Introduction
Thoracolumbar fracture is most common trauma in
spine surgery (1–3) and is usually a high energy trauma
caused by a traffic accident or fall. With the rapid development of
the economy and the popularization of cars, the thoracolumbar
fracture incidence rate is increasing year by year (2–4). The
aim of recovering vertebral height, maintaining vertebral stability
by internal fixation and enabling ambulation as early as possible
has become established among physicians in spine surgery. For the
conventional posterior reduction and fixation of thoracolumbar
fractures, long-term follow-up shows cases of vertebral height loss
of the affected vertebral body (5–7). In
addition, computer technology has improved rapidly in recent years.
Computer-assisted surgery (CAS) was first applied in spine surgery
in the 1990s (1) and is a novel
guidance mode of implant placement. CAS is an assistive technology
based on modern computer technology, stereo positioning techniques
and medical imaging technology that is used to guide surgeons in
precise surgical planning and surgery. The principle of a CAS
system is similar to that of a global positioning system; it
assembles a three-dimensional coordinate system of the
intraoperative anatomical structure and a three-dimensional
coordinate system of navigation images. Modern spinal surgery
navigation and positioning systems mainly use infrared technology
to identify anatomical structures of the patients and the mutual
spatial relationship of the surgical implant with the surgical
apparatus, and the computer provides virtual images of the internal
fixation in vivo in multiple directions. Therefore, CAS may
aid the surgeon in mastering the accurate positioning of the
internal fixation in vivo in real-time (8) and create a radiation-free,
multidimensional and virtual surgical environment during surgery
(9). Although there are certain
disputes, the use of CAS in spinal surgery, particularly in the
pedicle screw transplantation process, is safer, more accurate and
is accepted by an increasing number of spinal surgeons. Between
June 2005 and March 2011, surgeons at The Sixth People’s Hospital
of Shanghai (Shanghai, China) used CAS to conduct the reduction and
fixation of pedicle screws and conduct artificial vertebral bone
transplantation of 30 patients with thoracolumbar fractures via the
affected vertebral pedicle and obtained a satisfactory
efficacy.
Materials and methods
General data
In this study, there were a total of 30 cases,
including 18 males and 12 females, and their ages ranged from 21 to
57 years (mean, 35.5 years). Among them, there were 17 cases of
falling trauma, 9 cases of trauma caused by traffic accident and 4
cases of crashing traumas (high-energy injury). Of the fracture
sites, 3 cases occurred in T11, 11 cases occurred in T12, 14 cases
occurred in L1 and 2 cases occurred in L2. According to the Frankel
method (10) of neurological
dysfunction classification, 2 cases were classified as grade A, 3
cases were classified as grade B, 3 cases were classified as grade
C, 7 cases were classified as grade D and 15 cases were classified
as grade E. Fracture severity: intraspinal occupation was 5–70%
(mean, 37.5%), vertebral height compression was 40–70% (mean,
54.5%) and Cobb angle was 15.5–41.5° (mean, 29.5°). The navigation
equipment provided by Stryker Company (Kalamazoo, MI, USA) and used
during surgery included a space-positioning device, an image
workstation, a patient tracer and a surgical operation guide. This
study was conducted in accordance with the declaration of Helsinki
and with approval from the Ethics Committee of the Sixth People’s
Hospital of Shanghai, Shanghai Jiaotong University. Written
informed consent was obtained from all participants.
Preoperative preparation
For all patients, conventional X-ray photography was
conducted at the frontal and lateral positions, and MRI examination
was conducted to assess the spinal cord injury. In addition, CT
scanning was conducted for reconstruction. The CT scanning
thickness was 1 mm and scanning was continuous. CT plain scanning
of the vertebral body was 0.625–1.25 mm. CT scanning images
included the vertebral body, spinal process and transverse process.
In general, the images contained two adjacent upper and lower
vertebral bodies. The CT scanning image of a single-segment
vertebral fracture contained five vertebral bodies. CT scanning
data were stored via compact disc. Prior to surgery, CT data in the
compact disc were input into the computer navigational system for
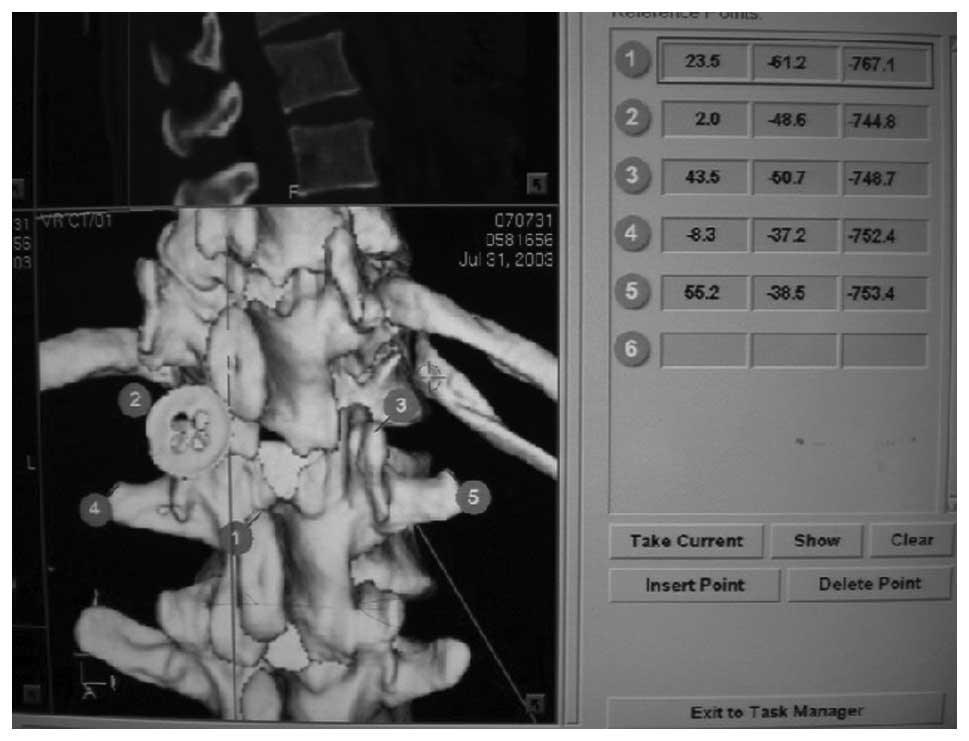
preoperative design. During registration, individual vertebral
bodies were registered separately. The reference points should not
be selected on the same plane. In our study, the superior margin of
the spinous process was a required point, with two points selected
on both sides of the spinous process. The reference points were not
on the same plane. The upper spinous process was selected and the
other four points were distributed at the two sides of the spinous
process (two points at each side). In general, articular and
transverse processes were selected as reference points. Vertebral
compression, spinal bone block occupation, fracture displacement
and deformity changes in the vertebral pedicle of the affected
vertebral body were observed, and the insertion point, track and
length of the screw channel were measured.
