Introduction
Thromboangiitis obliterans (TAO), or Buerger’s
disease, is characterized by chronic occlusive segmental lesions of
the peripheral blood vessels (1).
von Winiwarter provided the first description of a patient with TAO
in 1879 (2). In 1908, Buerger
produced a detailed description of the pathological findings of the
amputated limbs of patients diagnosed with TAO (3). TAO mainly affects small- and
medium-sized arteries and veins of the upper and lower extremities.
There are a limited number of case reports describing the influence
of the cerebral and coronary arteries, the aorta, the intestinal
vessels and even multiple-organ involvement in TAO. When TAO is
considered to have been identified in an unusual location, the
diagnosis may be confirmed only by the observation of acute-phase
lesions in the histopathological examination (4,5). TAO
occurs worldwide, with a high incidence in the Middle and Far East,
and a low incidence in South America and Eastern Europe (6).
It is considered that tobacco, immune system
disorders and genes are the three main contributors to TAO
(7). The main pathological
findings for the diagnosis of TAO are thrombus formation and
fibrosis in the blood vessel walls and the perivascular area. TAO
develops slowly and occurs periodically, with symptoms including
ischemic pain, intermittent claudication and skin ulcers. Symptoms
of thrombophlebitis and Raynaud’s phenomenon have been identified
in 40% of patients previously diagnosed with TAO (8,9). The
treatment of TAO is challenging, due to high disability rates;
however, the main treatment methods include microcirculation
improvement, and pain and claudication control (10). A limited number of studies
concerned with familial TAO exist worldwide, as this form of the
disease is rare. This case report describes two brothers who were
diagnosed with TAO. The present study was approved by the Guangzhou
Red Cross Hospital Medical Ethics Committee, Guangzhou, China.
Written informed consetn was obtained from the patients.
Case reports
Case 1
A 33-year-old male was diagnosed with erythema
nodosum in both legs. The disease had occurred repeatedly within
the last 8 years, and the patient’s symptoms had increased in
severity during the past 8 months.
Eight years earlier, the patient had been diagnosed
with erythema nodosum in the bilateral lower leg extensors and the
dorsum of the right foot, without evident causes. Following a
biopsy of the skin tissue from the right foot, the patient was
diagnosed with erythema nodosum. The patient’s symptoms were
marginally relieved and the tubercles partially disappeared
following immune suppression treatment with methotrexate (MTX),
triptriolide (TII), cyclophosphamide, prednisolone and
methylprednisolone, as well as microcirculation improvement
treatment with Xueshuantong tablets and Salvia tetramethylpyrazine.
