) was significantly lower in the
patients receiving lipo-PGE1 than in the controls. The
Kaplan-Meier curves for freedom from POM and any MALE are shown in
Fig. 2. The overall incidence of
all clinical events (POM+MALE+MACE) was significantly reduced in
the lipo-PGE1 patients compared with the control group.
Multivariable analysis revealed that the occurrence of POM or MALE
was less frequent for patients with ischemia of class I-IIa
(compared with IIb-III) and this was more related to bypass
procedures (compared with thromboembolectomy procedures; Table III). No serious adverse reactions
occurred following lipo-PGE1 administration.
 | Table IIOutcomes at the 6-month
follow-up. |
Table II
Outcomes at the 6-month
follow-up.
| Event | Control |
Lipo-PGE1 |
|---|
| POM | 8 (7.5) | 3 (3.1) |
| Major endpoints
(POM+MALE) | 14 (13.2) | 5 (5.1)a |
| MALE | 6 (5.7) | 2 (2.0) |
| POM+MALE+MACE | 22 (20.8) | 8 (8.2)a |
| Secondary endpoints
(MACE) | 8 (7.5) | 3 (3.1) |
 | Table IIICox proportional hazard regression
model analysis for the primary study endpoint (combined POM and
MALE). |
Table III
Cox proportional hazard regression
model analysis for the primary study endpoint (combined POM and
MALE).
| Variable | Effect | HR | 95% CI | P-value |
|---|
| Treatment
group | Control vs.
lipo-PGE1 | 2.15 | 1.01–4.57 | <0.05 |
| Class of
ischemia | ≥IIb vs. ≤IIa | 3.61 | 1.37–9.51 | <0.01 |
| Surgical
method | Bypass vs. TE | 3.33 | 1.33–8.31 | 0.01 |
Discussion
Previous studies have reported a 30-day amputation
rate of 5–12% and mortality risk of 9.9–42% for patients with ALLI
(12–15). The risk factors that may lead to
poor prognosis include the absorption of metabolic toxins
associated with acute ischemia, ischemia-reperfusion injury and the
incomplete restoration of perfusion (residual thrombi in distal
vessels not reached by the Fogarty catheter thromboembolectomy,
propagation of residual thrombus or presence of underlying
steno-occlusive lesions). Pemberton et al demonstrated that,
following thromboembolectomy, 24% patients required an immediate
further procedure and 15% patients underwent further limb salvage
surgery within 30 days (15). In
the present study, the combined incidence of POM and MALE in the
two groups (13.2 and 5.1% in the lipo-PGE1 and control
groups, respectively) was far lower than previous reports, which
may demonstrate the benefit of hybrid procedures. Intraoperative
completion angiography allowed the surgeons to discern the defects
of vessels following surgical revascularization and guided them to
repair lesions by means of endovascular techniques, which may
improve the primary patency (16).
Bosma and Jörning identified using angiography that following
thromboembolectomy, 30% of patients presented incomplete clearance
of the arterial tree, resulting in a requirement for further
embolectomy, as well as a reduced rate of amputation (17). In another study, the adoption of
routine intraoperative angiography following thromboembolectomy
confirmed that 53.4% patients required intraoperative
re-interventions for residual lesions with the result that 2-year
primary patency rates were improved (18). Through intraoperative angiography,
we identified a residual thrombus in 30.4% of patients, and 35.3%
patients required additional solutions (repeated thromboembolectomy
and/or endovascular interventions). These therapeutic strategies
are reliable methods for ensuring the patency of the whole arterial
tree.
In the present study, lipo-PGE1 was used
in emergency conditions and not in patients with chronic diseases
or as an adjuvant therapy to organ transplantation or elective
surgery, as in previously reported studies (19–21).
The patients who received intravenous lipo-PGE1
presented higher MALE-free and survival rates than the controls.
This may be due to the various pharmacological effects of
lipo-PGE1, which is known to interfere with inflammatory
responses and reduce systemic damage following ischemia and
reperfusion. The actions of lipo-PGE1 include
anti-inflammatory activity, reduction of free radicals and cytokine
production, protection against ischemia reperfusion injury,
improvement of endothelial function, reduced expression of
intercellular adhesion molecules, vasodilation, antiplatelet
activity, antithrombotic activity and thrombolytic activation,
improvement of the microcirculation and amelioration of the
rheological property of the blood (19–24).