Surgical techniques
General anesthesia was conducted and patients were
in the prone position. The posterior median incision was cut open
to expose the affected vertebral body, articular process joint and
the transverse process at the upper and lower segments. A tracer
was installed on the spinous process where pedicle screws would be
implanted. It was necessary to ensure the tracer was fixed solidly.
The position sensor was adjusted, and the radio calibrator (a
necessary instrument for a navigation surgery; Stryker Company,
USA) was registered and calibrated. According to the five reference
points designated prior to surgery, control matching was conducted.
It was essential that the reference point on the upper spinous
process was preserved. Two reference points (one reference point
with the largest matching error at each side of the spinous
process) were deleted. For the remaining three reference points,
the error range was automatically calculated by the navigational
system. If the error range was <1.0 mm, it was acceptable
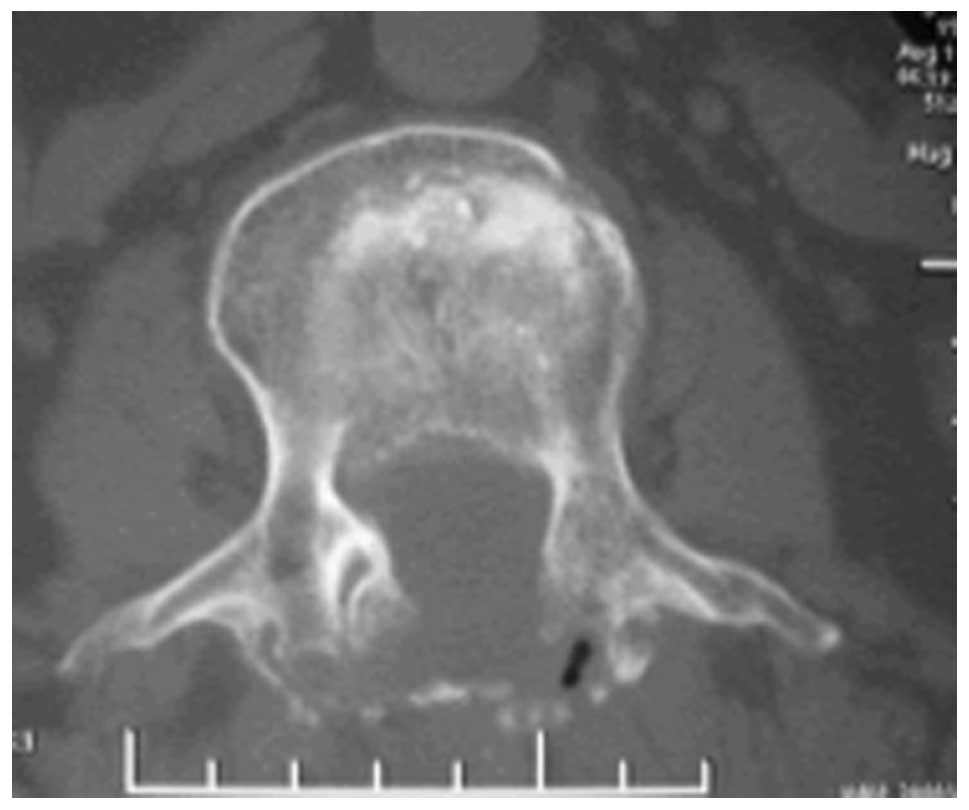
(Fig. 1) and it was feasible to
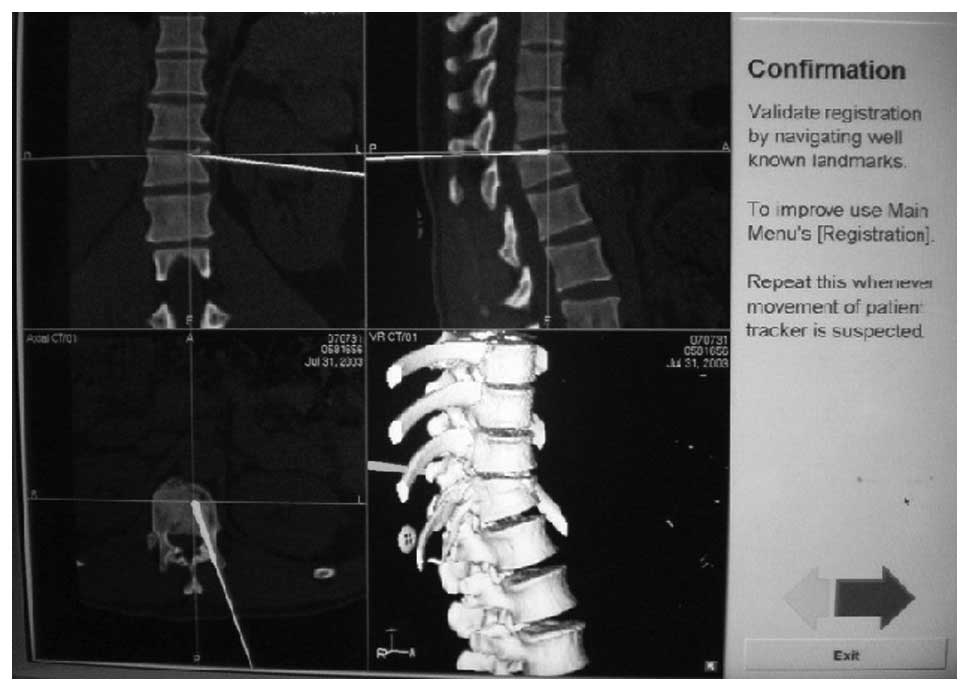
operate under the guidance of the navigation model. Each time,
intraoperative surgical tools were registered and calibrated in
order to be displayed on-screen following infrared receiver
reconnaissance (Fig. 2). Following
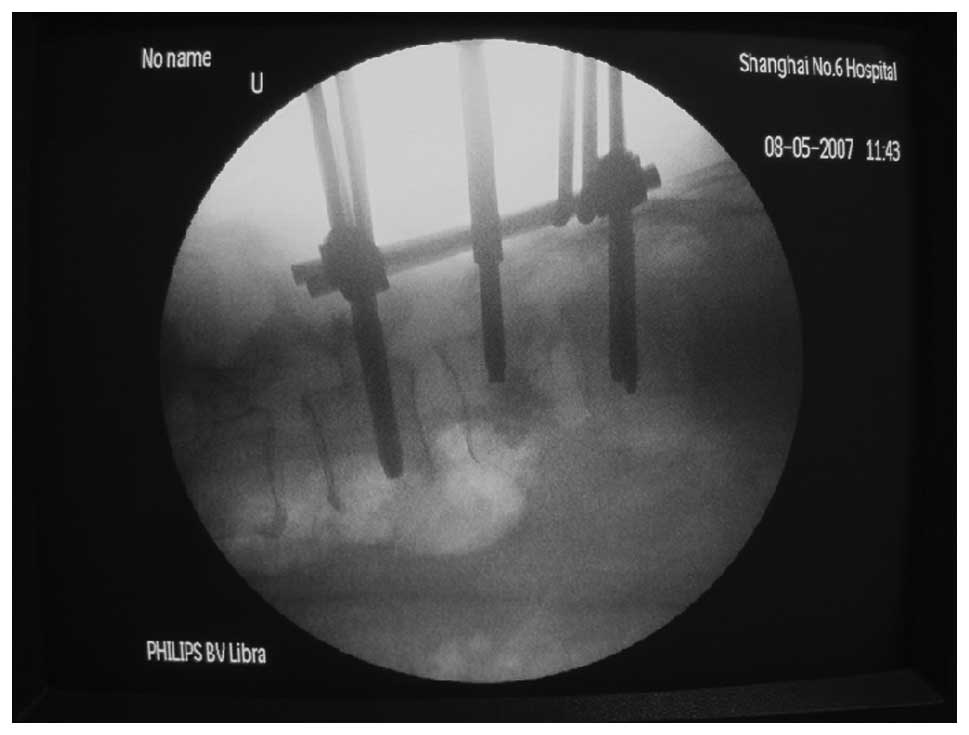
the implantation of two groups of four vertebral pedicle screws,
C-arm X-ray examination was conducted to confirm the position of
the vertebral pedicle. For the affected vertebral body, a small
hole was created at the vertebral pedicle by cutting under the
guidance of the navigation model and the bone-opening instrument
was placed into the vertebral body along the vertebral pedicle to
the empty cavity of the fracture. The screw channel was closed with