Eight months ago, inflammation had been identified on the first and
fifth toes, as well as a secretion of pus, which was diagnosed as
paronychia. Following the removal of the first toenail, the
severity of the patient’s symptoms increased. An ulcer developed on
the right foot, which gradually increased in size. Symptoms such as
arthralgia and fever did not occur. The patient had tested positive
for tuberculosis in a purified protein derivative (PPD) test five
years ago, but tuberculosis was not confirmed. Therefore, the
patient was treated with antituberculosis preventive therapy. The
patient was diagnosed with hepatitis B, due to a liver function
test demonstrating alanine aminotransferase (ALT) levels >1,000
U/l. Subsequent medical intervention with compound glycyrrhizin
tablets and diammonium glycyrrhizinate capsules resulted in the
normalization of ALT levels. The patient’s medical history did not
include hyper-tension, diabetes, coronary heart disease, chronic
gastritis, systemic lupus erythematosus (SLE), dermatomyositis,
scleroderma, drug allergy, trauma or blood transfusions. The
patient had a 10-year history of smoking 5–8 cigarettes per day,
and did not abuse alcohol. The physical examination results
revealed normal vital signs. The erythema had decreased in size,
leaving a small level of pigmentation on the skin; however, the
ulcer had increased in size (to 4×5 cm), with partial tendon
exposure and a reduction in the yellow purulent discharge. The
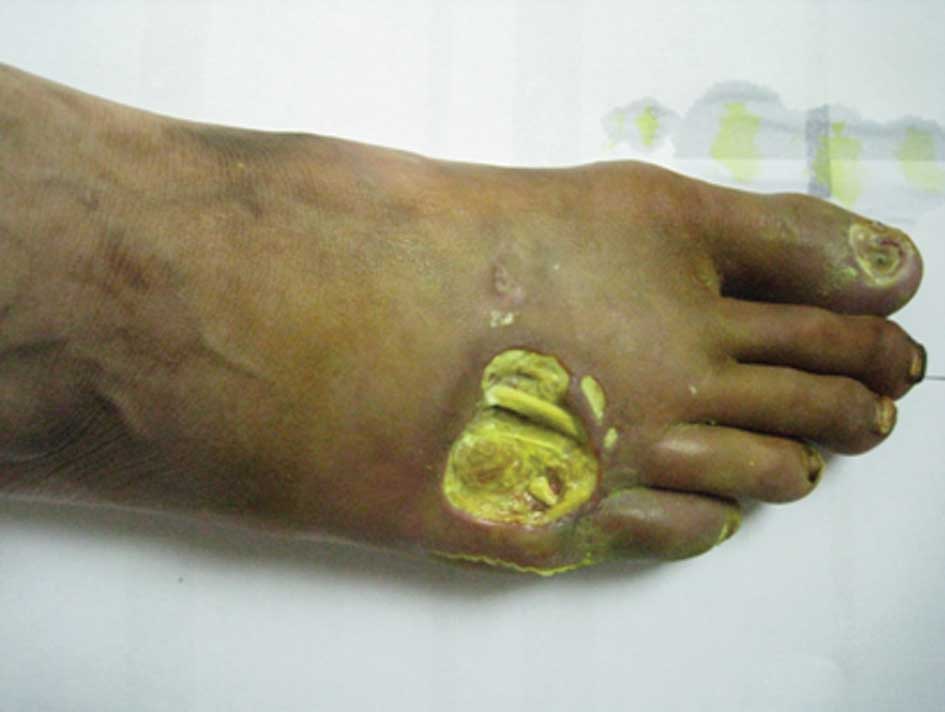
fifth toe of the right foot was swollen and infected (Fig. 1). In addition, the skin temperature
of the right foot was low and the pulse in the top of this foot was
weak. Examination identified low levels of touch sensitivity in the
right foot.
Case 2
A 29-year-old male had been diagnosed with erythema
nodosum 3 years earlier, and this condition had become increasingly
severe in the past 5 months. Three years ago, the patient was
diagnosed with erythema nodosum on the left shank and the top of
the left foot. This was accompanied by numbness and pain in the
feet, without evident reason. Following hormonal and
microcirculation therapy, the symptoms of erythema nodosum
decreased. Five months ago, the patient experienced pain in the
first toe of the left foot, and partial inflammation and an ulcer
were observed. The patient had a 4-year history of smoking 3–5
cigarettes per day. The physical examination revealed normal vital
signs. The erythema nodosum on the left shank had disappeared;
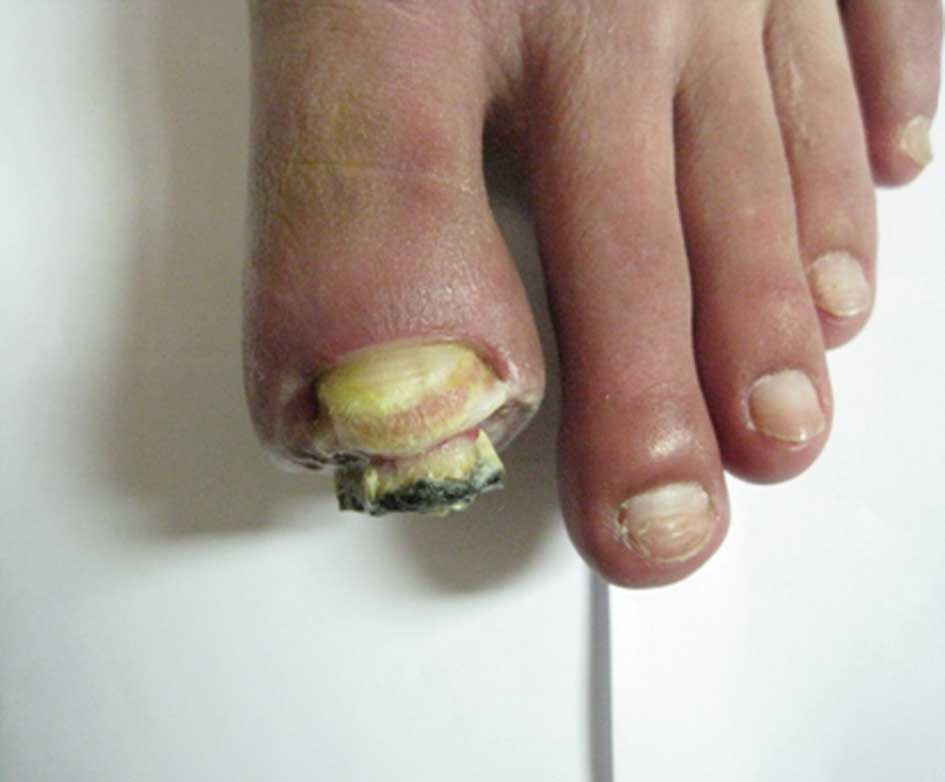
however, the nail of the first toe on the left foot had become
broken and an ulcer was identified on the toe. The ulcer was
partially black with a secretion of pus (Figs. 2 and 3). Furthermore, the skin temperature of
the foot was low, and the pulse in the top of the foot was weak. An
examination identified low levels of touch sensitivity in the left
foot.