The pharmacological effects may be conducive to dissolving residual
thrombi and thrombi in runoff and branch vessels inaccessible to
the balloon catheter, as well as increasing blood flow and reducing
the incidence of POM and MALE. Lipo-PGE1 also has
certain visceral protective effects, including reduction of hepatic
injury and myocardial infarction size and improvement of lung
function (19,24,25),
which may help to reduce the occurrence of MACE.
Lipo-PGE1 as an adjuvant to surgery greatly reduces the
incidence of adverse clinical events.
Thromboembolectomy procedures result in a better
prognosis than bypass procedures; however, the detailed mechanism
is not yet known. A possible explanation may be that bypass
procedures as a secondary choice are adopted when the Fogarty
catheter is not able to traverse the occluded segment, which may
indicate serious local vessel lesions or that a longer vessel is
involved. The other possible reasons include anastomotic stricture,
changes of blood flow direction resulting in shear stress and
intimal hyperplasia. Although the majority of patients (>60%
patients in the two groups) presented acute ischemia classified as
grade IIb-III or even irreversible (54.9%), the high survival and
MALE-free rates support a more aggressive treatment strategy in
patients with severe peripheral ischemia; a similar conclusion was
made by Mohler et al(26).
The current study also presents several limitations,
including the necessity of a longer-term follow-up survey and the
absence of a placebo group due to the clinical gravity of the
patients. Further studies are required to confirm our data and
reveal the mechanism by which lipo-PGE1 reduces the
incidence of adverse clinical events.
Hybrid procedures may improve the clinical outcomes
of patients with ALLI by permitting the identification of inflow,
outflow, conduit and anastomotic defects intraoperatively and
remedying the underlying lesions of vessels. Lipo-PGE1
as an adjuvant to surgical revascularization in ALLI significantly
lowered the combined incidence of POM and MALE. Further data and
studies are required to support the results.
References
|
1.
|
Dormandy JA and Rutherford RB: Management
of peripheral arterial disease (PAD). TASC Working Group
TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg.
31:S1–S296. 2000.PubMed/NCBI
|
|
2.
|
Murota H, Kotobuki Y, Umegaki N, Tani M
and Katayama I: New aspect of anti-inflammatory action of
lipo-prostaglandin E1 in the management of collagen
diseases-related skin ulcer. Rheumatol Int. 28:1127–1135. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Gao Y, Xu P, Chen L and Li Y:
Prostaglandin E1 encapsulated into lipid nanoparticles improves its
anti-inflammatory effect with low side-effect. Int J Pharm.
387:263–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Brass EP, Anthony R, Dormandy J, et al:
Parenteral therapy with lipo-ecraprost, a lipid-based formulation
of a PGE1 analog, does not alter six-month outcomes in patients
with critical leg ischemia. J Vasc Surg. 43:752–759. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Gabriel A, Werba A, Mares P, Grubhofer G,
Hrska F, Griesmacher A, et al: Influence of prostaglandin E1 on
tissue ischemia during surgical repair of the abdominal aorta. J
Cardiothorac Vasc Anesth. 2:201–206. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Willerson JT, Yao SK, McNatt J, Cui K,
Anderson HV, Swensen C, et al: Liposome-bound prostaglandin E1
often prevents cyclic flow variations in stenosed and
endothelium-injured canine coronary arteries. Circulation.
89:1786–1791. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
de Donato G, Gussoni G, de Donato G, Cao
P, Setacci C, Pratesi C, et al: Acute limb ischemia in elderly
patients: can iloprost be useful as an adjuvant to surgery? Results
from the ILAILL study Eur J Vasc Endovasc Surg. 34:194–198.
2007.PubMed/NCBI
|
|
8.
|
Matsagkas M, Kouvelos G, Arnaoutoglou E,
Papa N, Labropoulos N and Tassiopoulos A: Hybrid procedures for
patients with critical limb ischemia and severe common femoral
artery atherosclerosis. Ann Vasc Surg. 25:1063–1069. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Schrijver AM, Moll FL and De Vries JP:
Hybrid procedures for peripheral obstructive disease. J Cardiovasc
Surg (Torino). 51:833–843. 2010.PubMed/NCBI
|
|
10.
|
Conte MS, Geraghty PJ, Bradbury AW,
Hevelone ND, Lipsitz SR, Moneta GL, et al: Suggested objective
performance goals and clinical trial design for evaluating
catheter-based treatment of critical limb ischemia. J Vasc Surg.