bone wax temporarily to reduce vertebral hemorrhage. If the
fracture was accompanied by neurological dysfunction, full
decompressive laminectomy and spinal nerve exploration were
conducted. Following pedicle screw reduction, C-arm X-ray
examination was conducted to confirm the reduction status of the
affected vertebral body. Following satisfactory reduction, all
screws were tightened (Fig. 3). A
bone implant funnel was inserted from one side of the affected
vertebral pedicle screw channel and 5 g particulate artificial bone
was implanted into the funnel and pressurized into the vertebral
body with the filling rod. Subsequently, the screw channel opening
was closed with bone wax. For the contralateral screw channel, 5 g
particulate artificial bone was implanted in the same manner
(Fig. 4). The wounds were closed
layer by layer. Following surgery, negative pressure drainage was
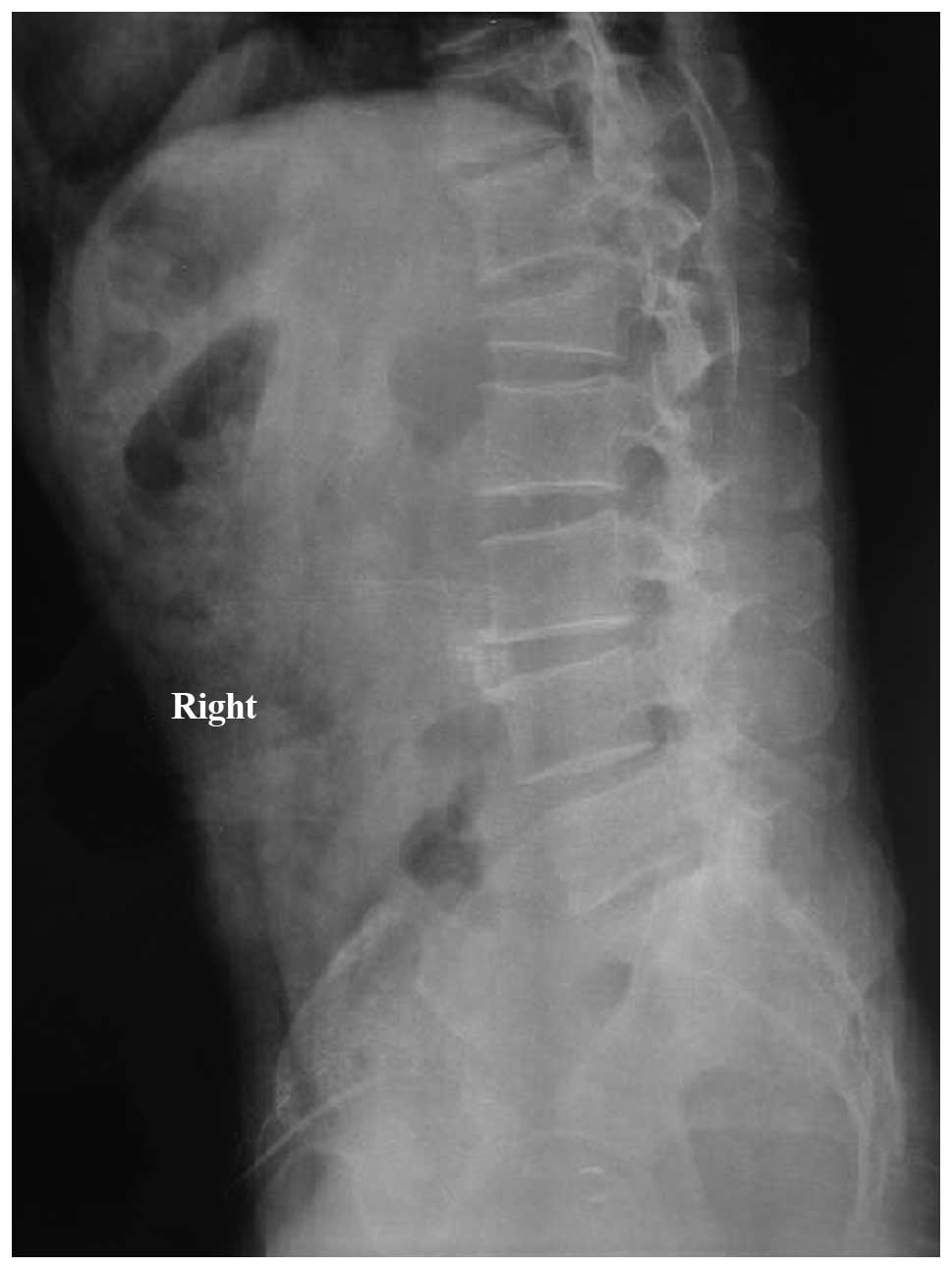
conducted for 48 h (Figs.
5–10).
Postoperative treatment
Following surgery, the patients lay in bed for 2–4
weeks, then started to ambulate under waist protection. For all
cases, postoperative plain CT scanning was conducted to observe the
accuracy of the vertebral pedicle screw implantation. According to
the possibility of the screw penetrating the vertebral pedicle and
penetration extent, the pedicle screw was classified into 4 grades.
If the pedicle screw was within the vertebral pedicle, it was
classified as grade 0; if the pedicle screw was involved in the
vertebral pedicle cortex, it was classified as grade I; if the
depth of the pedicle screw penetrating the cortex was <2 mm, it
was classified as grade II; and if the depth was >2 mm, it was
classified as grade III. In addition, the bone implantation status
of the affected vertebral body and the intravertebral fracture
block occupation and reduction status were observed.