Laboratory examination
Case 1
The blood, urine, stool routine, liver and kidney
function, electrocardiogram, chest radiography, arteriovenous color
Doppler ultrasound of bilateral extremities and foot X-ray results
were all normal. The hepatitis B test results were as follows:
HBsAg+, HBeAb+ and HBcAb+. The
immunity test revealed complement component 3 (C3) levels of 0.49
g/l. The T lymphocyte subsets and NK cell counts included
CD3+, 85%; CD4+, 49%; CD8+, 27%;
and NK cells, 8%. The T lymphocyte subset H/S ratio was 1.81. The
rheumatism, antinuclear antibody spectrum and antineutrophil
cytoplasmic antibody test results were negative, as were the
bacterial and fungal cultures. Following pathological examination
of the left shank, collagen-based dermis was identified, without
evident proliferation of the epidermis. An infiltration of
inflammatory cells was observed in the appendage, the vein wall and
fat tissues. A large blood vessel was identified in the injured
tissue. Moreover, inflammation was evident in the inner membrane
and the wall of the blood vessel and a thrombus was identified in
the lumen of the blood vessel (Fig.
4).
Case 2
The blood, urine, routine stool, liver and kidney
function, electrocardiogram, chest radiography, arteriovenous color
Doppler ultrasound of bilateral extremities and foot X-ray results
were all normal. The rheumatism, antinuclear antibody spectrum and
antineutrophil cytoplasmic antibody test results were all negative.
The T lymphocyte subsets and NK cell counts were as follows:
CD3+, 67%; CD4+, 38%; CD8+, 26%;
and NK cells, 12%. Additionally, the T lymphocyte subset H/S ratio
was 1.46, and the bacterial and fungal culture tests were
negative.
Diagnosis
Two patients were 29 and 33 year-old male smokers,
with lower limb involvement. In case 1, a 3×3 cm ulcer was
identified on the skin of the patient’s right foot, with partial
tendon exposure and yellow purulent discharge. Furthermore, the
right fifth toe was swollen and infected. The skin temperature of
the right foot was low, the sensitivity to touch was reduced and
the pulse at the top of the right foot was weak. In case 2,
pathological examination of the left shank revealed collagen-based
dermis, as well as an infiltration of inflammatory cells in
affiliated tissue, vein wall and fat tissues. A large blood vessel,
with a thrombus in the lumen, was observed in the injured tissue.
According to the above features, the two patients were diagnosed
with TAO.
Treatment
Treatments for TAO included stopping smoking,
resting and keeping warm. To promote blood circulation, the patient
was treated with compounds such as Danshen Dripping Pills, Fufang
Danshen Diwan and Salvia tetramethylpyrazine. The patients were
also treated with fibroblast growth factor to promote epidermal
growth and Bayaspirin enteric-coated tablets to reduce platelet
aggregation. The polysaccharide nucleic acid fraction of Bacillus
Calmette-Guérin and compound glycyrrhizin tablets were taken to
improve immune function. Following treatment, the patient in case 2
had reduced pain levels in the left foot. The ulcer on the first
toe of the left foot had decreased in size, with a reduction in pus
secretions and inflammation (Fig.