50:1462–1473. el–e3. 2009. View Article : Google Scholar
|
|
11.
|
Katzen BT: Clinical diagnosis and
prognosis of acute limb ischemia. Rev Cardiovasc Med. 3(Suppl 2):
S2–S6. 2002.PubMed/NCBI
|
|
12.
|
Aune S and Trippestad A: Operative
mortality and long-term survival of patients operated on for acute
lower limb ischaemia. Eur J Vasc Endovasc Surg. 15:143–146. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Braithwaite BD, Davies B, Birch PA,
Heather BP and Earnshaw JJ: Management of acute leg ischaemia in
the elderly. Br J Surg. 85:217–220. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nypaver TJ, Whyte BR, Endean ED, Schwarcz
TH and Hyde GL: Nontraumatic lower-extremity acute arterial
ischemia. Am J Surg. 176:147–152. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pemberton M, Varty K, Nydahl S and Bell
PR: The surgical management of acute limb ischaemia due to native
vessel occlusion. Eur J Vasc Endovasc Surg. 17:72–76. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lipsitz EC, Veith FJ and Wain RA: Digital
fluoroscopy as a valuable adjunct to open vascular operations.
Semin Vasc Surg. 16:280–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bosma HW and Jörning PJ: Intra-operative
arteriography in arterial embolectomy. Eur J Vasc Surg. 4:469–472.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zaraca F, Stringari C, Ebner JA and Ebner
H: Routine versus selective use of intraoperative angiography
during thromboembolectomy for acute lower limb ischemia: analysis
of outcomes. Ann Vasc Surg. 24:621–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gianetti J, De Caterina M, De Cristofaro
T, Ungaro B, Guercio RD and De Caterina R: Intravenous
prostaglandin E1 reduces soluble vascular cell adhesion molecule-1
in peripheral arterial obstructive disease. Am Heart J.
142:733–739. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Shin M, Song SH, Kim JM, Kim SJ, Joh JW,
Lee SK, et al: Effectiveness of intraportal prostaglandin E1
administration after liver transplantation. Transplant Proc.
44:500–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mitate E, Sasaguri M, Oobu K, Mitsuyasu T,
Tanaka A, Kiyosue T and Nakamura S: Postoperative changes of blood
flow in free microvascular flaps transferred for reconstruction of
oral cavity: effects of intravenous infusion of prostaglandin E1.
Asian J Oral Maxillofacial Surg. 23:113–116. 2011. View Article : Google Scholar
|
|
22.
|
Sim AK, McCraw AP, Cleland ME, Aihara A,
Otomo S, Hosoda K, et al: A preclinical assessment of the effect of
lipo-PGE1 on thrombus formation and thrombus disaggregation. Adv
Drug Deliv Rev. 20:165–170. 1996. View Article : Google Scholar
|
|
23.
|
Marchesi S, Pasqualini L, Lombardini R,
Vaudo G, Lupattelli G, Pirro M, et al: Prostaglandin E1 improves
endothelial function in critical limb ischemia. J Cardiovasc
Pharmacol. 41:249–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Luo CF, Wu X, Hu X, Wu GF, Hu CH and Du
ZM: Protective effect of lipid microspheres 1 on myocardial injury
following elective percutaneous coronary intervention in patients
with angina pectoris: a pilot study. J Cardiovasc Med (Hagerstown).
12:790–794. 2011. View Article : Google Scholar
|
|
25.
|
Taylor CJ, McGaw J, Rigby AS, Threlfall D
and Karmel J: Pilot safety study of liposomal prostaglandin (PGE1)
in respiratory exacerbations in cystic fibrosis. J Cyst Fibros.
1:90–93. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Mohler E III and Giri J; ACC; AHA:
Management of peripheral arterial disease patients: comparing the
ACC/AHA and TASC-II guidelines. Curr Med Res Opin. 24:2509–2522.
2008. View Article : Google Scholar : PubMed/NCBI
|