Efficacy evaluation and observation
indices
Following surgery, X-ray re-examination was
conducted regularly to measure the vertebral height and Cobb angle.
With the affected vertebral body positioned in the center, frontal
and lateral X-ray plain films were photographed at 3, 6, 9 and 12
months after surgery and at 3 months following removal of the
internal fixation. On the lateral X-ray plain film, two straight
lines were drawn along the upper endplate of the upper adjacent
vertebral body of the affected vertebral body and the lower
endplate of the lower adjacent vertebral body, and the crossing
angle of the two lines was the Cobb angle of the affected vertebral
body.
At 12 months following the surgery, plain CT
scanning was conducted for re-examination to measure the
intraspinal occupation and artificial bone replacement status.
Intraspinal occupation and vertebral height ratio following surgery
and at the final follow-up were compared with those prior to
surgery. According to the lower back pain efficacy assessment
standard of the Japanese Orthapaedic Association (JOA) (7), JOA scores prior to surgery and at the
final follow-up were assessed. According to the visual analog score
system (VAS) evaluation standard, VAS scores of pain prior to
surgery and at the final follow-up were assessed.
Statistical analysis
SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used and measurement data, including the vertebral
height, Cobb angle and intraspinal occupation, were expressed as
the mean ± standard deviation. Repeated measures analysis of
variance was conducted for comparisons of various indicators prior
to and following surgery and at the final follow-up, and least
significant difference t-test was used for intra-group pairwise
comparison. P<0.05 was considered to indicate a statistically
significant result.