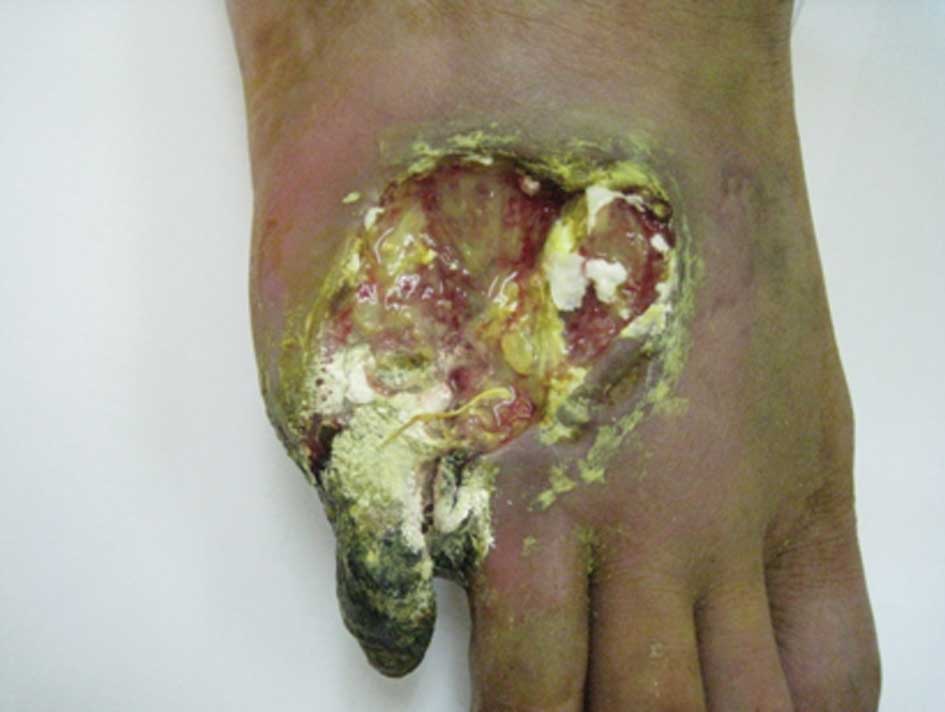
5). The patient in case 1 demonstrated a reduction in pus
secretion from the ulcer; however, the area of the ulcer had
increased, spreading to the fifth toe with gangrene. A tendon had
become exposed on the right foot, which was broken and inducing
severe pain (Fig. 6).
Discussion
The occurrence of TAO has been increasing and has a
marked effect on the health and quality of life of affected
individuals (11). The disease has
a high morbidity in China and frequently occurs in smokers <45
years of age (11). The cause of
TAO remains unknown; however, use or exposure to tobacco is related
to the onset and progression of the disease. Nicotine and
carboxyhemoglobin may cause damage to the structure of endothelial
cells and inflammation of the vascular endomembrane, resulting in
thrombus formation. Thus, an individual’s smoking history is an
important basis for the diagnosis of TAO (6,12).
The patients in cases 1 and 2, who were brothers, had a long-term
history of smoking. Furthermore, it was identified that the levels
of adhesion molecules in the plasma of the two patients, such as
intracellular adhesion molecule 1 (ICAM-1), vascular cell adhesion
molecule 1 (VCAM-1) and inflammatory factor, were significantly
higher than that in healthy individuals. Therefore, it was
concluded that an inflammatory response is significant in the
progression of TAO (13,14). Studies have demonstrated that
anti-collagen, -elastic protein, -endothelial cell, -cardiolipin
and -neutrophil cytoplasmic antibody levels increase during the
active period of TAO (15,16). Therefore, it has been suggested
that hypersensitivity responses and autoimmune disorders are
involved in the progression of TAO. However, the two patients in
the current case report had normal autoimmune antibodies.
Erythema nodosum is a type of skin inflammation with
the histopathology of small vessel vasculitis in the deep dermis
and panniculitis. It is characterized by painful nodules that are
located on the lower extremities. Erythema nodosum may resolve
spontaneously within 3–5 weeks (17). Takanashi et al previously
studied one patient with TAO who had erythema nodosum and a black
reticulum plaque in the early stages of the disease. An early
biopsy indicated that numerous mono-nuclear cells had
subcutaneously infiltrated the dermis and perivascular areas.