Results
Surgical duration and bleeding
volume
Surgical durations ranged from 90 to 130 min and the
mean duration was 105.2±26.3 min. The bleeding volumes ranged from
150 to 800 ml and the mean bleeding volume was 279.7±173.7 ml.
Intraoperative C-arm X-ray examination was conducted once or
twice.
Among 120 vertebral pedicle screws, 110 pieces were
classified as grade 0, 8 pieces were classified as grade I and 2
pieces were classified as grade II. Successful screw rate was 98.3%
and no piece was classified as grade III. Follow-up durations
ranged from 12 to 36 months (mean, 18 months). At 3, 6, 12 and 24
months after surgery, follow-ups were conducted. At each follow-up,
X-ray examination was conducted. Thoracolumbar fracture was more
common in young adults. At >1 year after surgery, the internal
fixation was removed in order to prevent vertebral pedicle screw
breakage and plain CT scanning was conducted. According to
postoperative Frankel classification, 2 cases were grade A, 2 cases
were grade B, 1 case was grade C, 7 cases were grade D and 18 cases
were grade E. Postoperative intraspinal occupations ranged from 0
to 20% (mean, 14.3%) and vertebral heights ranged from 80 to 100%
(mean, 91.3%). Cobb angles ranged from 1.3 to 9.1° (mean, 4.9°). At
the final follow-up, intraspinal occupations ranged from 0 to 25%
(mean, 14.3%), vertebral heights ranged from 80 to 100% (mean,
90.7%) and Cobb angles ranged from 1.6 to 8.6° (mean, 5.1°).
Following long-term follow-up, postoperative Cobb angle loss was
<1°, vertebral height loss was <2 mm and there was no screw
breakage or internal fixation loosening (Table I). Typical case images are shown in
Figs. 5–10.
 | Table I.Intraspinal occupation, vertebral
height ratio and Cobb angle of 32 patients before and after surgery
and at the final follow-up (mean ± SD, n=30). |
Table I.
Intraspinal occupation, vertebral
height ratio and Cobb angle of 32 patients before and after surgery
and at the final follow-up (mean ± SD, n=30).
| Time | Intraspinal
occupation (%) | Vertebral height
(%) | Cobb angle (°) |
|---|
| Preoperative | 37.5±32.5a | 54.5±14.5a | 29.5±12.5a |
| Postoperative | 14.3±10.7 | 91.3±9.7 | 4.9±3.6 |
| Final follow-up | 14.3±10.7 | 90.7±9.3 | 5.1±3.5 |
| F-value | 4.939 | 3.386 | 5.892 |
| P-value | 0.000 | 0.000 | 0.000 |
Surgical complications
Among the cases in the formation group (receiving
artificial bone transplantation into the injured vertebral body),
the weights of the individual artificial vertebral bone implants
ranged from 10 to 25 g and the mean weight was 18.64 g. Among them,
2 cases presented anterior vertebral body leakage and the leakage
was absorbed naturally over 3 months. No case presented intraspinal
leakage. Following surgery, no neurological complication and no
surgical complication in other vessels, nerves or organs
occurred.
JOA score results
The lower back pain efficacy assessment standard
used was that prepared by the JOA (7) which is mainly used for the evaluation
of postoperative efficacy in thoracolumbar vertebral diseases. This
standard is brief, clear and widely applied in the clinic. A normal
total score is 29 points; the higher the score, the greater the
efficacy. The preoperative mean JOA score was 11.73 (11.73±2.94)
and JOA score at the final follow-up was 27.53 (27.53±3.01). The
VAS score is mainly evaluated according to the subjective pain
sensation of patients (8). The
score range is 0–10 points and if the subjective pain sensation is
more severe, the score is higher. The mean preoperative VAS score
was 6.83 (6.83±0.91) and the mean VAS score at the final follow-up
score was 9.17 (9.17±0.27).
Discussion
Lordosis and kyphosis are different types of
postural disorders which cause a physiological curvature of the
thoracolumbar spine. Thoracic vertebrae are fixed relatively due to
rib support and lumbar vertebra are highly mobile. Such anatomical
features easily cause thoracolumbar vertebral fracture in casew of
trauma. In particular, vertebral fracture at T12-L1 is the most
common and is usually accompanied by injuries to the cauda equina
and other injuries. At present, unstable spinal cord injuries are
mostly treated by surgery (11–14).
The surgery restores the integrity and stability of the spinal
anatomical structure as far as possible in order to create
favorable conditions for recovery of neural function. Spinal
anatomical reduction and bone fusion may effectively prevent
malformation and reduce chronic disability to enable early
exercise. If the fracture is accompanied by neurological
dysfunction, decompressive laminectomy and spinal nerve exploration
are conducted according to the disease conditions. If damage to the
endorachis is visible, it is repaired as far as possible. However,
this is only to create favorable conditions for neurological
recovery and not all nerve injuries may be restored.
In cases of vertebral fracture, the lamina
terminalis is damaged, the intervertebral disc is pushed into the
vertebral body and the normal bone trabecula support system in the
vertebral body is damaged. Although the vertebral height may be
fully be restored, compressed bone trabeculae are not restorable,
which results in a vertebral body with lack of bone integrity. It
is difficult to form bone by hematoma organization and
cartilaginification mechanisms, and it is only feasible to fill the
empty defect (namely, a so-called ‘eggshell’ or ‘empty’ vertebral
body) with fibrous tissues (15–17).
On X-ray plain film, the manifestations may be normal.
Occasionally, mild depression of the affected vertebral lamina
terminalis may be visible. However, plain CT scanning shows an
empty cavity on the affected vertebral body. According to the
three-column spinal column theory by Denis (18), the vertebral body and the posterior
longitudinal ligament respectively constitute the anterior and
central columns of the spinal column. The stability and loading of
the spinal column primarily depend on the anterior and central
columns, which account for approximately 60% of the total load of
the spinal column. The spinous process and vertebral lamina
constitute the posterior column of the spinal column. Therefore,
pedicle screw fixation and lamina posterolateral bone graft fusion
during operation are not reliable for the stability maintenance of
the integral spinal column, namely that strengthening the stability
of the posterior column merely has no noticeable effect on the
stability of the anterior and central columns. Plain CT scanning
images of the fracture at the long-term follow-up show that the
empty intravertebral cavity is always present (19,20).
As vertebral stability is mainly involved in the anterior middle
spine (accounting for ∼60%), performing posterolateral fusion
during surgery is not reliable and is ineffective for the
stabilization of the anterior middle spine. Bone transplantation of
the affected vertebral body is controversial and not all vertebral
fractures require bone transplantation (21,22).
Bone transplantation of the affected vertebral body is recommended
for thoracolumbar unstable fractures, including vertebral fractures
with compression >1/3, vertebral burst fractures, fracture
fragment intrusion into the spinal canal and cases of a larger
empty cavity in the vertebral body following vertebral body
reduction and in which long-term vertebral height loss is easily
generated (23–26). The artificial bone transplanted
into the vertebral body is used as filler to strengthen and support
the affected vertebral body and thus reconstruct the stability of
the anterior middle spine. Therefore, the artificial bone may
effectively resist axial load to avoid the ‘eggshell’ effect and
effectively prevent long-term vertebral height loss to reduce
kyphosis deformity and to reduce the incidence rate of long-term
complications (27). Artificial
bone may induce bone growth and act as a bone support for bone
creeping substitution. For the affected vertebral body, spinal
stability may be fully restored only by autologous bone fusion. It
is undesirable to conduct internal fixation without bone
fusion.
Internal fixation technology has developed greatly,
and simple anterior and posterior approach surgeries have certain
advantages and shortcomings (28–30).
We applied short-segment posterior fixation reduction combined with
artificial bone transplantation via the affected vertebral pedicle
to treat thoracolumbar fractures. This combines the advantages of
the anterior and posterior approach surgeries and overcomes their
shortcomings to conduct reduction, decompression and reconstruction
of the injured spine in a one-step process. The bone implant funnel
may be directly inserted into the anterior middle spine of the
vertebral body via the affected vertebral pedicle. The filling rod
for bone transplantation is used to directly place the artificial
bone into the empty cavity and uniformly distribute it in the empty
cavity of the anterior middle spine of the vertebral body. Its
effect is to fully fill the intravertebral empty cavity following
reduction, which improves the load-bearing capacity of the affected
vertebral body and creates more reliable vertebral stability. Solid
artificial bone has no toxicity and low leakage. In instances of
leakage, solid artificial bone does not cause risks and may
naturally degrade and be absorbed. In addition, its biological
compatibility with tricalcium phosphate is good. Once the solid
artificial bone has been fully degraded by body fluid degradation
and cytophagy in vivo, it is absorbed. Following
degradation, released calcium and phosphorus are able to directly
participate in new bone mineralization or enter the calcium and
phosphorus banks for further use.
There are three methods of image acquisition using
navigational systems: CT navigation, X-ray navigation and
intraoperative real-time three-dimensional imaging (31). The advantages of the CT
navigational system are that it enables preoperative design and
planning to be conducted and may be used for intraoperative 3D
image guidance. The shortcomings of the CT navigational system
include that it is necessary to acquire images prior to surgery and
it is impossible to update images during surgery. The advantages of
the X-ray navigational system include that it is not necessary to
acquire images prior to surgery and it is possible to update images
during surgery. Shortcomings include that it is impossible to
conduct preoperative planning and the image definition is poor. In
addition, no three-dimensional image is provided for reference.
Intraoperative three-dimensional imaging combines the advantages of
the other two methods and overcomes their shortcomings. The quality
of the three-dimensional images obtained during the operation was
restricted by the adopted machine and software. They were less
clear than those obtained by the navigator before CT plain
scanning. In particular, the three-dimensional reconstruction image
is much worse. Due to the characteristics of the CT and X-ray
navigational systems, a CT navigational system was used for all
cases in this study. As the vertebral pedicle diameter of the
thoracolumbar vertebral body is relatively large and the anatomic
structure of the vertebral body has less variation, surgical
navigation is relatively simple. However, as vertebral fracture
situations differ according to the injury extent of the affected
vertebral body, particularly in cases of fracture displacement of
the affected vertebral pedicle and the loss of important anatomical
landmarks, including the articular process joint and fracture
displacement of the transverse process, the drilling difficulty and
risk associated with the affected vertebral pedicle screw are
markedly increased (32). During
surgery, it is impossible to comprehensively and accurately master
the actual situations of the affected vertebral body according to
C-arm X-ray examination. The surgery conducted at this time has a
certain lack of visibility and risk. The use of a CT navigational
system may comprehensively and accurately indicate the extent of
injury of the affected vertebral body, the involvement of the
vertebral pedicle, articular process joint and transverse process
in the fractures and the possibility of malformation. Therefore, it
is feasible to prepare a detailed preoperative plan for the
determination of screw channel length, placement site and screw
channel track, which make bone implantation more accurate,
effective and safe. During surgery, it is feasible to conduct
real-time monitoring of the screw channel under the visual guide of
a three-dimensional image and change the three-dimensional
direction of the drilling equipment and drilling depth in a timely
manner to accurately reach the bone transplantation site.