Following treatment with prednisolone, the erythema nodosum and
livedo reticularis improved; however, the necrosis and ulceration
of the lower limbs repeatedly occurred. The patient underwent
amputation due to a severe ulcer 1.5 years later. The postoperative
pathological examination demonstrated anterior tibial artery
inflammation and the formation of a thrombus in accordance with
TAO. Therefore, symptoms of erythema nodosum and livedo reticularis
may suggest a diagnosis of TAO (18).
The present case report identified two brothers who
had painful nodules and erythema in the leg. Administration of
immunosuppressive agents resulted in a reduction in the symptoms of
TAO, before the symptoms repeatedly occurred. The pathological
biopsy demonstrated that the vessels had undergone inflammatory
changes. At a later stage, the erythema was significantly reduced,
although increased ulceration was present. Both patients
demonstrated reduced touch sensitivity. A primary biopsy identified
a thrombus in the vascular cavity; therefore, the diagnosis of TAO
was confirmed. TAO is rarely identified in individuals of the same
family. However, due to the present two cases, we propose that
familial factors ought to be considered when conducting TAO
treatment. Wysokinski et al described two brothers who were
simultaneously diagnosed with TAO. It was considered that there was
a correlation between a gene mechanism and TAO (19). Therefore, a complete family history
may be required in clinical practice. Chen et al performed a
gene polymorphism analysis in 131 patients with TAO and 227 healthy
individuals, in order to test the promoters of HLA-DPB1, -DRB1 and
-B, and CD14. The genotype frequency of CD14 TT was significantly
higher in TAO patients than in the healthy controls. Chen et
al suggested that at least three genes were associated with the
occurrence of TAO, and that TAO may be regulated by genes, with the
involvement of innate and acquired immunity(20).
It has been demonstrated that the most effective way
to prevent and treat TAO is to stop smoking (2). Amputation and vascular reconstruction
are not routine treatments, due to chronic ischemia of the distal
extremities. Olin et al revealed that 94% of patients did
not undergo amputation subsequent to stopping smoking, whereas 43%
of patients who smoked underwent amputation surgery (6). Adjuvant drugs, including iloprost,
prostaglandin vasodilator agents, antiplatelet drugs, aspirin and
vasodilator agents (such as, α-adrenergic blocking agents and
calcium antagonists), may effectively reduce the symptoms of pain
associated with TAO. Vascular endothelial growth factor aids the
recovery of ischemic ulcers. In addition, spinal cord stimulation
and surgical sympathectomy have also been reported to be
beneficial; however; the long-term effects are yet to be confirmed
(2,21).
The two patients presented in this case report
stopped smoking due to the diagnosis of TAO. Following treatment
with vasodilator agents, microcirculation improvement interventions
and surgical excision, the ulcer on the left foot of the patient in
case 2 decreased. The patient in case 1 developed dry gangrene and
partial ischemia in the right foot. This patient underwent
amputation of the fifth toe and skin grafting treatment.
References
|
1.
|
Małecki R, Zdrojowy K and Adamiec R:
Thromboangiitis obliterans in the 21st century - a new face of
disease. Atherosclerosis. 206:328–334. 2009.PubMed/NCBI
|
|
2.
|
von Winiwarter F: Über eine eigenthümliche
form von endarteritis und endophlebitis mit gangrän des fusses.
Arch Klin Chir. 23:202–206. 1879.(In German).
|
|
3.
|
Buerger L: Thrombo-angiitis obliterans: a
study of the vascular lesions leading to presenile spontaneous
gangrene. Am J Med Sci. 136:567–580. 1908. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kurata A, Nonaka T, Arimura Y, Nunokawa M,
Terado Y, Sudo K and Fujioka Y: Multiple ulcers with perforation of
the small intestine in Buerger’s disease: a case report.