Subsequently, the surgery is accurate and effective, the risk
associated with the surgery is greatly reduced, and intraoperative
accidents are reduced. In addition, the surgery duration is
shortened.
The CT navigational system has clear advantages for
bone transplantation, but also has shortcomings. The preoperative
CT body position of a patient may be different from the
intra-operative body position to a certain extent and the fracture
situations may be slightly different. If the difference is great,
navigation accuracy will be affected, requiring the operator to
conduct a multiple-point registration of the anatomical structure
of the patient to correct the difference and increase the real-time
accuracy of this navigation mode (33,34).
If necessary, C-arm X-ray examination is conducted for
confirmation. Numerous physicians speculate that the use of
navigational aids is likely to increase surgical duration, but that
was not observed to be the case in the present study. The main
difference between surgical navigation and routine surgery is that
individual vertebral bodies must be registered individually. If the
operator is skilled in surgery, registration only takes a few
minutes and the number of C-arm X-ray examinations is reduced.
Certain scholars consider that the relative degeneration of
fracture patients is milder and the anatomical landmarks are clear
and question whether it is necessary to apply a navigation system
for a non-fractured vertebral body. We consider that since a
navigational aid is used during surgery, it is feasible to apply
the navigational system to non-fractured vertebral bodies. Since it
is possible to conduct multi-point surgery, there are no risk or
cost issues, although the registration time is increased.
In summary, the instantaneous tracking function of
the navigational system enables the surgeon to monitor the surgical
tool in real-time and accurately guide the arrival of the implant.
It allows surgery to be visualized in multi-dimensions and
real-time, making it an ideal implant guide. It is likely that
navigational systems will be accepted by an increasing number of
physicians. For physicians in spine surgery, vertebral pedicle
screw implantation is a basic skill which is mastered expertly. At
present, computer navigation technology plays only an auxiliary
role and not a leading role. Navigational systems should not be
excessively relied on, however, they should not be ignored. With
the rapid development of computer technology, navigational aids
will be used more widely. The treatment of posterior vertebral
pedicle screw system fixation plus intravertebral bone
transplantation via the vertebral pedicle for thoracolumbar
fracture aids the restoration of normal spinal physiological
structure and curvature. In addition, intravertebral bone
transplantation with particulate artificial bone via the affected
vertebral pedicle enables effective filling of the intravertebral
bone defect cavity and strengthens the affected vertebral body to
avoid postoperative vertebral height loss.
References
|
1.
|
Steinmann JC, Herkowitz HN, el-Kommos H
and Wesolowski DP: Spine pedicle fixation. Confirmation of an
image-based technique for screw placement. Spine (Phila Pa 1976).
18:1856–1861. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rampersaud YR and Lee KS: Fluoroscopic
computer-assisted pedicle screw placement through a mature fusion
mass: an assessment of 24 consecutive cases with independent
analysis of computed tomography and clinical data. Spine (Phila Pa
1976). 32:217–222. 2007. View Article : Google Scholar
|
|
3.
|
Kawahara N, Tomita K, Baba H, Kobayashi T,
Fujita T and Murakami H: Closing-opening wedge osteomy to correct
kyphotic deformity by a single posterior approach. Spine (Phila Pa
1976). 26:391–402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Parker JW, Lane GR, Karaikovic EE and
Gaines RW: Successful short-segment instrumentation and fusion for
thoracolumbar spine fracture: A consecutive 41/2-year series. Spine
(Phila Pa 1976). 25:1157–1170. 2000.PubMed/NCBI
|
|
5.
|
Stadhouder A, Buskens E, de Klerk LW, et
al: Traumatic thoracic and lumbar spine fractures: operative or
nonoperative treatment: comparison of two treatment strategies by
means of surgeon equipoise. Spine (Phila Pa 1976). 33:1006–1017.
2008. View Article : Google Scholar
|
|
6.
|
Hitchon PW, Torner J, Eichoiz KM and
Beeler SN: Comparison of anterolateral and posterior approaches in
the management of thoracolumbar burst fractures. J Neurosurg Spine.
5:117–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Siebenga J, Leferink VJ, Segers MJ, et al:
Treatment of traumatic thoracolumbar spine fracture: a multicenter
prospective treatment. Spine (Phila Pa 1976). 31:2881–2890. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lad SP, Patil CG, Lad EM, Hayden MG and
Boakye M: National trends in vertebral augmentation procedures for
the treatment of vertebral compression fractures. Surg Neurol.
71:580–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Laredo JD and Hamze B: Complications of
percutaneous vertebroplasty and their prevention. Semin Ultrasound
CT MR. 26:65–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Frankel HL, Hancock DO, Hyslop G, et al:
The value of postural reduction in the initial management of closed
injuries of the spine with paraplegia and tetraplegia. I
Paraplegia. 7:179–192. 1969. View Article : Google Scholar
|
|
11.
|
Patel AA, Vaccaro AR, Martyak GG, et al:
Neurologic deficit following percutaneous vertebral stabilization.
Spine (Phila Pa 1976). 32:1728–1734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Monticelli F, Meyer HJ and Tutsch-Bauer E:
Fatal pulmonary cement embolism following percutaneous
vertebroplasty (PVP). Forensic Sci Int. 149:35–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Majdouline Y, Aubin CE, Sangole A and
Labelle H: Computer simulation for the optimization of
instrumentation strategies in adolescent idiopathic scoliosis. Med
Biol Eng Comput. 47:1143–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Klein S, Whyne CM, Rush R and Ginsberg HJ:
CT-based patient-specific simulation software for pedicle screw
insertion. J Spinal Disord Tech. 22:502–506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
von Jako RA, Carrino JA, Yonemura KS, et
al: Electromagnetic navigation for percutaneous guide-wire
insertion: accuracy and efficiency compared to conventional
fluoroscopic guidance. Neuroimage. 47(Suppl 2): T127–T132.
2009.PubMed/NCBI
|
|
16.
|
Mizu-Uchi H, Matsuda S, Miura H, Higaki H,
Okazaki K and Iwamoto Y: Three-dimensional analysis of computed
tomography-based navigation system for total knee arthroplasty: the
accuracy of computed tomography-based navigation system. J
Arthroplasty. 24:1103–1110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Campos WK, Gasbarrini A and Boriani S:
Case report: Curetting osteoid osteoma of the spine using combined
video-assisted thoracoscopic surgery and navigation. Clin Orthop
Relat Res. 471:680–685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Denis F: The three column spine and its
significance in the classification of acute thoracolumbar spinal
injuries. Spine (Phila Pa 1976). 8:817–831. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yson SC, Sembrano JN, Sanders PC, Santos
ER, Ledonio CG and Polly DW Jr: Comparison of cranial facet joint
violation rates between open and percutaneous pedicle screw
placement using intraoperative 3-D CT (O-arm) computer navigation.
Spine (Phila Pa 1976). 38:E251–E258. 2013. View Article : Google Scholar
|
|
20.
|
Van de Kelft E, Costa F, Van der Planken D
and Schils F: A prospective multicenter registry on the accuracy of
pedicle screw placement in the thoracic, lumbar, and sacral levels
with the use of the O-arm imaging system and StealthStation
Navigation. Spine (Phila Pa 1976). 37:E1580–E1587. 2012.PubMed/NCBI
|
|
21.
|
Yang BP, Wahl MM and Idler CS:
Percutaneous lumbar pedicle screw placement aided by
computer-assisted fluoroscopy-based navigation: perioperative
results of a prospective, comparative, multicenter study. Spine
(Phila Pa 1976). 37:2055–2060. 2012. View Article : Google Scholar
|
|
22.
|
Tian W, Weng C, Li Q, et al: Occipital-C2
transarticular fixation for occipitocervical instability associated
with occipitalization of the atlas in Klippel-Feil syndrome
patients by using intraoperative 3-dimensional navigation system.
Spine (Phila Pa 1976). Nov 2–2012.(Epub ahead of print).
|
|
23.
|
Cho JY, Chan CK, Lee SH and Lee HY: The
accuracy of 3D image navigation with a cutaneously fixed dynamic
reference frame in minimally invasive transforaminal lumbar
interbody fusion. Comput Aided Surg. 17:300–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Guan HG, Wang G, Huo ZM, Shen YB, Chen C
and Liang LK: Minimally invasive surgical treatment for lumbar
degenerative disease with IsoC-3D navigation under Mast Quadrant
system. Zhongguo Gu Shang. 25:451–454. 2012.(In Chinese).
|
|
25.
|
Allam Y, Silbermann J, Riese F and
Greiner-Perth R: Computer tomography assessment of pedicle screw
placement in thoracic spine: comparison between free hand and a
generic 3D-based navigation techniques. Eur Spine J. 22:648–653.
2013. View Article : Google Scholar
|
|
26.
|
Waschke A, Walter J, Duenisch P, Reichart
R, Kalff R and Ewald C: CT-navigation versus fluoroscopy-guided
placement of pedicle screws at the thoracolumbar spine: single
center experience of 4,500 screws. Eur Spine J. 22:654–660. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Tian W, Liu Y, Zheng S and Lv Y: Accuracy
of lower cervical pedicle screw placement with assistance of
distinct navigation systems: a human cadaveric study. Eur Spine J.
22:148–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Yoshida G, Kanemura T and Ishikawa Y:
Percutaneous pedicle screw fixation of a Hangman’s fracture using
intraoperative, full rotation, three-dimensional image
(O-arm)-based navigation: A technical case report. Asian Spine J.
6:194–198. 2012.
|
|
29.
|
Ohnsorge JA, Salem KH, Ladenburger A, Maus
UM and Weißkopf M: Computer-assisted fluoroscopic navigation of
percutaneous spinal interventions. Eur Spine J. 22:642–647. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Fan Chiang CY, Tsai TT, Chen LH, et al:
Computed tomography-based navigation-assisted pedicle screw
insertion for thoracic and lumbar spine fractures. Chang Gung Med
J. 35:332–338. 2012.PubMed/NCBI
|
|
31.
|
Larson AN, Polly DW Jr, Guidera KJ, et al:
The accuracy of navigation and 3D image-guided placement for the
placement of pedicle screws in congenital spine deformity. J
Pediatr Orthop. 32:e23–e29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Dekomien C, Roeschies B and Winter S:
System architecture for intraoperative ultrasound registration in
image-based medical navigation. Biomed Tech (Berl). 57:229–237.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Ungi T, Abolmaesumi P, Jalal R, et al:
Spinal needle navigation by tracked ultrasound snapshots. IEEE
Trans Biomed Eng. 59:2766–2772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Cho JY, Lee SH, Jang SH and Lee HY:
Oblique paraspinal approach for thoracic disc herniations using
tubular retractor with robotic holder: a technical note. Eur Spine
J. 21:2620–2625. 2012. View Article : Google Scholar : PubMed/NCBI
|