Gastroenterology. 125:911–916. 2003.PubMed/NCBI
|
|
5.
|
Arkkila PE: Thromboangiitis obliterans
(Buerger’s disease). Orphanet J Rare Dis. 1:142006.
|
|
6.
|
Olin JW: Thromboagiitis obliterans
(Buerger’s disease). N Engl J Med. 343:864–869. 2000.
|
|
7.
|
Pereira de Godoy JM and Braile DM:
Buerger’s disease and anticardiolipin antibodies. J Cardiovasc Med
(Hagerstown). 10:792–794. 2009.
|
|
8.
|
Olin JW, Young JR, Graor RA, Ruschhaupt WF
and Bartholomew JR: The changing clinical spectrum of
thromboangiitis obliterans (Buerger’s disease). Circulation.
82(Suppl): IV3–IV8. 1990.PubMed/NCBI
|
|
9.
|
de Godoy JM, de Godoy MF and Braile DM:
Recurrent thrombosis in patients with deep vein thrombosis and/or
venous thromboembolism associated with anticardiolipin antibodies.
Angiology. 57:79–83. 2006.
|
|
10.
|
Patwa JJ and Krishnan A: Buerger’s disease
(thromboangiitis obliterans) - Management by Ilizarov’s technique
of horizontal distraction. A retrospective study of 60 cases.
Indian J Surg. 73:40–47. 2011.
|
|
11.
|
Darnige L, Helley D, Fischer AM, Emmerich
J, Smadja DM and Fiessinger JN: Platelet microparticle levels: a
biomarker of thromboangiitis obliterans (Buerger’s disease)
exacerbation. J Cell Mol Med. 14:449–451. 2010.PubMed/NCBI
|
|
12.
|
Lawall H, Bramlage P and Amann B:
Treatment of peripheral arterial disease using stem and progenitor
cell therapy. J Vasc Surg. 53:445–453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lee T, Seo JW, Sumpio BE and Kim SJ:
Immunobiologic analysis of arterial tissue in Buerger’s disease.
Eur J Vasc Endovasc Surg. 25:451–457. 2003.PubMed/NCBI
|
|
14.
|
Slavov ES, Stanilova SA, Petkov DP and
Dobreva ZG: Cytokine production in thromboangiitis obliterans
patients: new evidence for an immune-mediated inflammatory
disorder. Clin Exp Rheumatol. 23:219–226. 2005.
|
|
15.
|
Eichhorn J, Sima D, Lindschau C, Turouski
A, Schmidt H, Schneider W, Haller H and Luft FC: Antiendothelial
cell antibodies in thromboangiitis obliterans. Am J Med Sci.
315:17–23. 1998. View Article : Google Scholar
|
|
16.
|
Saito Y, Sasaki K, Katsuda Y, Murohara T,
Takeshita Y, Okazaki T, Arima K, Katsuki Y, Shintani S, Shimada T,
et al: Effect of autologous bone-marrow cell transplantation on
ischemic ulcer in patients with Buerger’s disease. Circ J.
71:1187–1192. 2007.
|
|
17.
|
Wang Z, Liu LQ, Ling HZ and Yang JM: Value
of serum anti-neutrophil cytoplasmic autoantibodies and
anti-vascular endothelial cell antibodies in diagnosis of erythema
nodosum. China Trop Med. 8:1554–1555. 2008.
|
|
18.
|
Takanashi T, Horigome R, Okuda Y, Nose M,
Matsuda M and Ikeda S: Buerger’s disease manifesting nodular
erythema with livedo reticularis. Intern Med. 46:1815–1819.
2007.
|
|
19.
|
Wysokinski WE, Kwiatkowska W, Maslowski L,
Witkiewicz W and Kowal-Gierczak B: Buerger’s disease in two
brothers: iliac artery occlusion by thromboangiitis obliterans -
case reports. Angiology. 49:409–414. 1998.
|
|
20.
|
Chen Z, Takahashi M, Naruse T, Nakajima T,
Chen YW, Inoue Y, Ishikawa I, Iwai T and Kimura A: Synergistic
contribution of CD14 and HLA loci in the susceptibility to Buerger
disease. Hum Genet. 122:367–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Isner JM, Baumgartner I, Rauh G,
Schainfeld R, Blair R, Manor O, Razvi S and Symes JF: Treatment of
thromboangiitis obliterans (Buerger’s disease) by intramuscular
gene transfer of vascular endothelial growth factor: preliminary
clinical results. J Vasc Surg. 28:964–973. 1998.
|